Abstract
A novel commercially available enzyme-linked immunosorbent assay (ELISA) for prevaccination screening and diagnosis of Q fever (PanBio Coxiella burnetii immunoglobulin G [IgG] ELISA) was compared to the complement fixation test (CFT), and the indirect fluorescent-antibody test (IFAT) was used to resolve discrepant results between the other two tests. A total of 214 serum samples was tested. The ELISA demonstrated a specificity of 96% (46 of 48 samples) and a sensitivity of 71% (95 of 134 samples). Of the six serum pairs showing CFT seroconversion, three pairs showed a corresponding ELISA seroconversion. No cross-reactivity was observed in the ELISA with serum samples from patients with mycoplasma, brucella, and chlamydia infections. One of the 13 patients with leptospirosis demonstrated a positive result in the ELISA but not in the CFT or the IFAT, and Legionella pneumophila serogroup 4 antibody was found in one of the two sera that were false-positive by ELISA. The results presented in this study suggest that the PanBio Q fever IgG ELISA is a specific alternative method for prevaccination testing and an aid for the diagnosis of Q fever. This test is suitable for use as a screening assay, with CFT and/or IFAT used to confirm negative results.
Q fever is the most common occupational zoonotic disease of livestock handlers and abattoir workers in Australia. The disease is usually acquired by inhalation of contaminated aerosols from animals, mainly cattle, sheep, and goats, infected with the causative agent, Coxiella burnetii. Q fever usually presents as an influenza-like illness, but asymptomatic infection and shedding of the organism into products of conception may occur. Occasionally, a chronic disease form, subacute endocarditis, may develop months or years later (5). Other chronic complications are granulomatous changes in liver, lesions in other organs, and the post-Q fever fatigue syndrome.
Due to possible severe local or systemic reactions, prevaccination screening to assess prior exposure before vaccination is mandatory for occupational groups at risk of Q fever infection. Adverse reactions are rare (<0.05%) in subjects who are screened to assess prior exposure. Even though 25 to 50% of abattoir workers have immune markers after previous clinical or subclinical infection, the risk of adverse reaction in this group is much higher (9). Screening comprises serological tests for antibodies and a skin test for cellular immunity. Immunoglobulin G (IgG) antibodies may persist for 10 or more years, as measured by the indirect fluorescent-antibody test (IFAT) and the enzyme-linked immunosorbent assay (ELISA), but may occasionally fall below detectable levels over a long period of time (16). After an attack of Q fever, complement fixation test (CFT) antibodies fall to low levels some 3 years postillness and eventually to undetectable levels (11). To take account of this eventuality, a skin test was also performed to determine cell-mediated immunity before vaccination. Similarly, as a safeguard against incorrectly performed skin tests, an antibody test was done. Although the skin test, if performed correctly, is highly sensitive for subjects who have previously been infected with C. burnetii, only about 60% of vaccinated subjects will subsequently develop a positive skin test reaction. If either the antibody test or the skin test is positive, the vaccine must not be given (9).
The traditional serological methods for assessing C. burnetii antibody status have been the CFT and IFAT (3). Both tests are subjective and are not standardized between laboratories. They are also inconvenient for large-scale screening and cannot be automated (4). These limitations led to the development of ELISAs that detected antibodies to C. burnetii (3, 14), including a commercial ELISA (PanBio, Brisbane, Australia) for the detection of IgG antibodies (2, 13). In this study, we compared the PanBio C. burnetii (Q fever) IgG ELISA to the CFT with sera from patients with past or acute Q fever or other infections and used the IFAT to resolve discrepant results between the other two tests.
A total of 214 serum samples was included in this study. Of these, 78 specimens were single-serum samples from subjects being investigated for Q fever prevaccination immunity, 92 specimens were from patients investigated for Q fever infection, and 6 paired sera were from patients showing Q fever CFT seroconversion. An additional 32 convalescent-phase sera from patients with serologically confirmed infections other than Q fever were also tested. They comprised sera from patients with infections due to Mycoplasma pneumoniae (n = 6), Chlamydia psittaci (n = 7), Legionella sp. (n = 4), Leptospira sp. (n = 13), and Brucella sp. (n = 2).
All 214 serum samples were tested by the PanBio Q fever IgG ELISA according to the manufacturer's instructions. Sera were diluted 1/100 in the serum diluent provided, and 100 μl of each diluted sample was transferred to microwells coated with C. burnetii whole-cell phase II antigen and incubated for 30 min at 37°C. The microwells were then washed six times with phosphate-buffered saline (PBS) containing 0.05% Tween 20. After washing, 100 μl of horseradish peroxidase-conjugated anti-human IgG was added to each well and incubated for another 30 min at 37°C. The microwells were again washed six times, and 100 μl of tetramethylbenzidine was pipetted into each well. After 10 min, this reaction was stopped by the addition of 100 μl of 1 M phosphoric acid. The microwells were then read in a microtiter plate reader at a wavelength of 450 nm. The results were determined by comparison with a provided IgG reference serum sample which contains a borderline level of Q fever IgG phase II antibody (cutoff calibrator). A positive sample was defined as having a sample absorbance/calibrator absorbance ratio (ELISA ratio) of ≥1.0; a negative sample had a ratio of <1.0.
