Abstract
The production of the bundle-forming pilus subunit BfpA was investigated in 44 enteropathogenic Escherichia coli (EPEC) diarrheal isolates after growth on several conventional bacteriological media. In all, the use of brucella agar supplemented with 4.5 mM sodium bisulfite resulted in a higher detection of BfpA-producing EPEC.
Enteropathogenic Escherichia coli (EPEC) is the most important bacterial cause of diarrheal disease in infants 0 to 6 months of age living in developing countries (11, 15, 23). EPEC strains are characterized by the presence of a 92-kb plasmid that codes for a type IV bundle-forming pilus (Bfp) (12) associated with the formation of tight microcolonies on tissue culture cells, a phenotype referred to as the localized adherence pattern, and virulence (3, 8, 29). Antibodies against BfpA were demonstrated to exist in the sera of children with EPEC infections (22, 25). The Bfp contains a structural subunit of 19.5 kDa, termed BfpA or bundlin, and its expression can be manifested by growth of the bacteria on tryptic soy agar supplemented with 5% defibrinated sheep blood in tissue culture media (e.g., Dulbecco's minimal essential medium with Earle salts [DMEM]) or in the course of infection of cultured epithelial cells (9, 12).
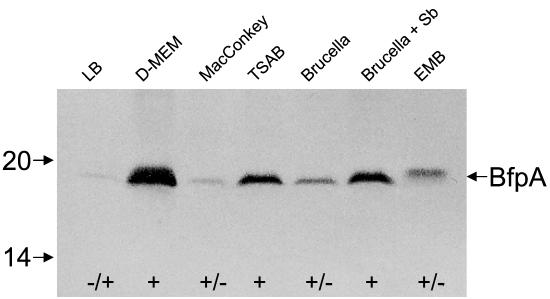
The goal of this study was to find alternative bacteriological media suitable for the induction of Bfp expression and thereby to improve the identification of Bfp-producing EPEC. Towards this end, we analyzed the production of Bfp in a collection of 44 well-defined strains belonging to classical EPEC serotypes isolated from the feces of children with diarrheal disease between 1954 and 1993 in Brazil, Congo, Chile, and Mexico (Tables 1 and 2) (6, 17, 26, 28). Within this bacterial collection, 36 strains showed the EPEC adherence factor (EAF)-positive bfpA+ eae+ genotype typical of EPEC (Table 1) and 8 strains showed the EAF-negative bfpA+ eae+ genotype (Table 2) (6, 17, 26, 28, 30). The prototypical EPEC strains B171 (O111:NM) and E2348/69 (O127:H6) were used as positive controls. JPN15, a plasmidless derivative of E2348/69 that does not produce Bfp, was used as negative control (12, 13). First, we studied the production of BfpA in E2348/69 after growth at 37°C in several conventional laboratory solid media, such as eosin-methylene blue (EMB), MacConkey, brucella, and Mueller-Hinton agars, and compared it to that observed in DMEM by immunoblotting as follows. Equal amounts of bacteria were denatured, electrophoresed, and reacted with polyclonal anti-Bfp antibodies as previously described (13, 14, 21). Obvious differences in the levels of expression of BfpA by E2348/69 were noted in the various growth conditions (Fig. 1). Namely, BfpA was highly induced in DMEM, followed by Mueller-Hinton, EMB, and brucella agars. Small amounts of BfpA were detected in Luria-Bertani and MacConkey agars as well. A slight variation in the molecular migration of BfpA was observed when this strain was grown on EMB agar. However, no obvious morphological differences were noted in the Bfp structures produced by this strain on EMB agar (data not shown). It is possible that the BfpA polypeptide undergoes biochemical modification (e.g., amino acid substitutions) when grown in this medium, resulting in a slower migration in sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels. Such variability in migration has been attributed to differences in the lengths of the amino acid chains of BfpA subunits of diverse EPEC strains (4, 13). Antigenic heterogeneity in BfpA has also been noted previously (14).
TABLE 1.
