Abstract
Mycobacterium microti (vole tuberculosis) infections in small wild mammals were first described more than 60 years ago in several populations in Great Britain. Few studies of vole tuberculosis have been undertaken since then, and little is known about the relationship between M. microti isolates originating from different populations or at different times or of the prevalence of this infection in wild rodent populations, despite human cases of M. microti infections being increasingly reported. In this study, field voles (Microtus agrestis), bank voles (Clethrionomys glareolus), and wood mice (Apodemus sylvaticus) were found to be infected, with up to 8% having external tuberculous signs, in wild populations in Northumberland and Cheshire, England. Spoligotyping applied directly to the clinical material simultaneously detected and typed M. microti bacteria in skin lesions, lymph glands, and internal abcesses. IS6110 restriction fragment length polymorphism typing of cultured bacteria was used to compare these isolates with previously isolated strains from both animals and humans. This demonstrated that although the current rodent isolates were distinct from those isolated from voles in the 1930s in Great Britain, they had a high degree of similarity to these strains and were distinct from the M. microti isolates from humans, a pig, and a ferret from The Netherlands. Thus, M. microti infection seems to be widespread in wild rodent populations, but more studies are needed to understand how M. microti might be transmitted from animals to humans and to determine better the zoonotic risk posed.
Tuberculosis (TB) in small wild mammals, namely, field voles (Microtus agrestis), bank voles (Clethrionomys glareolus), wood mice (Apodemus sylvaticus), and shrews (Sorex araneus), was first reported by Wells and Oxon in 1937 (25). The causative agent was named Mycobacterium tuberculosis subsp. muris (4) and later Mycobacterium microti (or vole tuberculosis), a member of the M. tuberculosis complex (24). The other members of this complex are M. tuberculosis, Mycobacterium bovis (including the attenuated BCG vaccine strains), Mycobacterium africanum, and the recently described subspecies Mycobacterium canetti (22, 23). M. microti has a characteristic pleomorphic microscopic morphology, with sickle-shaped, spiral, or S-like forms being seen in fresh material. This typical curved appearance is, however, usually lost during in vitro culture (18). It is difficult to distinguish M. microti from other members of the M. tuberculosis complex on the basis of biochemical properties, but they are readily identified by PCR-based spoligotyping and/or IS6110 restriction fragment length polymorphism (RFLP) typing (23). The latter requires large numbers of cultivated bacteria but facilitates more detailed phylogenetic comparisons.
Well's early studies showed a varying prevalence of M. microti of 9 to 31% in field voles, depending on the place and season of capture, and overall prevalences of 11% in bank voles, 2% in wood mice, and 1.5% in shrews (26). No further data are available on the prevalence of M. microti in wild mammals, and in general, little attention has been given to M. microti as a causative agent of tuberculosis, with only sporadic cases being documented. It was isolated from a rock hyrax (Procavia capensis) in South Africa in 1960 (19) and from a pig in The Netherlands in 1965 (9). Pattyn et al. (17) described a disseminated M. microti infection in a llama (Llama vicugna molina) from the Antwerp zoo in 1970, and it was isolated from a pet ferret, also in The Netherlands, in 1993 (23).
In the late 1990s, as part of a project to develop a standardized method for the identification and nomenclature of M. bovis, spoligotype patterns of M. tuberculosis complex strains from the Central Veterinary Laboratory in the United Kingdom were compared to those in an international database at the National Institute of Public Health and the Environment in The Netherlands. The patterns of 11 M. tuberculosis complex isolates from the Central Veterinary Laboratory database were identical or highly similar to the spoligotypes of M. microti isolates. These isolates were from eight domestic cats, a cow, a wild badger (Meles meles), and one human (12, 23), raising the possibility that the prevalence of M. microti is greater than was hitherto assumed.
