Abstract
Aspergilloma and invasive aspergillosis are important opportunistic infections caused by Aspergillus species, among which Aspergillus fumigatus is the most common species associated with human disease. We developed an enzyme-linked immunosorbent assay (ELISA)-based antibody assay with Afmp1p, a purified recombinant antigenic cell wall galactomannoprotein of A. fumigatus. Evaluation of the test with guinea pig sera against A. fumigatus and other pathogenic fungi indicated that this assay was specific for A. fumigatus. Clinical evaluation revealed that the assay was 100% sensitive for patients with aspergilloma and 33.3% sensitive for patients with invasive aspergillosis. No false-positive results were found for serum samples from 80 healthy blood donors, 6 patients with typhoid fever, 4 patients with melioidosis, 20 patients with penicilliosis marneffei, 5 patients with candidiasis, and 4 patients with cryptococcosis, indicating a high specificity of the test. Thus, this ELISA-based test for the detection of anti-Afmp1p antibody can be of significant value as a diagnostic for aspergillosis.
Since the last decade, Aspergillus spp. are gaining prominence as opportunistic pathogens. In immunocompetent hosts, Aspergillus species rarely causes serious illnesses, except for aspergilloma in patients with preexisting chronic lung diseases. On the other hand, invasive aspergillosis is one of the most important infectious causes of mortality in patients with hematological malignancies and bone marrow transplant (BMT) recipients, with an incidence of 6% in our recent study on 230 BMT recipients (13). Furthermore, up to 2.5% of solid organ transplant recipients, 12% of patients with AIDS, and 40% of patients with chronic granulomatous disease could be affected by this infection (6). The mortality rate in patients with invasive aspergillosis with pulmonary involvement and persistent neutropenia was 95% (4). Of all the known Aspergillus spp., Aspergillus fumigatus is the most common species associated with human disease.
The successful management of invasive aspergillosis is hampered by difficulties in establishing diagnosis. The “gold standard” for making a diagnosis is to obtain a positive culture of A. fumigatus and to demonstrate histological evidence of mycelial invasion from tissue biopsy. Due to the very sick nature of these patients and often the presence of bleeding diathesis, tissue biopsy is often not possible or acceptable by patients. For serological diagnosis of invasive aspergillosis, although commercial kits for antigen detection assay using monoclonal antibody against the galactomannan antigen extract is available for clinical use, no commercially available antigen or antibody detection kit based on recombinant antigens of Aspergillus is currently available. Recombinant antibody and antigen detection tests may offer a higher specificity and reproducibility. Moreover, recombinant antigens and generated antibodies are easy to standardize.
Recently, we have described the cloning of the AFMP1 gene, which encodes an antigenic cell wall galactomannoprotein of A. fumigatus (Afmp1p), and immunoprecipitation studies showed that patients with invasive A. fumigatus infections develop specific antibody against Afmp1p (12). In this study, we report the development of an enzyme-linked immunosorbent assay (ELISA)-based antibody test for the serodiagnosis of invasive A. fumigatus infection with a purified recombinant Afmp1p protein. The sensitivities and specificities of such an assay in patients with aspergilloma and invasive aspergillosis are also compared.
MATERIALS AND METHODS
Strains and growth conditions.
A. fumigatus, A. flavus, A. niger, and A. terreus were clinical isolates from patients with invasive aspergillosis after BMT at Queen Mary Hospital, Hong Kong (13). Penicillium marneffei was a clinical isolate from a patient with systemic penicilliosis at Queen Mary Hospital. Candida albicans was a blood culture isolate from a patient with systemic candidiasis at Queen Mary Hospital. Histoplasma capsulatum (ATCC 26032) and Blastomyces dermatitidis (ATCC 26199) were obtained from the American Type Culture Collection (Manassas, Va.).
The fungi were grown first on Sabouraud agar plates at 37°C for 2 to 3 days to get single colonies. Broth cultures were obtained by inoculating fungal cells from plates into the synthetic medium RPMI (Gibco-BRL, Gaithersburg, Md.) and further shaking at 37°C for 1 to 5 days to achieve a cell density of >105/ml of culture.