Production of BfpA by EAF- and bfpA probe-positive EPEC strains in different bacterial growth media and correlation with production of Bfp filaments on HEp-2 cells
| Serogroup | Serotype | Isolate | Production of BfpA in indicated bacterial mediuma
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DMEM
|
EMB
|
Brucella
|
MacConkey
|
Mueller-Hinton
|
|||||||||
| −CO2 | +CO2 | −CO2 | +CO2 | +Sb | +Sbb | +Sb | −CO2 | +CO2 | −Sb | +Sb | |||
| O55 | H6 | 7 | + | + | + | + | + | + | + | + | + | + | + |
| H6 | 17 | + | + | + | + | + | + | + | + | + | + | + | |
| H6 | 18 | + | + | + | + | + | + | + | + | + | + | + | |
| H6 | 21 | + | + | + | + | + | + | + | + | + | − | + | |
| H6 | 38 | + | + | + | + | + | + | + | + | + | + | + | |
| H− | 34 | + | + | + | + | + | + | + | + | + | + | + | |
| H− | 72 | + | + | + | + | + | + | + | + | + | + | + | |
| H− | 26 | − | − | − | − | − | − | + | − | + | − | + | |
| H− | 55 | − | + | − | − | − | − | + | + | − | + | + | |
| H− | 61 | − | + | + | − | − | − | − | + | − | − | − | |
| H− | 62 | + | + | + | − | − | + | + | − | − | − | + | |
| O86 | H34 | 24 | + | + | + | + | + | − | + | + | + | + | + |
| H34 | 26 | + | + | + | + | + | + | + | + | + | − | − | |
| H34 | 27 | + | + | − | + | + | − | + | + | + | + | + | |
| O111 | H2 | 30 | + | + | + | + | + | + | + | + | + | + | + |
| H2 | 249 | + | + | + | + | + | + | + | + | + | − | + | |
| H2 | 254 | + | + | + | + | + | + | + | + | + | + | + | |
| H2 | 257 | + | + | + | + | + | − | + | + | + | + | + | |
| H2 | 261 | + | + | + | + | + | − | + | + | + | + | + | |
| O114 | H− | 3 | + | + | + | + | + | − | + | + | + | + | + |
| H2 | 2 | + | + | + | + | + | − | + | + | + | + | + | |
| O119 | H6 | 43 | + | − | + | + | + | − | + | + | + | + | − |
| H6 | 49 | + | + | + | + | + | − | + | + | + | + | − | |
| H6 | 70 | + | − | + | + | + | − | + | + | + | − | + | |
| O127 | H6 | 11 | + | − | + | − | + | + | + | + | + | − | + |
| H6 | 12 | + | + | + | + | + | + | + | + | + | − | + | |
| H6 | 13 | + | − | + | + | + | + | + | + | + | + | − | |
| H40 | 6 | + | + | + | − | − | + | + | + | + | − | + | |
| H40 | 7 | + | + | − | + | + | − | + | + | + | + | + | |
| H40 | 8 | + | − | + | − | − | + | + | + | − | + | − | |
| H40 | 9 | + | + | − | − | − | + | + | + | + | − | − | |
| O142 | H34 | 21 | + | + | + | − | + | + | + | + | + | + | + |
| H34 | 23 | + | + | + | + | + | + | + | + | + | + | + | |
| H34 | 24 | + | + | + | + | + | + | + | + | + | + | + | |
| H34 | 25 | + | + | + | − | + | + | + | − | − | + | + | |
| H34 | 26 | + | + | − | − | + | + | + | − | − | + | + | |
| Total positive | 36 | 33 | 30 | 30 | 25 | 29 | 23 | 35 | 32 | 30 | 25 | 29 | |
| % | 100 | 91 | 83 | 83 | 69 | 80 | 64 | 97 | 89 | 83 | 69 | 80 | |
+ and − indicate detection and no detection, respectively, of BfpA in immunoblots or by immunofluorescence. −CO2, without CO2; +CO2, with CO2; etc. CO2 was used at a concentration of 5%. Sb, sodium bisulfite. Sodium bisulfite was used at a concentration of 4.5 mM except where indicated.
Sodium bisulfite at a concentration of 0.9 mM.
TABLE 2.