M. microti has long been considered unimportant as a human pathogen (13). However, the diagnosis of M. microti infections is severely hampered by the slow growth in vitro of the bacteria on primary isolation, and routine diagnostic laboratories that culture for M. tuberculosis bacteria for up to 4 weeks, as is standard, will probably miss M. microti, since at least 8 weeks of incubation is needed (23, 26). When a database of nearly 6,000 IS6110 RFLP patterns of M. tuberculosis complex isolates from human isolates was searched, two M. microti infections were diagnosed for the first time by van Soolingen et al. (23). In the same study, two other human M. microti infections were diagnosed by their M. microti-specific spoligopatterns (23). Three of these four cases were in immuncompromised individuals, and one was in a 39-year-old immunocompetent man. Later, a fifth case caused by M. microti was diagnosed in another immunocompetent patient in the Netherlands (D. van Soolingen, unpublished observation). More recently still, severe forms of tuberculosis caused by M. microti have been diagnosed in two patients in Germany (16) and in one patient in Switzerland (I. Fengels, R. Mayer, B. Frauchiger, D. van Soolingen, B. Villiger, and G. E. Pfyffer, poster from the Congress of the Swiss Society of Pneumology, 2000), all of whom were immunocompetent. Since these M. microti infections were recognized through the application of molecular techniques, it is conceivable that they represent only a small proportion of the true number of human cases.
The present study describes the identification, characterization, and distribution of M. microti in wild rodents at two sites in England. Such studies are needed to provide some insight into how M. microti might be transmitted from animals to humans and to understand better the zoonotic risk posed. Furthermore, this paper describes the genetic relatedness of the vole isolates of the present study to the previously published M. microti strains on the basis of IS6110 RFLP.
MATERIALS AND METHODS
Isolation of M. microti from rodents.
Samples were collected from Kielder, Northumberland, in northeastern England and Cheshire, northwestern England. In Kielder, field voles were trapped monthly, using Ugglan special mousetraps, at six 0.3-ha sites, located in clear-fell areas of 5 to 10 ha, in March to October 1998 and March to October 1999 (inclusive) and in March and April 2000 (I. M. Graham and X. Lambin, unpublished data). The numbers of voles in Kielder with clinical signs of TB infection, that is, the characteristic skin lesions described by Wells or obvious lymphadentitis (26), were recorded. Material from skin lesions was sampled from nine live field voles, and enlarged lymph nodes were removed from four field voles and one bank vole that had died in the traps. Additionally, 20 fecal and two urine samples were collected in April 2000 from live field voles with clinical signs of TB.
A snap-trap study was undertaken in Kielder from April to July 2000 as part of a separate project. Postmortems were performed on 180 field voles captured, and gross internal lesions typical of TB (25, 26) were recorded. The lungs from nine voles and internal caseous lesions from seven voles were removed for detection and typing of M. microti.
In Cheshire, bank voles and wood mice were trapped in woodland on a monthly basis as part of a long-term study of host-pathogen dynamics (2, 3, 5, 8), but for most of this time TB was not suspected and therefore was not monitored. On the basis of the results from Kielder, however, TB-like skin lesions were removed from five animals (three bank voles and two wood mice) in 1998 and 1999 for laboratory analysis. Since the clinical samples were collected solely for microbiological examination and since lesions at the Cheshire sites were not routinely recorded, prevalence studies were not carried out here.
Culture and DNA extraction.
Clinical samples were decontaminated and grown in two different ways. Five samples were homogenized in N-acetylcysteine-NaOH and inoculated into MB/BacT Process bottles. These were incubated at 37°C in the MB/BacT mycobacteria detection system (Organon Teknika Corp., Durham, N.C.). Thirteen samples of external lesions were each sonicated in 0.1 ml of phosphate-buffered saline for approximately 1 min using an ultrasonic power unit operating at 1.7 A. Thereafter the samples were decontaminated in 0.3 ml of 6% H2SO4 at room temperature for 10 min and neutralized with 1.5 ml of neutralization reagent. This reagent was prepared by dissolving 89 g of Na2HPO4 · 2H2O, 68 g of KH2PO4, and 20 mg of phenol red in 1,000 ml of distilled water and adjusting the pH to 11.0 with 5 M NaOH. Of this solution, 0.5 ml was incubated on Löwenstein-Jensen medium supplemented with 12.5 g of pyruvate per liter and on modified Dubos medium (24) at 35.5°C.