IFAT was done as previously described (3, 8). Phase II antigen (Nine Mile strain; Commonwealth Serum Laboratories, Melbourne, Australia) was diluted, dropped onto the wells of a glass microscope slide, allowed to dry, and fixed with acetone. Five fourfold dilutions of serum (from 1/10 to 1/2,560) in PBS were reacted with antigen on the slides for half an hour at 37°C and then washed with PBS. Bound antibody was then detected via a 30-min incubation with fluorescein-labeled sheep anti-human IgG F(ab′)2 fragment conjugate (Amersham, Melbourne, Australia). After the slides were washed and dried, they were mounted with a coverslip and examined by using an incident-light fluorescence microscope (Carl Zeiss, Oberkochen, Germany). Antibody titers were defined as the inverse of the highest dilution with definite staining of C. burnetii membranes. A positive IgG result was defined as having an endpoint titer of 10 or greater.
CFT was performed as previously described (3, 12). After the optimal dilutions of C. burnetii phase II antigen, complement, and hemolysin were determined via checkerboard titrations, serial dilutions of serum were prepared in Veronal-buffered saline and 2 U each of antigen and guinea pig complement were added. After an overnight incubation at 4°C, sensitized sheep cells (2%) were added and incubated for 45 min at 37°C with intermittent shaking. The highest dilution with ≥75% fixation was defined as the endpoint (12). A positive result was defined as having an endpoint titer of ≥2.5 for prevaccination sera (9) and ≥4.0 for diagnostic specimens (11).
Analysis of variation was used to compare the mean ELISA ratios for different CFT titers. Receiver operator curve (ROC) analysis was performed to compare sensitivity and specificity at different cutoff values (10). The cutoff for optimal assay performance was determined by using two-graph ROC analysis (6, 17). Statistics were performed by using InstatR (Graphpad Software Inc., San Diego, Calif.).
Of the 214 serum samples, 184 were tested in the Q fever CFT with phase II antigen. These included all sera submitted for investigation of Q fever immunity and infection and two positive sera from the specificity panel. Any serum that showed discrepant results was retested by ELISA and tested in the Q fever phase II IgG IFAT, which is the reference method. In those samples with conflicting CFT and IFAT results, the latter was used to classify the sample. A CFT antibody titer of 2.5 or greater, a positive IgG IFAT result with a titer of 10 or greater, and a positive IgG ELISA result against phase II antigen are suggestive of recent or past infection.
In the comparison with the CFT, and with the use of IFAT as the reference method to classify discrepant results, the Q fever IgG ELISA was found to have a specificity of 96%. In contrast, the sensitivity was only 71% (Table 1). This low sensitivity means that, if the ELISA is used for Q fever prevaccination screening, then there is a risk that some meat workers who have a negative Q fever IgG ELISA result, and in whom the skin test is also negative, may be inadvertently vaccinated. Such vaccinees have a low risk of developing a severe local or systemic reaction. The combined prevaccination antibody and skin test is not an absolute test of immunity. It is done primarily to exclude those individuals who may develop severe reactions when vaccinated at the inoculation site (9). Minor or threshold levels of immunity or sensitization to C. burnetii may not be detected by the serological and skin tests but only by lymphocyte stimulation testing (16).
TABLE 1.
Comparison of Q fever CFT and IFAT with PanBio IgG ELISA (n = 182)
| CFT and/or IFAT result | No. of results (%)
|
|
|---|---|---|
| ELISA positivea | ELISA negative | |
| Positiveb (n = 134) | 95c (71) | 39 (29) |
| Negative (n = 48) | 2 (4) | 46d (96) |
ELISA cutoff of ≥1.0.
CFT cutoffs of ≥2.5 for prevaccination screening and ≥4.0 for investigation of Q fever infection; IFAT cutoff of ≥10.0.
ELISA sensitivity = 71% (95 of 134 samples).
ELISA specificity = 96% (46 of 48 samples).