Production of BfpA by EAF probe-negative and bfpA probe-positive EPEC strains in different bacterial growth media and correlation with production of Bfp filaments on HEp-2 cells
| Serogroup | Serotype | Isolate | Production of BfpA in indicated bacterial mediuma
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DMEM
|
EMB
|
Brucella
|
MacConkey
|
Mueller-Hinton
|
|||||||||
| −CO2 | +CO2 | −CO2 | +CO2 | +Sb | +Sbb | +Sb | −CO2 | +CO2 | −Sb | +Sb | |||
| O55 | H− | 23 | − | − | + | − | − | − | + | − | − | − | − |
| O119 | H− | 18 | + | + | − | − | − | − | − | − | − | − | − |
| H− | 56 | + | + | + | + | + | − | + | + | + | − | − | |
| H6 | 73 | + | + | + | + | + | − | + | + | − | − | − | |
| O142 | H6 | 15 | − | + | + | + | + | + | + | + | + | + | + |
| H6 | 16 | − | − | − | − | − | + | − | − | + | − | − | |
| H6 | 17 | + | − | + | + | + | + | + | − | − | + | + | |
| H6 | 18 | + | + | + | + | + | + | + | + | + | + | + | |
| Total positive | 8 | 5 | 5 | 6 | 5 | 5 | 4 | 6 | 4 | 4 | 3 | 3 | |
| % | 100 | 62 | 62 | 75 | 62 | 62 | 50 | 75 | 50 | 50 | 37 | 37 | |
+ and − indicate detection and no detection, respectively, of Bfp in immunoblots and by immunofluorescence. −CO2, without CO2; +CO2, with CO2; etc. CO2 was used at a concentration of 5%. Sb, sodium bisulfite. Sodium bisulfite was used at a concentration of 4.5 mM except where indicated.
Sodium bisulfite at a concentration of 0.9 mM.
FIG. 1.
Expression and detection of BfpA after growth on different bacteriological media. E2348/69 was grown on several solid media and adjusted to the same bacterial concentration on each medium, and the proteins were denatured in sodium dodecyl sulfate-polyacrylamide gel electrophoresis sample buffer. Immunoblots were reacted with anti-Bfp antibodies. Mass standards (in kilodaltons) are indicated on the left, and the BfpA pilin subunit is indicated by an arrow on the right. Note the different levels of synthesis of BfpA in the different media and the difference in the mobility of BfpA for bacteria grown on EMB agar. LB, Luria-Bertani; TSAB, tryptic soy agar supplemented with 5% defibrinated sheep blood; Sb, sodium bisulfite; +, positive; +/−, weakly positive.
We then tested all 36 typical (EAF-positive bfpA+) EPEC strains for the production of bundlin on the selected media. Among these, 33 (91%) produced BfpA in DMEM (Table 1). No difference was found between DMEM containing 5% fetal bovine serum (a common component of tissue culture medium) and DMEM alone (data not shown), suggesting that serum factors do not influence BfpA expression. A considerable number of strains did produce BfpA after growth on solid media, such as MacConkey (89%), EMB (83%), Mueller-Hinton (69%), and brucella (64%) agars. These results are particularly interesting since MacConkey and EMB agars are routinely used for the identification of lactose-positive E. coli isolated from diarrheal stools. These media offer the possibility of identifying EPEC directly in stool samples spotted onto colony blots and detecting BfpA by an immunological assay.
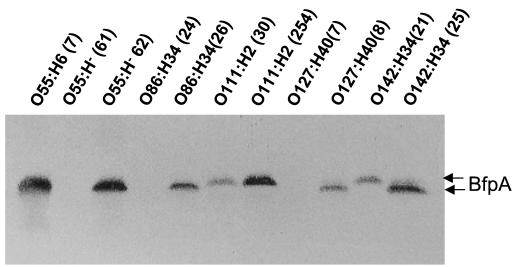
Analysis of Bfp production in the different growth media revealed interesting findings. Some strains produced Bfp selectively in some of the media, and differences were also noted within strains of a particular serotype (Tables 1 and 2). Figure 2 shows the variability of BfpA production between serotypes and between strains of the same serotype after growth on brucella agar.
FIG. 2.
Detection of BfpA produced by EPEC strains of heterologous serotypes. The indicated EPEC strains were grown on brucella agar, and BfpA was detected as described in the text. Note that strains of the same serotype behaved differently in terms of BfpA production. The levels and sizes of BfpA produced varied among the isolates.