DNA was extracted from all samples of clinical material (with the exception of the feces and urine samples) for spoligotyping by incubation overnight at 60°C in digestion buffer (1:1), consisting of 500 mM Tris (pH 9.0), 20 mM EDTA, 10 mM NaCl, 1% sodium dodecyl sulfate, and 0.5 mg of proteinase K per ml, followed by DNA purification using the QIAamp DNA mini kit (Qiagen Ltd., Crawley, United Kingdom) as specified by the manufacturer for purification of tissue material. DNA was extracted from the feces and urine as specified by the manufacturer of the QIAamp mini kit for stool samples.
Molecular typing.
Spoligotyping was performed as described previously (10, 21, 23). Extracted DNA was tested both neat and diluted 1:100. A total of 60 samples were subjected to spoligotyping. They included material from 2 of the cultures of lesions from Kielder field voles; external lesions from 9 Kielder field voles, 3 Cheshire bank voles, and 2 Cheshire wood mice; lymph node material from 4 Kielder field voles (5 samples, since lymph nodes were taken from both the retropharyngeal region and abdomen of one of the voles) and 1 Kielder bank vole; 20 fecal, 2 urine, and 9 lung samples from Kielder field voles; and internal caseous lesions from an additional 7 Kielder field voles.
To control for the occurrence of possible PCR inhibitory effects, 10 of the 60 samples were randomly chosen to test for the presence of inhibitory compounds. Each of these 10 samples was subjected to spoligotyping twice: one subsample was tested in the standard way together with the other samples, and the second subsample was spiked with 1 ng of M. tuberculosis DNA.
Standard IS6110 RFLP typing, as described previously (20), was performed on successful cultures of M. microti (three Kielder field voles and one Cheshire bank vole).
Computer-assisted analysis of DNA fingerprints.
Using Gelcompar (version 4.1; Applied Maths, Kortrijk, Belgium), the IS6110 RFLP patterns of the current isolates were compared with those of previous M. microti isolates including those isolated from voles in the United Kingdom by Wells in the 1930s (23, 25). Furthermore, the IS6110 RFLP patterns of all M. microti isolates were compared with the international database consisting of 5,909 IS6110 RFLP patterns of M. tuberculosis isolates originating in 33 different countries worldwide, held at the National Institute of Public Health and the Environment in The Netherlands.
Pathology.
Frozen material from four skin lesions was fixed in 10% formalin. In addition, one male field vole, also from Kielder, with a large external skin lesion between the scapulae was killed following removal of lesion material (for molecular typing) and fixed in formalin. At necropsy, axillary, mandibular, and mesenterial lymph nodes, lungs, heart, liver, spleen, kidneys, adrenals, colon, and femur were processed by standard procedures for embedding in paraffin. Paraffin sections were stained with hematoxylin-eosin [HE], Auramine-rhodamine [AR] or Ziehl-Neelsen stain [ZN]. HE and ZN pictures were taken with an SV-MICRO digital microphotocamera (Sound Vision, Wayland, Mass.). AR fluorescence was imaged with a Bio-Rad 1024 confocal laser-scanning microscope equipped with an argon/krypton laser. The 568-nm line was used to excite rhodamine.
RESULTS
Prevalence of clinical TB in wild rodents.
Animals were designated as having clinical TB if they had the characteristic skin lesions described by Wells (26) or obvious lymphadentitis. Most sampling from the Cheshire sites was carried out without reference to TB. Thus, prevalence has been estimated only for the Kielder samples. There, 101 cases of clinical TB were recorded throughout the study period from a total of 4,852 field voles examined (ca. 2% prevalence). There was a significantly higher prevalence in 1999 (67 of 2,495 = 2.7%) than in 1998 (12 of 1946 = 0.6%; χ2 = 26.8; 1 degree of freedom; P < 0.001), and this increase appeared, from the two months sampled, to have continued in 2000 (22 of 411 = 5.4%; χ2 = 3.4; 1 degree of freedom; P = 0.066), reaching around 8% (14 of 172) in April 2000. Detailed analysis of the influence of site, season, and vole density on prevalence will be described elsewhere (R. Cavanagh, X. Lambin, T. Ergon, M. Bennett, I. M. Graham, D. van Soolingen, and M. Begon, unpublished data).