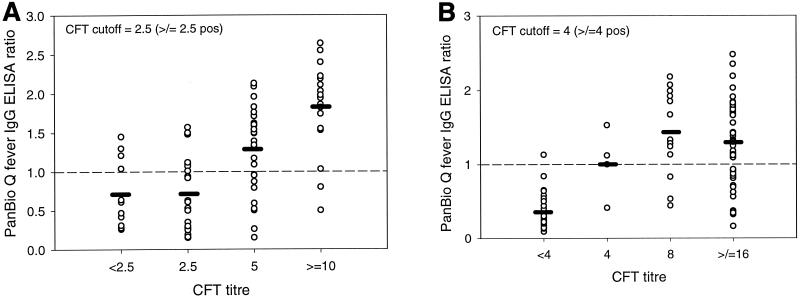
A correlation was shown between the individual Q fever IgG ELISA assay values and the CFT results with the sera submitted for prevaccination screening (r = 0.634, P < 0.0001) and for investigation of active infection (r = 0.2965, P = 0.0012) (Fig. 1). An analysis of the distribution of index values in the ELISA showed that 28 of the 39 sera (71.8%) with false-negative ELISA results had absorbance values within 50% of the cutoff. However, lowering the cutoff ratio of the ELISA below 1.0 (F value, 166.73) did not significantly improve its performance. Although the highest F value (174.50) was obtained with a cutoff of 0.7, the specificity was reduced to 91.7%. As the specificity of the test is clinically more important in prevaccination screening, it is preferable to use a cutoff of 1.0 or 0.9, which results in a specificity of 96% and a sensitivity of 71 or 77%, respectively (6). The low sensitivity of the IgG ELISA for the diagnosis of recent infection using paired sera is of lesser concern. This is because the formation of IgM antibodies is transient and may precede that of IgG antibodies (3); consequently, a specific IgM IFAT or IgM ELISA is preferable for the diagnosis of Q fever infection (4).
FIG. 1.
Comparison of Q fever IgG ELISA ratios with CFT titers in sera for prevaccination screening (A) and investigation of Q fever infection (B). Mean ELISA ratios are shown by horizontal bars. The cutoff values (ratio = 1.0) are shown by broken lines. Note that in panel A two sera that had CFT titers of <2.5 had IFAT titers of ≥10 and that in panel B one serum sample that had a CFT titer of <4.0 had an IFAT titer of ≥10. pos, positive.
Of the six serum pairs showing CFT seroconversion or rising titers, three pairs showed a corresponding ELISA seroconversion (Table 2).
TABLE 2.
Detection of Q fever in six paired sera
| Testa | No. positive (%)
|
|
|---|---|---|
| Acute-phase sera | Convalescent-phase sera | |
| CFT | 0/6 (0) | 6/6b (100) |
| IgG ELISA | 0/6 (0) | 3/6c (50) |
CFT cutoff, ≥4.0; ELISA cutoff, ≥1.0.
All paired sera showed a fourfold rise in antibody titer by CFT.
Three convalescent-phase sera showed ELISA seroconversion.
No cross-reactivity was observed in the ELISA with serum samples from patients with mycoplasma, brucella, and chlamydia infections. However, cross-reactivity was suggested in a sample from one of the 13 patients with Leptospira interrogans serovar Pomona infection, which gave a false-positive ELISA result. Samples from one patient with confirmed Mycoplasma pneumoniae infection and one with confirmed Leptospira interrogans serovar Hardjo infection were repeatedly ELISA reactive and were confirmed to be positive by CFT and IFAT. This suggests that these patients had past Q fever infections. In a recent study of the PanBio Leptospira IgM ELISA (15), sera from 3 of 34 patients with Q fever infections were reactive, although it could not be determined whether this was due to cross-reactivity or persistent antibody from a past leptospiral infection. Legionella pneumophila serogroup 4 antibody was found in one of the two sera giving false-positive ELISA results. This may represent cross-reactivity, as certain C. burnetii epitopes have extensive homology with proteins from other prokaryotes (7). Further studies with larger sample sizes are needed to validate these preliminary findings.
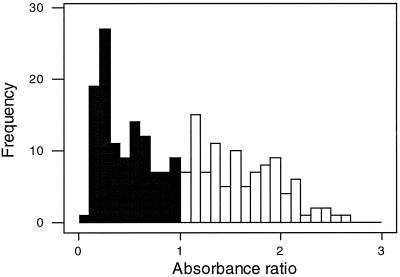
The frequency distribution of the absorbance ratios was not bimodal and showed no clear discrimination between positive and negative ELISA values (1). The dynamic range of positive ELISA values was largely restricted to samples with lower absorbance ratios (between 1.0 and 2.64) (Fig. 2), which suggests that some positive values could be incorrectly categorized as negative. Some changes to the ELISA components may be needed to improve the bimodal distribution of positive and negative values and extend the dynamic range of positive values.
FIG. 2.
Frequency distribution of PanBio Q fever IgG ELISA absorbance ratios. Absorbance ratios of ≤1.0 are negative (filled columns) and those of ≥1.0 are positive (open columns).
The results presented in this study suggest that the PanBio Q fever IgG ELISA is a specific alternative method for prevaccination testing and the diagnosis of Q fever. It provides a standardized method with a total incubation time of 70 min, is suitable for large-scale screening, and has the potential for automation. However, this test is suitable as a screening assay provided that CFT and/or IFAT is used to confirm negative results.