Compared to typical EPEC strains, fewer strains within the EAF-negative group were able to produce BfpA, including those that grew in DMEM. Only 62% of these showed BfpA in DMEM, while 6 (75%), 4 (50%), 4 (50%), and 3 (37%) strains produced BfpA in EMB, MacConkey, brucella, and Mueller-Hinton agars, respectively (Table 2). Similar to the case for strains in the typical group, various profiles of BfpA production were noted for strains in the EAF-negative group. Only isolate number 18 (O142:H6) produced BfpA in all of the media tested. Some isolates that were BfpA negative in DMEM were positive in other media, such as brucella, EMB, and MacConkey agars. Blank et al. (4) identified eight different bfpA alleles: the α group contains three alleles found in strains of the O55, O86, O111, O119, O127, and O128 serogroups, and the β group contains five alleles found in strains of the O55, O119, O128, and O142 serogroups and in strains with nontypeable antigens. We have employed strains belonging to these serogroups, but we could not find an association between the serotype and a specific pattern of BfpA expression with the media tested.
It is known that the expression of some virulence factors is affected negatively or positively by carbon dioxide (10, 19, 20). Thus, we tested the production of bundlin after growth under an atmosphere of 5% carbon dioxide. Although this was not the case for all of the isolates, a few of the typical and EAF-negative EPEC strains which produced BfpA in some of the media tested (7 to 17% in both groups) became BfpA negative after growth in the presence of 5% carbon dioxide (Tables 1 and 2). This effect was especially noticeable when EMB agar was employed. However, this was not a general phenomenon, since we also encountered a few strains that became BfpA positive after growth in the carbon dioxide-containing atmosphere.
Detailed analysis of the nutritional and chemical compositions of all of the media employed revealed that neither NaCl, yeast extract, nor beef infusions were key factors required for Bfp production. We noted that sodium bisulfite (a reducing agent present at a concentration of 0.9 mM) was one of the components present in brucella agar but not in the rest of the media tested. Therefore, we investigated whether higher concentrations (two- to fivefold) of sodium bisulfite added to brucella agar or any other medium, such as Mueller-Hinton or EMB agar, would induce BfpA production in the isolates, especially in those of the EAF-negative EPEC group, which contained many BfpA-negative strains. We did not test MacConkey agar supplemented with sodium bisulfite, because it already showed good stimulation of BfpA production alone. E2348/69 produced more bundlin when grown on brucella plus 4.5 mM sodium bisulfite (hereafter designated Bfp agar) than when grown on brucella agar alone (Fig. 1). We could rescue BfpA production in several of the isolates that were repeatedly negative for BfpA in some of the media, including DMEM and brucella agar. In the typical EPEC group, 97% of the strains produced BfpA on Bfp agar compared to only 64% on brucella agar (Table 1). The positive effect of sodium bisulfite on BfpA production was confirmed with Mueller-Hinton agar, as well. An increase was observed in the number of BfpA-producing EPEC isolates compared to that observed for the media without this compound (Table 1). In the EAF-negative EPEC group, we could rescue BfpA production in three isolates when they were grown on Bfp agar (Table 2). To determine whether sodium bisulfite was exerting any metabolic effect, we measured growth curves in the presence and absence of this compound and noted, as expected, that higher (>4.5 mM) concentrations of this chemical had deleterious effects on the bacteria (data not shown). It appears that Bfp agar is a better alternative to DMEM for promoting BfpA production in both typical and EAF-negative EPEC (Tables 1 and 2).
The environmental factors involved in the regulation of BfpA have been studied in two different EPEC strains, namely B171 (O111:NM) and E2348/69 (O127:H6) (9, 27). In strain B171, the presence of glucose enhances BfpA expression whereas the presence of ammonium, but not urea, nitrate, or nitrite, significantly reduces BfpA expression (2, 7, 27, 32). In contrast, the presence of calcium and magnesium ions has been shown to influence the local adherence pattern phenotype and the production of Bfp (2, 7, 27, 32). The observation that sodium bisulfite enhances BfpA expression adds yet another factor to the overall regulation scheme of this pilus. The identification of the true signals that activate Bfp expression in the small intestine of young infants remains an important issue to address.