From the snap-trap study, 13 of 180 voles had clinical signs of TB (7% compared to the 8% prevalence in animals live trapped at a similar time [see above]). However, dissection revealed that a further 25 had internal tuberculous lesions in the form of cream-colored caseous abscesses under the skin or in the abdomen (overall prevalence, 21%).
Detection and identification of mycobacteria by spoligotyping.
The characteristic spoligotype pattern for M. microti, in which spacer sequences 37 and 38 are present (23), was obtained for 24 of the 60 samples tested. The spoligotypes of 13 of the 14 skin lesions clearly indicated M. microti infection, and 1 was negative. The negative result was from a small lesion, less characteristic in appearance than the others. Spoligotyping of all lymph node samples showed the M. microti pattern except for one mesenteric lymph node from a field vole, and five of the internal caseous abscess samples also had the characteristic pattern, as did the two cultures. The fecal, urine, and lung samples were all negative, however.
Of the 10 samples spiked with M. tuberculosis DNA, there was complete inhibition of the spoligotyping PCR when the DNA was used neat for 7 samples, and when it was diluted, 2 of the samples were still inhibited.
Culture and RFLP typing.
After 12 weeks, cultures were successfully obtained from lesion material from three field voles and one bank vole. Of these, 2 were included in the 13 samples that were incubated on Löwenstein-Jensen and modified Dubos medium. Growth of the two samples was detected on both media, with the Dubos medium showing somewhat better and faster growth. The other two successfully grown strains were from the five samples that were incubated in the MB/BacT mycobacteria detection system. Culture has so far been unsuccessful from other lesion and lymph node material tested in this study.
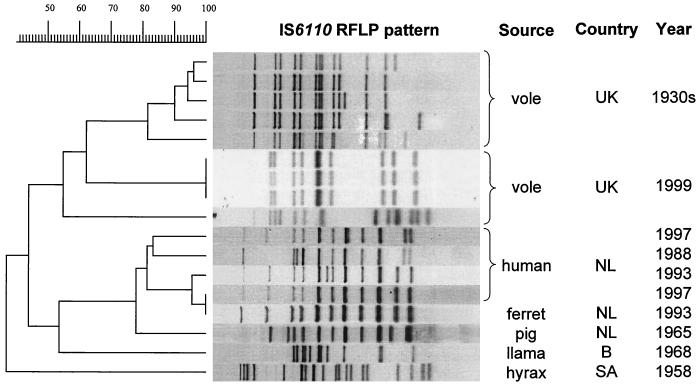
The results of the IS6110 RFLP typing of the four cultured isolates (voles UK 1999) are shown in Fig. 1. The three field vole isolates from Kielder all displayed identical IS6110 banding patterns, which exhibited 62% similarity to those for the five vole strains from Wells's collection from the 1930s. The bank vole isolate from Cheshire had a different pattern, which exhibited an overall similarity of 55% to those for the other eight vole strains. The comparison of all IS6110 RFLP patterns of M. microti isolates revealed two distinct groups: one of isolates from the United Kingdom and one of isolates from The Netherlands. The isolates within each of these two groups showed a high degree of similarity as can be seen in Fig. 1. There is more genetic diversity between the two groups, with a similarity of only 45%. The hyrax isolate was the most different of all.
FIG. 1.
Comparison of the IS6110 RFLP patterns of the M. microti strains identified in this study and those of the M. microti strains from previous studies.
Comparison of all IS6110 RFLP patterns of M. microti isolates, from this study and a previous study (23), with those of the M. tuberculosis complex isolates present in the international database showed that the RFLP patterns of the M. microti isolates were unique: all other M. tuberculosis complex patterns had less than 80% similarity to M. microti. The M. microti isolates formed four different groups, quite distantly related, in the dendrogram of the international database. The first group consisted of the three vole isolates from Kielder, with identical IS6110 RFLP patterns and a similarity of 68% to the RFLP pattern of the llama isolate from the Antwerp zoo. The second group consisted of the scab isolate from Cheshire together with the vole isolates from the 1930s, which matched with a similarity of 56.9%. The third group was formed by the isolates from a pig, a ferret, and four humans from The Netherlands. The RFLP pattern of the hyrax isolate was found to be unique in the international database.
Pathology.