Expression and purification of recombinant Afmp1p protein from Escherichia coli.
To produce a fusion plasmid for protein purification, primers AMPF1 (5′-TCTCCTCCTACAACGGTGGT-3′) and AMPR1 (5′-AGAGGTCAGAGCCAGAGCAT-3′) were used to amplify the AFMP1 gene from the pBSK-AFMP1 plasmid. The sequence coding for amino acid residues 18 to 284 of Afmp1p was amplified and cloned into the BamHI and EcoRI sites of expression vector pGEX-2T in frame and downstream of the glutathione S-transferase (GST) coding sequence. The GST-Afmp1p fusion protein was expressed and purified with the GST Gene Fusion System (Pharmacia) according to the manufacturer's instructions. Approximately 10 mg of purified protein was routinely obtained from 1 liter of E. coli carrying the fusion plasmid.
Animal and human sera.
Guinea pig antiserum against Afmp1p was produced by injecting 250 μg of purified Afmp1p, along with an equal volume of complete Freund adjuvant, intramuscularly into the thighs of three guinea pigs. Incomplete Freund adjuvant was used in subsequent immunizations in a procedure identical to the first immunization in which complete Freund adjuvant was used. A total of four inoculations per guinea pig were completed in 2 months, with one injection done every 2 weeks.
Guinea pig antisera against A. fumigatus, A. flavus, A. niger, A. terreus, P. marneffei, C. albicans, H. capsulatum, and B. dermatitidis were produced as follows. After growth in RPMI medium for 1 to 5 days, the fungal cells were harvested by centrifugation at 3,000 rpm. The cells were then resuspended in phosphate-buffered saline (13.7 mM sodium chloride, 0.27 mM potassium chloride, and 1 mM phosphate buffer [pH 7.4] with 0.05% phenol) at a McFarland turbidity standard of 3. An equal volume of complete Freund adjuvant was mixed with 500 μl of fungal cell suspension, and 500 μl of the final suspension was injected intramuscularly into the thighs of the guinea pigs. Incomplete Freund adjuvant was used in subsequent immunizations in a procedure identical to the first immunization in which complete Freund adjuvant was used. A total of four inoculations were completed in 2 months, with one injection done every 2 weeks.
Human sera were obtained from patients with computed tomography- and culture-documented aspergilloma caused by A. fumigatus (n = 9; 6 from Queen Mary Hospital and 3 from Grantham Hospital). Sera were obtained from BMT recipients and patients with hematological maligancies with culture- and histology-documented invasive aspergillosis caused by A. fumigatus (n = 15; Queen Mary Hospital). Control sera were obtained from healthy blood donors (n = 80) and patients with culture-documented typhoid fever (n = 6, Queen Mary Hospital), meliodiosis (n = 4; Queen Mary Hospital), penicilliosis marneffei (n = 20; Queen Mary Hospital), candidiasis (n = 5; Queen Mary Hospital), and cryptococcosis (n = 4; Queen Mary Hospital).
Western blot analysis.
One hundred nanograms of purified GST-Afmp1p protein was loaded onto a sodium dodecyl sulfate-10% polyacrylamide gel and subsequently electroblotted onto a nitrocellulose membrane (Bio-Rad, Hercules, Calif.). The blot was incubated with a 1:2,000 dilution of a guinea pig anti-A. fumigatus antibody and detected with an ECL Fluorescence System (Amersham Life Sciences, Little Chalfont, Buckinghamshire, England).
Serological test.