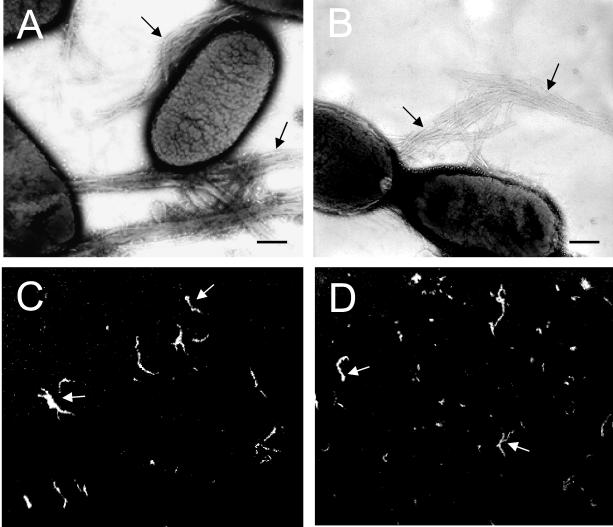
To visualize Bfp structures, bacterial colonies growing on Bfp agar were harvested and negatively stained for transmission electron microscopy or reacted with anti-Bfp antibodies on glass slides for immunofluorescence (12). Among the strains studied, 34 (94%) typical EPEC and 4 (50%) EAF-negative EPEC produced Bfp structures (data not shown). Typical Bfp rope-like structures produced by two EPEC strains (O55:H6 number 7 and O142:H34 number 21) are shown in Fig. 3. In agreement with the immunoblotting data, the level of production of Bfp structures varied significantly among the strains, and a few of the isolates of both typical and EAF-negative strains failed to produce BfpA. This could be explained by the lack of accessory genes involved in the regulation, export, anchoring, or polymerization of pilins on the bacterium (4, 5, 31, 32). Bortoloni et al. reported that clinical isolates of EPEC serotypes O128ab:H2 and O119:H2 possess large deletions of bfp genes, do not produce Bfp structures, and only adhere to epithelial cells after 6 h of infection (5). Okeke et al. (24) reported that a subset of O119:H2, O128:H2, and O142:H6 strains carry frameshift mutations in perA (a transcriptional activator of Bfp expression [16]), leading to premature truncation. As with bfpA, molecular variations were found in the perA gene.
FIG. 3.
Visualization of Bfp structures produced by EPEC O55:H6 (7) (A and C) and O142:H34 (21) (B and D) strains grown on Bfp agar. (A and B) Negative staining of EPEC showing Bfp rope-like structures; (C and D) detection of Bfp fibers produced by EPEC grown on Bfp agar by immunofluorescence. Arrows indicate Bfp structures.
Several immunological, DNA-based (PCR and hybridization with specific DNA probes), and adherence assays have been proposed to detect EPEC strains in epidemiological settings (1, 8, 13, 18, 29). An enzyme-linked immunosorbent assay was reported to detect localized adherent EPEC employing EPEC-specific antibodies (1). Further characterization of these antibodies indicated that they were directed against the Bfp (J. Girón and J. M. Albert, unpublished data). Direct detection of the gene is simple nowadays and more accurate than immunological assays that rely on expression. However, in areas of the world where EPEC is a frequent cause of childhood diarrhea, the use of molecular diagnostic tools and tissue culture cells is unaffordable. Thus, the use of Bfp agar is an alternative for promoting Bfp production and detection by an immunoassay when Bfp production is being investigated in epidemiological settings or in basic research laboratories. Based on the data obtained, we recommend the use of Bfp agar to obtain maximal production of BfpA in most EPEC strains.
Acknowledgments
We thank Alejandro Ruiz-Tagle for assistance, Isabel Scaletsky for critical discussion, and James B. Kaper for support during preparation of this manuscript.
J. A. Girón thanks Lilia Cedillo (BUAP), Conacyt (Grant 32777-M), and the Fundação de Apoio à Pesquisa do Estado de São Paulo (FAPESP) for support.