All four dermal lesions showed necrotic histiocytosis with some calcification. One lesion showed exudative tuberculosis, with necrotic macrophages and large numbers of intracellular mycobacteria. In other lesions, more proliferative tuberculosis was observed, with fewer bacteria in macrophages.
The killed vole appeared to be in a good nutritional state despite the presence of widespread tuberculous lesions. All its lung lobes contained firm light yellow nodules up to 1 cm in diameter. Histological examination revealed granulomatous interstitial and bronchoalveolar lesions. In ZN- and AR-stained sections, mycobacteria were abundant, especially in alveolar lumina bordering necrotic lesions and bronchioles. Periarterial plasmacellular cuffs were prominent. All lymph nodes showed lymphodepletion, plasmacytosis, and a variable number of microgranulomas. Draining axillary lymph nodes were more severely affected, with classic tubercles containing larger numbers of mycobacteria, than were nondraining lymph nodes. With the exception of the intestine and bone marrow, all other organs or tissues showed histopathological abnormalities, such as microgranulomas and calcification and variable numbers of mycobacteria.
DISCUSSION
This study confirms that 60 years after they were first discovered, M. microti infections are prevalent in wild rodent populations, although there are few reports of the condition in the intervening period (23). We suspect that, as in human cases, M. microti in wild rodents has simply been overlooked, despite increasing interest in M. bovis infections in wildlife (11, 15).
Wells (25, 26) obtained higher prevalences than those reported here, perhaps because he examined all animals postmortem, enabling him to record internal tuberculous lesions, which appear to develop earlier in the pathogenesis of the condition than external skin lesions do. In support of this, our postmortem study of snap-trapped field voles revealed a prevalence of infection in April to July 2000 of 21% rather than the 7% obtained based on external signs alone.
The optimal approach to diagnosing infection in the field, without sacrificing animals, would be the detection of M. microti in feces or urine of infected rodents. Wells (26) found a high prevalence of tuberculous lesions in the gastrointestinal and urinary tracts, and feces and urine can be collected directly from captured rodents. In this study, however, we failed to detect M. microti in fecal and urine samples from voles with clinical signs of TB by using spoligotyping. Pathogenesis studies are therefore essential to establish whether M. microti is excreted in feces and/or urine and, if so, whether it is excreted throughout the infection. Further work is also necessary to optimize DNA extraction to remove PCR inhibitors. In addition, oral swabbing might be tried, since Wells also found a high prevalence of pulmonary lesions (26).
The spoligopatterns of M. microti in field voles, bank voles, and wood mice in this study were indistinguishable from those of strains isolated from British voles in the 1930s and, indeed, most other M. microti isolates. However, RFLP typing using IS6110 enabled finer differentiation of the successfully cultured isolates. The fingerprint patterns of isolates from three recently captured Kielder field voles were indistinguishable from each other but different from that of the Cheshire bank vole isolate and from those of the vole isolates from various British sites in the 1930s. The isolates from The Netherlands appear to cluster separately from the British isolates, but they differ according to the geographic source within each of these countries (unfortunately, the information regarding the exact geographic location within Britain of the five 1930s isolates is not known). This suggests long-term evolutionary divergence between the bacteria found in Great Britain and mainland Europe. In contrast, the strains isolated with a time interval of 60 years in Britain showed a high degree of similarity (62%). The half-life of IS6110 RFLP, which was determined on the basis of the rate of transpositions in 546 serial patient isolates, of 3 to 5 years could be in range with this divergence (6). Least closely related to the other M. microti isolates was that from a South African hyrax: this isolate also had an unusual spoligopattern (23). In contrast to the findings by IS6110 RFLP, on the basis of spoligotyping alone, all M. microti isolates in the database form a group clearly separate from other members of the M. tuberculosis complex. This indicates that M. microti, regardless of host or geographic origin, constitutes an evolutionarily conserved group of bacteria. Clearly, more work is required to clarify the nature of strain variation with host, region, and time of isolation.
Although more isolates from a variety of host species need to be studied, RFLP typing did not provide any evidence for the existence of a specific M. microti strain adapted to the human host, although human-to-human transmission may occur (7). This, together with the high prevalences of M. microti found in wild rodents, both in this study and by Wells (25, 26), suggests that these animals are likely to be the reservoir for the disease in humans. Furthermore, there is strong circumstantial evidence for direct zoonotic transmission of M. microti infection from rodents to humans (23; Fengels et al., Poster). Sometimes, however, transmission may occur via liaison hosts, such as domestic cats or pet ferrets: such routes can be important for other zoonotic infections of rodents, for example, cowpox virus (1) and plague (14).