The ELISA-based aspergillosis antibody test was modified from our previous publication (3). Briefly, each well of a Nunc immunoplate (Roskilde, Denmark) was coated with 0.5 ng of purified GST-Afmp1p protein for 12 h and then blocked in phosphate-buffered saline with 2% bovine serum albumin. Next, 100 μl of serially diluted guinea pig serum (1:100, 1:500, 1:1,000, 1:5,000, 1:10,000, 1:50,000, and 1:100,000) or human serum at 1:3,000 dilution was added to the wells of the GST-Afmp1p-coated plates in a total volume of 100 μl and incubated at 37°C for 2 h. After three washes with washing buffer, 100 μl of 1:10,000-diluted horseradish peroxidase-conjugated goat anti-guinea pig immunoglobulin G (H+L; Zymed, S. San Francisco, Calif.) or alkaline phosphatase-conjugated goat anti-human antibody (Cappel ICN Pharmaceuticals, Aurora, Ill.) was added to the wells, followed by incubation at 37°C for 30 min. After three washes with washing buffer, 100 μl of 3,3′,5,5′-tetramethylbenzidine (TMB) Single Solution (Zymed) or p-nitrophenylphosphate substrate (Sigma Immuno Chemicals, St. Louis, Mo.) was added to each well, followed by incubation at room temperature for 15 min. Then, 100 μl of 0.3 M H2SO4 was added, and the absorbance at 405 nm of each well was measured. Each sample was tested in duplicate, and the mean absorbance for each serum was calculated.
RESULTS
ELISA-based antibody test for aspergillosis.
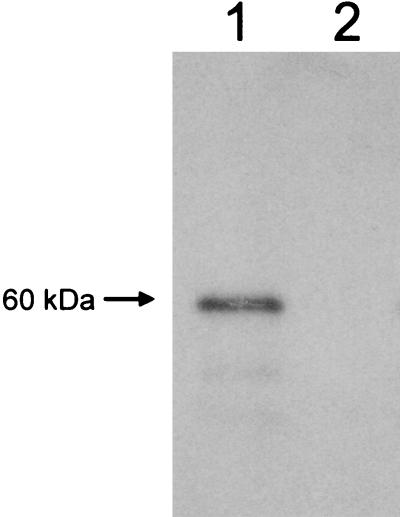
To produce recombinant Afmp1p protein, the GST-Afmp1p fusion protein was expressed in E. coli and subsequently purified. The purified fusion protein was separated on sodium dodecyl sulfate-polyacrylamide gels, followed by Western blot analysis with serum from a guinea pig inoculated with A. fumigatus cells. A prominent immunoreactive protein band of ca. 60 kDa was visible on the Western blot (Fig. 1, lane 1). This size was consistent with the expected size of 57.4 kDa for the full-length GST-Afmp1p fusion protein. This result confirmed the immunoreactivity of the purified GST-Afmp1p recombinant protein.
FIG. 1.
Western blot analysis of the purified recombinant Afmp1p protein antigen with serum from an A. fumigatus-inoculated guinea pig (lane 1) and preimmune serum (lane 2).
An ELISA-based A. fumigatus serology test was developed with this recombinant Afmp1p protein for the detection of specific antibodies against this protein. Box titration was carried out with different dilutions of Afmp1p coating antigen and a guinea pig anti-Afmp1p specific antibody. The results identified 0.5 ng of purified GST-Afmp1p protein per ELISA well as the ideal amount for plate coating. Subsequently, this antibody test was evaluated for its sensitivity and specificity in an animal model and in clinical specimens.
Fungal specificity of the ELISA-based antibody test in a guinea pig model.
An animal model system was developed to examine this aspergillosis antibody test for potential cross-reactivity with antibodies to other fungal pathogens. Sera were collected from guinea pigs inoculated with A. fumigatus, A. flavus, A. niger, A. terreus, P. marneffei, C. albicans, H. capsulatum, and B. dermatitidis. Western blot assays with cell lysates of the fungi and the sera obtained from guinea pigs immunized with the corresponding fungi were performed to confirm the immune response elicited by the various fungi (data not shown). Serial dilutions of these antisera were made and subjected to the apergillosis serology study, and the results are shown in Fig. 2. The results indicated that the Afmp1p- and A. fumigatus-inoculated guinea pigs developed high levels of specific antibody in this assay.
FIG. 2.