REFERENCES
- 1.Albert, J. M., M. Ansaruzzaman, S. M. Faruque, P. K. Neogi, K. Haider, and S. Tzipori. 1991. An ELISA for the detection of localized adherent classic enteropathogenic Escherichia coli serogroups. J. Infect. Dis. 164: 986-989. [DOI] [PubMed] [Google Scholar]
- 2.Andrade, J. R. C., and M. R. De Santa Rosa. 1986. Investigation on an adhesive property (localized adherence) characteristic of classic enteropathogenic serogroups of Escherichia coli. Rev. Microbiol. São Paulo 17:116-125. [Google Scholar]
- 3.Bieber, D., S. W. Ramer, C. Y. Wu, W. J. Murray, T. Tobe, R. Fernandez, and G. K. Schoolnik. 1998. Type IV pili, transient bacterial aggregates and virulence in enteropathogenic Escherichia coli. Science 280:2114-2118. [DOI] [PubMed] [Google Scholar]
- 4.Blank, T. E., H. Zhong, A. L. Bell, T. S. Whittman, and M. S. Donnenberg. 2000. Molecular variation among type IV pilin (bfpA) genes from diverse enteropathogenic Escherichia coli strains. Infect. Immun. 68:7028-7038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bortoloni, M. R., L. R. Trabulsi, R. Keller, G. Frankel, and V. Sperandio. 1999. Lack of expression of bundle-forming pili in some clinical isolates of enteropathogenic Escherichia coli (EPEC) is due to a conserved large deletion in the bfp operon. FEMS Microbiol. Lett. 179:169-174. [DOI] [PubMed] [Google Scholar]
- 6.Campos, L. C., T. S. Whittam, T. A. T. Gomes, J. R. C. Andrade, and L. R. Trabulsi. 1994. Escherichia coli group O111 includes several clones of diarrheagenic strains with different virulence properties. Infect. Immun. 62:3282-3288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chart, H., J. Spencer, H. Smith, and B. Rowe. 1997. Magnesium ions are required for HEp-2 adhesion by enteroaggregative strains of Escherichia coli O126:H27 and O44:H18. FEMS Microbiol. Lett. 148:49-52. [DOI] [PubMed] [Google Scholar]
- 8.Cravioto, A., R. Gross, S. Scotland, and B. Rowe. 1979. An adhesive factor found in strains of Escherichia coli belonging to the traditional infantile enteropathogenic serotypes. Curr. Microbiol. 3:95-99. [Google Scholar]
- 9.Donnenberg, M. S., J. A. Girón, J. P. Nataro, and J. B. Kaper. 1992. A plasmid-encoded type IV fimbrial gene of enteropathogenic Escherichia coli associated with localized adherence. Mol. Microbiol. 6:3227-3237. [DOI] [PubMed] [Google Scholar]
- 10.Edwards, R. A., and J. L. Puente. 1998. Fimbrial expression in enteric bacteria: a critical step in intestinal pathogenesis. Trends Microbiol. 6:282-287. [DOI] [PubMed] [Google Scholar]
- 11.Fagundes-Neto, U. 1996. Enteropathogenic Escherichia coli infection infants: clinical aspects and small bowel morphological alterations. Rev. Microbiol. São Paulo 27:117-119. [Google Scholar]
- 12.Girón, J. A., A. S. Y. Ho, and G. K. Schoolnik. 1991. An inducible bundle-forming pilus of enteropathogenic Escherichia coli. Science 254:710-713. [DOI] [PubMed] [Google Scholar]
- 13.Girón, J. A., M. S. Donnenberg, W. C. Martin, K. G. Jarvis, and J. B. Kaper. 1993. Distribution of the bundle-forming pilus structural gene (bfpA) among enteropathogenic Escherichia coli (EPEC). J. Infect. Dis. 168: 1037-1041. [DOI] [PubMed] [Google Scholar]
- 14.Girón, J. A., F. Qadri, T. Azim, K. G. Jarvis, J. B. Kaper, and M. J. Albert. 1995. Monoclonal antibodies specific for the bundle-forming pilus of enteropathogenic Escherichia coli. Infect. Immun. 63:4949-4952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gomes, T. A. T., V. Rassi, K. L. MacDonald, S. R. T. S. Ramos, L. R. Trabulsi, M. A. M. Vieira, B. E. C. Guth, J. A. N. Candeias, C. Ivey, M. R. F. Toledo, and P. A. Blake. 1991. Enteropathogens associated with acute diarrheal disease in urban infants in São Paulo, Brazil. J. Infect. Dis. 164: 331-337. [DOI] [PubMed] [Google Scholar]