Further study of the epidemiology of vole tuberculosis is timely, with the increased recognition of M. microti infections in humans. We suspect that particularly in environments with a high level of human-rodent interaction and less than optimal hygiene conditions, many cases of tuberculosis due to M. microti infections may remain undiagnosed.
Acknowledgments
We thank Walter van Ginkel, Mimount Enaimi, and Piet Overduin (RIVM) for help with the molecular typing and Andy Wattret (University of Liverpool) and Siska Gielis (RIVM) for histotechnical assistance.
R.C. was funded by National Environmental Research Council (NERC) Studentship GT04/97/149/TS. The laboratory work in The Netherlands was funded by EU grant BMH 4-97-2102-247070. I.M.G. was funded by NERC Studentship GT04/97/TS/10, and T.E. was funded by the Norwegian Research Council and the Norwegian Academy of Science.
REFERENCES
- 1.Baxby, D., M. Bennett, and B. Getty. 1994. Human cowpox: a review based on 54 cases, 1969-93. Br. J. Dermatol. 131:598-607. [DOI] [PubMed] [Google Scholar]
- 2.Begon, M., S. Feore, K. Bown, J. Chantrey, T. Jones, and M. Bennett. 1998. The population dynamics of cowpox virus infection in bank voles: testing fundamental assumptions. Ecol. Lett. 1:82-86. [Google Scholar]
- 3.Begon, M., S. M. Hazel, D. Baxby, K. Bown, R. Cavanagh, J. Chantrey, T. Jones, and M. Bennett. 1999. Transmission dynamics of a zoonotic pathogen within and between wildlife host species. Proc. R. Soc. London Ser. B 266:1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brooke, W. S. 1941. The vole acid-fast bacillus. Am. Rev. Tuberc. 43:806-816. [Google Scholar]
- 5.Chantrey, J., H. Meyer, D. Baxby, M. Begon, K. Bown, S. Feore, T. Jones, W. I. Montgomery, and M. Bennett. 1999, Cowpox: reservoir hosts and geographic range. Epidemiol. Infect. 122:455-460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Boer, A. S., M. W. Borgdorff, P. E. W. de Haas, N. J. D. Nagelkerke, J. D. A. van Embden, and D. van Soolingen. 1999. Analysis of rate of change of IS6110 RFLP patterns of Mycobacterium tuberculosis based on serial patient isolates. J. Infect. Dis. 180:1238-1244. [DOI] [PubMed] [Google Scholar]
- 7.Foudraine, N. A., D. van Soolingen, G. T. Noordhoek, and P. Reiss. 1998. Pulmonary tuberculosis due to Mycobacterium microti in a human immunodeficiency virus-infected patient. Clin. Infect. Dis. 27:1543-1544 [DOI] [PubMed] [Google Scholar]
- 8.Hazel, S. M., D. Baxby, M. Bennett, K. Bown, R. Cavanagh, J. Chantrey, T. R. Jones, and M. Begon. 2000. A longitudinal study of endemic disease in its wildlife reservoir: cowpox and wild rodents. Epidemiol. Infect. 124:551-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huitema, H., and F. H. J. Jaartsveld. 1967. Mycobacterium microti infection in a cat and some pigs. Antonie Leeuwenhoek 33:209-212. [DOI] [PubMed] [Google Scholar]
- 10.Kamerbeek, J., L. M. Schouls, M. van Agterveld, D. van Soolingen, A. E. Bunschoten, A. H. J. Kolk, S. Kuijper, and J. D. A. van Embden. 1997. Rapid detection and simultaneous strain differentiation of Mycobacterium tuberculosis for diagnosis and tuberculosis control. J. Clin. Microbiol. 35:907-914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krebs, J. R., R. Anderson, T. Clutton-Brock, I. Morrison, D. Young, and C. Donnelly. 1997. Bovine tuberculosis in cattle and badgers. MAFF Publications, London, United Kingdom.