Acknowledgments
We thank Eric Kapsalis and Felicity Jones for technical assistance.
REFERENCES
- 1.Crofts, N., W. Maskill, and I. D. Gust. 1988. Evaluation of enzyme-linked immunosorbent assays: a method of data analysis. J. Virol. Methods 22:51-59. [DOI] [PubMed] [Google Scholar]
- 2.D’Harcourt, S. C., A. B. Soto, V. C. Burgos, D. L. Calero, and R. Martínez-Zapico. 1996. Comparison of immunofluorescence with enzyme immunoassay for detection of Q fever. Eur. J. Clin. Microbiol. Infect. Dis. 15:749-752. [DOI] [PubMed] [Google Scholar]
- 3.Field, P. R., J. G. Hunt, and A. M. Murphy. 1983. Detection and persistence of specific IgM antibody to Coxiella burnetii by enzyme-linked immunosorbent assay: a comparison with immunofluorescence and complement fixation tests. J. Infect. Dis. 148:477-487. [DOI] [PubMed] [Google Scholar]
- 4.Field, P. R., J. L. Mitchell, A. Santiago, D. J. Dickeson, S.-W. Chan, D. W. T. Ho, A. M. Murphy, A. J. Cuzzubbo, and P. L. Devine. 2000. Comparison of a commercial enzyme-linked immunosorbent assay with immunofluorescence and complement fixation tests for detection of Coxiella burnetii (Q fever) immunoglobulin M. J. Clin. Microbiol. 38:1645-1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fournier, P. E., T. J. Marrie, and D. Raoult. 1998. Diagnosis of Q fever. J. Clin. Microbiol. 36:1823-1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Greiner, M., D. Sohr, and P. Gobel. 1995. A modified ROC analysis for the selection of cut-off values and the definition of intermediate results of serodiagnostic tests. J. Immunol. Methods 185:123-132. [DOI] [PubMed] [Google Scholar]
- 7.Hoffman, P. S., L. Houston, and C. A. Butler. 1990. Legionella pneumophila htpAB heat shock operon: nucleotide sequence and expression of the 60-kilodalton antigen in L. pneumophila-infected HeLa cells. Infect. Immun. 58:3380-3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hunt, J. G., P. R. Field, and A. M. Murphy. 1983. Immunoglobulin responses to Coxiella burnetii (Q fever): single-serum diagnosis of acute infection, using an immunofluorescence technique. Infect. Immun. 39:977-981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marmion, B. P. 1999. Q fever: your questions answered. CSL Ltd., Melbourne, Victoria, Australia.
- 10.Metz, C. E. 1978. Basic principles of ROC analysis. Semin. Nucl. Med. 8:283-298. [DOI] [PubMed] [Google Scholar]
- 11.Murphy, A. M., and P. R. Field. 1970. The persistence of complement-fixing antibodies to Q fever (Coxiella burnetii) after infection. Med. J. Aust. 1:1148-1150. [DOI] [PubMed] [Google Scholar]
- 12.Murphy, A. M., and L. Magro. 1980. IgM globulin response in Q fever (Coxiella burnetii) infections. Pathology 12:391-396. [DOI] [PubMed] [Google Scholar]
- 13.Pérez-Trallero, E., G. Cilla, M. Montes, J. R. Saénz-Dominguez, and M. Alcorta. 1995. Prevalence of Coxiella burnetii infection among slaughterhouse workers in northern Spain. Eur. J. Clin. Microbiol. Infect. Dis. 14:71-73. [DOI] [PubMed] [Google Scholar]
- 14.Péter, O., G. Dupuis, M. G. Peacock, and W. Burgdorfer. 1987. Comparison of enzyme-linked immunosorbent assay and complement fixation and indirect fluorescent-antibody tests for detection of Coxiella burnetii antibody. J. Clin. Microbiol. 25:1063-1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Winslow, W. E., D. J. Merry, M. L. Pirc, and P. L. Devine. 1997. Evaluation of a commercial enzyme-linked immunosorbent assay for detection of immunoglobulin M antibody in the diagnosis of leptospiral infection. J. Clin. Microbiol. 35:1938-1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Worswick, D., and B. P. Marmion. 1985. Antibody responses in acute and chronic Q fever and in subjects vaccinated against Q fever. J. Med. Microbiol. 19:281-296. [DOI] [PubMed] [Google Scholar]
- 17.Xu, H., J. Lohr, and M. Greiner. 1997. The selection of ELISA cut-off points for testing antibody to Newcastle disease by two-graph receiver-operating characteristic (TG-ROC) analysis. J. Immunol. Methods 13:61-64. [DOI] [PubMed] [Google Scholar]