- 16.Gómez-Duarte, O., and J. B. Kaper. 1995. A plasmid-encoded regulatory region activates chromosomal eaeA expression in enteropathogenic Escherichia coli. Infect. Immun. 63:1767-1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goncalves, A. G., L. C. Campos, T. A. T. Gomes, J. Rodrigues, V. Sperandio, T. S. Whittam, and L. R. Trabulsi. 1997. Virulence properties and clonal structures of strains of Escherichia coli O119 serotypes. Infect. Immun. 65:2034-2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gunzburg, S. T., N. G. Tornieporth, and L. W. Riley. 1995. Identification of enteropathogenic Escherichia coli by PCR-based detection of the bundle-forming pilus gene. J. Clin. Microbiol. 33:1375-1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haigh, R., T. Baldwin, S. Knutton, and P. H. Williams. 1995. Carbon dioxide regulated secretion of the EaeB protein of enteropathogenic Escherichia coli. FEMS Microbiol. Lett. 129:63-68. [DOI] [PubMed] [Google Scholar]
- 20.Kenny, B., A. Abe, M. Stein, and B. B. Finlay. 1997. Enteropathogenic Escherichia coli protein secretion is induced in response to conditions similar to those in the gastrointestinal tract. Infect. Immun. 65:2606-2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 22.Martinez, M. B., C. R. Taddei, A. Ruiz-Tagle, L. R. Trabulsi, and J. A. Girón. 1999. Antibody response of children with enteropathogenic Escherichia coli infection to the bundle-forming pilus and LEE-encoded virulence determinants. J. Infect. Dis. 179:269-274. [DOI] [PubMed] [Google Scholar]
- 23.Nataro, J. P., and J. B. Kaper. 1998. Diarrheagenic Escherichia coli. Clin. Microbiol. Rev. 5:109-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Okeke, I. N., J. A. Borneman, S. Shin, J. L. Mellies, L. E. Quinn, and J. B. Kaper. 2001. Comparative sequence analysis of the plasmid-encoded regulator of enteropathogenic Escherichia coli strains. Infect. Immun. 69:5553-5564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parissi, A., J. Parissi, and J. A. Girón. 2000. Recognition of virulence determinants of enteropathogenic Escherichia coli by human colostrum and serum antibodies. J. Clin. Microbiol. 38:2696-2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pelayo, S., I. C. A. Scaletsky, M. Z. Pedroso, V. Sperandio, J. A. Girón, G. Frankel, and L. R. Trabulsi. 1998. Virulence properties of atypical EPEC strains. J. Med. Microbiol. 48:41-49. [DOI] [PubMed] [Google Scholar]
- 27.Puente, J. L., D. Bieber, S. W. Ramer, W. Murray, and G. K. Schoolnik. 1996. The bundle-forming pili of enteropathogenic Escherichia coli transcriptional regulation by environmental signals. Mol. Microbiol. 20:87-100. [DOI] [PubMed] [Google Scholar]
- 28.Rodrigues, J., I. C. A. Scaletsky, L. C. Campos, T. A. T. Gomes, T. S. Whittam, and L. R. Trabulsi. 1996. Clonal structure and virulence factors in strains of Escherichia coli of the classic serogroups O55. Infect. Immun. 64:2680-2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scaletsky, I. C. A., M. L. Silva, and L. R. Trabulsi. 1984. Distinctive patterns of adherence of enteropathogenic Escherichia coli to HeLa cells. Infect. Immun. 45:534-536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sohel, I., J. L. Puente, S. W. Ramer, D. Bieber, C. Y. Wu, and G. K. Schoolnik. 1996. Enteropathogenic Escherichia coli: identification of a gene cluster coding for bundle-forming pilus morphogenesis. J. Bacteriol. 178:2613-2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stone, K. D., H. Z. Zhang, L. K. Carlson, and M. S. Donnenberg. 1996. A cluster of 14 genes from enteropathogenic Escherichia coli is sufficient for the biogenesis of a type IV pilus. Mol. Microbiol. 20:325-337. [DOI] [PubMed] [Google Scholar]
- 32.Vanmaele, R. P., and G. D. Armstrong. 1997. Effect of carbon source on localized adherence of enteropathogenic Escherichia coli. Infect. Immun. 65:1408-1413. [DOI] [PMC free article] [PubMed] [Google Scholar]