- 12.Kremer, K., D. van Soolingen, J. van Embden, S. Hughes, J. Inwald, and G. Hewinson. 1998. Mycobacterium microti: more widespread than previously thought. J. Clin. Microbiol. 36:2793-2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liebana, E., A. Aranaz, B. Francis, and D. Cousins. 1996. Assessment of genetic markers for species differentiation within the Mycobacterium tuberculosis complex. J. Clin. Microbiol. 34: 933-938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Macy, D. W. 1998. Plague, p. 295-300. In C. E. Greene (ed.), Infectious diseases of the dog and cat. The W. B. Saunders Co., Philadelphia, Pa.
- 15.Ministry of Agriculture, Fisheries and Food. 1999. TB in cattle. Reducing the risk. MAFF Publications, London, United Kingdom.
- 16.Niemann, S., E. Richter, H. Dalugge-Tamm, H. Schlesinger, D. Graupner, B. Konigstein, G. Gurath, U. Greinert and S. Rusch-Gerdes. 2000. Two cases of Mycobacterium microti-derived tuberculosis in HIV-negative immunocompetent patients. Emerg. Infect. Dis. 6:539-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pattyn, S. R., F. A. Portaels, P. Kageruka, and P. Gigase. 1970. Mycobacterium microti infection in a zoo-llama: Lama vicugna molina. Acta Zool. Pathol. Antwerp. 51:17-24. [PubMed] [Google Scholar]
- 18.Pattyn, S. R., F. A. Portaels, L. Spanogne, and J. Magos. 1970. Further studies on African strains of Mycobacterium tuberculosis. Comparison with M. bovis and M. microti. Ann. Soc. Belge Med. Trop. 50:211-228. [PubMed] [Google Scholar]
- 19.Smith, N. 1960. The ′dassie' bacillus. Tubercle 41:203-212. [DOI] [PubMed] [Google Scholar]
- 20.van Embden, J. D., M. D. Cave, J. T. Crawford, J. W. Dale, K. D. Eisenach, B. Gicquel, P. Hermans, C. Martin, R. McAdam, T. M. Shinnick, et al. 1993. Strain identification of Mycobacterium tuberculosis by DNA fingerprinting: recommendations for a standardized methodology. J. Clin. Microbiol. 31:406-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Soolingen, D., L. Qian, P. E. de Haas, J. T. Douglas, H. Traore, F. Portaels, H. Z. Qing, D. Enkhsaikan, P. Nymadawa, and J. D. van Embden. 1995. Predominance of a single genotype of Mycobacterium tuberculosis in countries of east Asia. J. Clin. Microbiol. 33:3234-3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Soolingen, D., T. Hoogenboezem, P. E. W. de Haas, P. W. M. Hermans, M. A. Koedam, K. S. Teppema, P. J. Brennan, G. S. Besra, F. Portaels, J. Top, L. M. Schouls, and J. D. A. van Embden. 1997. A novel pathogenic taxon of the Mycobacterium tuberculosis complex, Canetti: characterization of an exceptional isolate from Africa. Int. J. Syst. Bacteriol. 47:1236-1245. [DOI] [PubMed] [Google Scholar]
- 23.van Soolingen, D., A. G. M. van der Zanden, P. E. W. de Haas, G. T. Noordhoek, A. Kiers, N. A. Foudraine, F. Portaels, A. H. J. Kolk, K. Kremer, and J. D. A. van Embden. 1998. Diagnosis of Mycobacterium microti infections among humans by using novel genetic markers. J. Clin. Microbiol. 36:1840-1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wayne, L. G., and G. P. Kubica. 1986. The mycobacteria, p. 1435-1457. In P. H. A. Sneath and J. G. Holt (ed.), Bergey's manual of systematic bacteriology, vol. 2. The Williams & Wilkins Co., Baltimore, Md.
- 25.Wells, A. Q., and D. M. Oxon. 1937. Tuberculosis in wild voles. Lancet i:1221.
- 26.Wells, A. Q. 1946. The murine type of tubercle bacillus (the vole acid-fast bacillus). Sir William Dunn School of Pathology, University of Oxford. Spec. Rep. Ser. Med. 259:1-48.