ELISA-based antibody test detects high levels of specific antibodies only in A. fumigatus- and Afmp1p-inoculated guinea pigs. Serial dilutions of the guinea pig sera were made, and an ELISA was performed to determine the antibody levels in the animal sera. OD405 values were obtained and plotted. One guinea pig was used for each fungus, and three were used for Afmp1p (with the SD indicated).
High specificity of ELISA-based antibody test for aspergillosis.
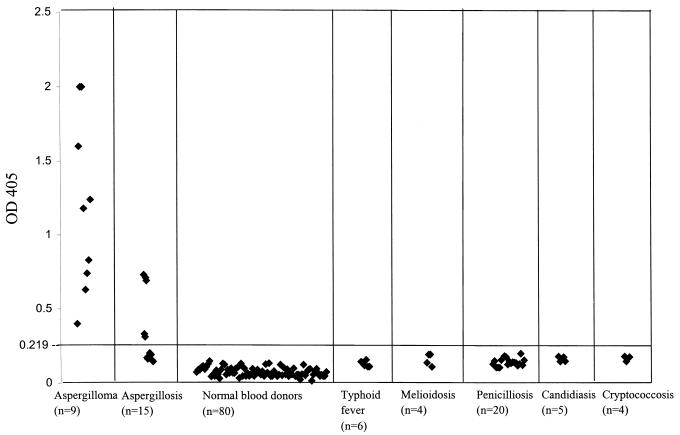
To establish the baseline for the test, serum samples from 80 healthy blood donors were tested in the aspergillosis antibody ELISA. For the 80 specimens from healthy blood donors, the mean ELISA optical density at 405 nm (OD405) was 0.074, with a standard deviation (SD) of 0.029. An absorbance value of 0.219 was selected as the cutoff value that equals the sum of the mean value from the healthy control (0.074) and five times the SD (0.145) (Fig. 3).
FIG. 3.
Evaluation of sensitivity and specificity of A. fumigatus antibody test of aspergillosis patients. Serum specimens were obtained from 9 patients with culture-documented aspergilloma and from 15 patients with culture-documented invasive aspergillosis. The control serum specimens for the test came from 80 normal blood donors, 6 patients with typhoid fever, 4 patients with melioidosis, 20 patients with penicilliosis marneffei, 5 patients with candidemia, and 4 patients with cryptococcosis. The test results were plotted as OD405s. The cutoff line for positive diagnosis is drawn at a value that equals the sum of the mean value and 5 times the SD of normal blood donors.
Sera from patients with infections caused by other fungi (P. marneffei, C. albicans, and Cryptococcus neoformans) and bacteria (Salmonella enterica serovar Typhi and Burkholderia pseudomallei) endemic in our locality were used to determine the specificity of the ELISA-based antibody for aspergillosis. Using 0.219 as the cutoff value, the specificity of the test was 100%. None of the sera obtained from the 80 healthy blood donors, 6 patients with typhoid fever, 4 patients with melioidosis, 20 patients with penicilliosis marneffei, and 5 patients with candidiasis had an OD405 of more than 0.219 (Fig. 3).
High antibody titer in patients with aspergilloma.
The mean OD405 value for the sera obtained from the 9 patients with computed tomography- and culture-documented aspergilloma caused by A. fumigatus was 1.18, with an SD of 0.586. All 9 sera had an OD405 of over 0.219 (range, 0.4 to 2), achieving a sensitivity of 100% (Fig. 3).
Clinical evaluation of the antibody test for BMT recipients and patients with hematological malignancy with invasive aspergillosis.
The mean OD405 value for the sera obtained from the 15 patients with culture- and histology-documented invasive aspergillosis caused by A. fumigatus was 0.298, with an SD of 0.22 (range, 0.144 to 0.73). A total of 5 and 10 sera had an OD405 of above and below 0.219, respectively, achieving a sensitivity of 33.3% (Fig. 3). The mean OD405 value of sera obtained from BMT recipients and patients with hematological malignancy with invasive aspergillosis is significantly lower than that of sera obtained from patients with aspergilloma (P < 0.005 by Student's t test).
DISCUSSION
We report the development of a recombinant antigenic protein-based ELISA for the detection of specific antibodies in patients with aspergillosis. We have previously described the cloning and characterization of a highly antigenic cell wall mannoprotein (Mp1p) in P. marneffei (1) and have shown that ELISA based on recombinant Mp1p is very useful for the serodiagnosis of penicilliosis marneffei (2, 3). Since there are no commercially available recombinant antigen-based kits for serodiagnosis of A. fumigatus infections, it would be logical to search for the Mp1p homologue in A. fumigatus and to examine its potential for serodiagnostic purposes. Recently, we have reported the cloning of the AFMP1 gene, which encodes the Mp1p homologue of A. fumigatus (12). Indirect immunofluorescent and immunoelectron microscopic studies indicate that Afmp1p is specifically located in the cell walls of A. fumigatus. Furthermore, Western blot studies showed that patients with invasive A. fumigatus infections develop high levels of specific antibody against Afmp1p. In this study, we evaluated the clinical usefulness and the corresponding sensitivity and specificity of recombinant Afmp1p-based ELISA for the serodiagnosis of aspergillosis.
Recombinant Afmp1p-based ELISA had high specificities in both guinea pig and human. None of the sera obtained from guinea pigs immunized with other Aspergillus species (A. flavus, A. niger, and A. terreus), dimorphic fungi (P. marneffei, a locally endemic dimorphic fungus, and H. capsulatum and B. dermatitidis, two dimorphic fungi endemic in North America), and a yeast that causes pandemic infections in both immunocompetent and immunocompromised hosts (C. albicans) had OD405 values of over 0.1 at serum dilutions of ≥1:500. As for the human subjects, none of the sera obtained from the normal blood donor controls and diseases controls had an OD405 of >0.219. It is interesting that there is no cross-reactivity between the Mp1p in P. marneffei and Afmp1p in A. fumigatus in both guinea pigs and humans, since these two proteins had 34.9% amino acid identity and 77.8% amino acid similarity. Furthermore, even for the different Aspergillus species, there is no cross-reactivity among the potential homologous proteins in A. flavus, A. niger, and A. terreus with Afmp1p in guinea pigs. As for the specificity of the Afmp1p-based ELISA, it showed high specificity (100% negative for normal blood donors, patients with other fungal infections, and patients with nonfungal infections) compared to antibody assays based on other antigens developed in the past 20 years (Table 1) (5, 7-9). Further studies have to be performed to determine the cross-reactivity among the potential homologous proteins in A. flavus, A. niger, and A. terreus in humans. However, such sera are difficult to obtain since infections caused by Aspergillus species other than A. fumigatus are not common.
TABLE 1.
Comparison of Afmp1p-based ELISA and other assays for antibody detection in patients with aspergilloma and invasive aspergillosis developed in the past 20 years
| Reference | Antigen(s) | Method of antibody detection | Cutoff values | % Specificity in:
|
% Sensitivity for:
|
|||
|---|---|---|---|---|---|---|---|---|
| Normal blood donors | Patients with other fungal infections | Patients with nonfungal infections | Aspergilloma | Invasive aspergillosis | ||||
| Mishra et al. (7) | Ammonium sulfate-precipitated protein-glycoprotein prepared from A. fumigatus | ELISA | NAa | 98 | 75 | 95.7 | 100 | 75 |
| Schonheyder et al. (8) | Crude antigen fraction of A. fumigatus containing catalase | Immunoelectrophoretic assay | NA | 100 | NA | 94.2 | 92.2 | NA |
| Holdom et al. (5) | Recombinant Cu,Zn superoxide dismutase | Western blot | NA | 100 | 100 | NA | 100 | 25 |
| Weig et al. (9) | Recombinant mitogillin protein | ELISA | Mean + 2 SD | 95.4 | NA | NA | 100 | 62.2 |
| The present study | Recombinant Afmp1p protein | ELISA | Mean + 5 SD | 100 | 100 | 100 | 100 | 33.3 |
NA, not available.
The present Afmp1p-based ELISA is more sensitive and specific than the mitogillin-based ELISA reported recently (Table 2) (9, 11). For rare infections with low prevalence, highly specific tests are always desirable. In our recently published studies on ELISA for antibody and antigen detection in patients with penicilliosis marneffei, a cutoff value at mean plus 10 SD was chosen, achieving a specificity of 100% (3). In the present study on aspergillosis, a cutoff value of mean plus 5 SD, which resulted in a specificity of 100%, was chosen. At this cutoff, the Afmp1p-based ELISA has sensitivities of 100 and 33.3%, respectively, for patients with aspergilloma and invasive aspergillosis. This is in contrast to the significantly lower sensitivities (84.4 and 7.3%) of the mitogillin-based ELISA for aspergilloma and invasive aspergillosis when mean plus 5 SD is used as the cutoff. This sacrifice of specificity for sensitivity was also reflected in an ELISA developed in the early 1980s, in which ammonium sulfate-precipitated protein-glycoprotein prepared from A. fumigatus was used as the antigen (Table 1) (7). In that ELISA, although the authors reported a relatively high sensitivity of 75% in patients with invasive aspergillosis, this is also probably due to the choice of a low cutoff, leading to relatively low specificity of down to 75% for patients with fungal infections other than aspergillosis.
TABLE 2.
Comparison of specificities and sensitivities of the Afmp1p-based ELISA and mitogillin-based ELISA at different cutoff values
| Cutoff | Specificity (%)
|
Sensitivity (%)
|
||||
|---|---|---|---|---|---|---|
| Mitogillin-based ELISA | Afmp1p-based ELISA | Aspergilloma
|
Invasive aspergillosis
|
|||
| Mitogillin-based ELISA | Afmp1p-based ELISA | Mitogillin-based ELISA | Afmp1p-based ELISA | |||
| Mean + 2 SD | 95.4 | 97.5 | 100 | 100 | 62.2 | 100 |
| Mean + 3 SD | 98.7 | 100 | 96.9 | 100 | 31.7a | 80a |
| Mean + 4 SD | ≥98.7 | 100 | 87.5 | 100 | 13.4b | 60b |
| Mean + 5 SD | ≥98.7 | 100 | 84.4 | 100 | 7.3a | 33.3a |
The sensitivity of the Afmp1p-based ELISA is significantly higher than that of the mitogillin-based ELISA (P < 0.05 by the chi-square test).
The sensitivity of the Afmp1p-based ELISA is significantly higher than that of the mitogillin-based ELISA (P < 0.005 by the chi-square test).
Despite the higher sensitivity of the Afmp1p-based ELISA than the mitogillin-based ELISA, a sensitivity of only 33.3% for patients with invasive aspergillosis is still far from ideal. This low sensitivity in patients with invasive aspergillosis is not unexpected since most patients with invasive aspergillosis, such as BMT recipients, solid organ transplant recipients, and patients with hematological malignancies undergoing chemotherapy, are highly immunosuppressed and therefore are unable to generate good antibody response. In contrast to the low sensitivity in patients with invasive aspergillosis, the present ELISA showed a perfect sensitivity of 100% for patients with aspergilloma. This phenomenon of differential antibody response in patients with invasive aspergillosis and aspergilloma is probably related to the difference in immune status of the two groups of patients and is analogous to the situation demonstrated in our study on antibody production in human immunodeficiency virus (HIV)-positive and HIV-negative patients with P. marneffei infections (10). In that study, Mp1p-based ELISA showed a low sensitivity for HIV-positive (more immunocompromised) and a high sensitivity for HIV-negative (less immunocompromised) patients. On the other hand, detection of serum Mp1p antigen with polycloncal Mp1p antibody-based ELISA was able to achieve a higher sensitivity in HIV-positive patients but a lower sensitivity in HIV-negative patients with P. marneffei infections. Therefore, a combination of antibody and antigen detection is much more sensitive than either test alone for the serodiagnosis of penicilliosis marneffei. Using a similar approach, further studies should be carried out to delineate the usefulness of polyclonal Afmp1p antibody-based ELISA for the detection of serum Afmp1p in patients with invasive aspergillosis.
Acknowledgments
This work was partly supported by a Research Grant Council grant, the AIDS Trust Fund, the University Development Fund, and the Committee of Research and Conference Grant of the University of Hong Kong.
REFERENCES
- 1.Cao, L., C. M. Chan, C. Lee, S. S. Wong, and K. Y. Yuen. 1998. MP1 encodes an abundant and highly antigenic cell wall mannoprotein in the pathogenic fungus Penicillium marneffei. Infect. Immun. 66:966-973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cao, L., K. M. Chan, D. Chen, N. Vanittanakom, C. Lee, C. M. Chan, T. Sirisanthana, D. N. Tsang, and K. Y. Yuen. 1999. Detection of cell wall mannoprotein Mp1p in culture supernatants of Penicillium marneffei and in sera of penicilliosis patients. J. Clin. Microbiol. 37:981-986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cao, L., D. L. Chen, C. Lee, C. M. Chan, K. M. Chan, N. Vanittanakom, D. N. Tsang, and K. Y. Yuen. 1998. Detection of specific antibodies to an antigenic mannoprotein for diagnosis of Penicillium marneffei penicilliosis. J. Clin. Microbiol. 36:3028-3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Denning, D. W., and D. A. Stevens. 1990. Antifungal and surgical treatment of invasive aspergillosis: review of 2,121 published cases. Rev. Infect. Dis. 12:1147-1201. [DOI] [PubMed] [Google Scholar]
- 5.Holdom, M. D., B. Lechenne, R. J. Hay, A. J. Hamilton, and M. Monod. 2000. Production and characterization of recombinant Aspergillus fumigatus Cu, Zn superoxide dismutase and its recognition by immune human sera. J. Clin. Microbiol. 38:558-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Latge, J. P. 1999. Aspergillus fumigatus and aspergillosis. Clin. Microbiol. Rev. 12:310-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mishra, S. K., S. Falkenberg, and K. N. Masihi. 1983. Efficacy of enzyme-linked immunosorbent assay in serodiagnosis of aspergillosis. J. Clin. Microbiol. 17:708-710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schonheyder, H., P. Andersen, and J. C. Petersen. 1985. Rapid immunoelectrophoretic assay for detection of serum antibodies to Aspergillus fumigatus catalase in patients with pulmonary aspergillosis. Eur. J. Clin. Microbiol. 4:299-303. [DOI] [PubMed] [Google Scholar]
- 9.Weig, M., M. Frosch, K. Tintelnot, A. Haas, U. Gross, B. Linsmeier, and J. Heesemann. 2001. Use of recombinant mitogillin for improved serodiagnosis of Aspergillus fumigatus-associated diseases. J. Clin. Microbiol. 39:1721-1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wong, S. S., K. H. Wong, W. T. Hui, S. S. Lee, J. Y. Lo, L. Cao, and K. Y. Yuen. 2001. Differences in clinical and laboratory diagnostic characteristics of penicilliosis marneffei in human immunodeficiency virus (HIV)- and non-HIV-infected patients. J. Clin. Microbiol. 39:4535-4540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Woo, P. C. Y., A. S. P. Leung, S. K. P. Lau, K. T. K. Chong, and K. Y. Yuen. 2001. Use of recombinant mitogillin for serodiagnosis of Aspergillus fumigatus-associated disease. J. Clin. Microbiol. 39:4598-4600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yuen, K. Y., C. M. Chan, K. M. Chan, P. C. Woo, X. Y. Che, A. S. Leung, and L. Cao. 2001. Characterization of AFMP1: a novel target for serodiagnosis of aspergillosis. J. Clin. Microbiol. 39:3830-3837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yuen, K. Y., P. C. Woo, M. S. Ip, R. H. Liang, E. K. Chiu, H. Siau, P. L. Ho, F. F. Chen, and T. K. Chan. 1997. Stage-specific manifestation of mold infections in bone marrow transplant recipients: risk factors and clinical significance of positive concentrated smears. Clin. Infect. Dis. 25:37-42. [DOI] [PubMed] [Google Scholar]