Abstract
We determined the value of four serological assays for the diagnosis of canine monocytic ehrlichiosis by comparing them to the indirect fluorescent-antibody assay “gold standard.” The specificity of Dip-S-Ticks was significantly lower than that of all of the other tests evaluated. The sensitivity of Dip-S-Ticks was significantly higher than that of Snap3Dx or the Snap Canine Combo. The sensitivity of the rMAP2 enzyme-linked immunosorbent assay (ELISA) was significantly higher than that of the Snap Canine Combo. The accuracy levels of the rMAP2 ELISA, Snap3Dx, Dip-S-Ticks, and Snap Canine Combo were 97.0, 89.8, 85.1, and 82.9%, respectively.
Canine monocytic ehrlichiosis, caused by tick-transmitted Ehrlichia canis, has been reported in the United States and throughout most of the world, causing extensive morbidity and mortality (12). Clinical and hematologic abnormalities are often nonspecific during E. canis infections, and coinfections with other tick-transmitted agents such as E. chaffeensis may be common (7); thus, a definitive diagnosis may be difficult to make. The indirect fluorescent-antibody assay (IFA) is the method most widely used to diagnose E. canis infection and is considered the “gold standard” (15). However, it can only be performed in specialized laboratories, reading of results is subjective (8, 14), and it does not differentiate consistently between E. canis and E. chaffeensis infections (3). There is a tremendous need for other serological assays for the diagnosis of E. canis infection in dogs. A variety of serodiagnostic tests are commercially available, but the diagnostic value of many of these tests remains unevaluated. The objective of the present study was to determine the value of four serological assays for the diagnosis of canine monocytic ehrlichiosis by comparing them to the IFA gold standard. A total of 97 canine serum samples was obtained from the College of Veterinary Medicine, University of Florida, Gainesville, or the College of Veterinary Medicine, North Carolina State University, Raleigh. Eighteen IFA-positive serum samples from five dogs experimentally infected with E. canis during previous studies were used (4). Thirty-five serum samples were from naturally infected dogs that had clinical signs consistent with canine ehrlichiosis and positive IFA titers (≥1:40). Forty-four IFA-negative serum samples were obtained from clinically healthy dogs during well-patient visits or preinfection from experimentally infected dogs. All serum samples were tested blindly in the following serological assays.
All serum samples, diluted 1/300, were tested with the rMAP2 indirect enzyme-linked immunosorbent assay (ELISA) as described previously (1). The samples were tested for canine ehrlichiosis with the Snap Canine Combo test kit, the new Snap3Dx assay (IDEXX Laboratories, Inc.), and the InDx canine multitest Dip-S-Ticks assay (PanBio InDx, Inc., Baltimore, Md.) in accordance with the manufacturers' recommendations. The data were analyzed by using SigmaStat, version 2.03, for Windows (SPSS Inc.) and calculated as described by Courtney and Cornell (6).
As observed in Table 1, a sensitivity of 96.2% was obtained with the rMAP2 ELISA. Its specificity was 97.7%, as one false-positive reaction was detected. The latter serum sample was positive with the Dip-S-Tick assay but negative by both Snap tests. The sensitivity of the rMAP2 ELISA was significantly higher than that of the Snap Canine Combo (P = 0.001) (Table 2). Also, the sensitivity of the Dip-S-Ticks was significantly higher than that of the Snap3Dx (P = 0.003) or the Snap Canine Combo (P ≤ 0.001). The specificity of the Dip-S-Ticks was significantly lower than that of all of the other tests evaluated (P ≤ 0.001).
TABLE 1.
Comparison of serological assays to detect antibodies in dogs infected with E. canis
| Dog status (no. of dogs) | No. (%) of dogs with detectable antibodies in the following tests:
|
||||
|---|---|---|---|---|---|
| IFA | rMAP2 ELISA | Dip-S-Ticks | Snap Combo | Snap3Dx | |
| Experimentally infected (18) | 18 (100) | 17 (94.4) | 18 (100) | 15 (83.3) | 14 (77.8) |
| Naturally infected (35) | 35 (100) | 34 (97.1) | 35 (100) | 19 (54.3) | 28 (80) |
| Total infected (53) | 53 (100) | 51 (96.2) | 53 (100) | 34 (64.2) | 42 (79.2) |
| Preinfectiona (3) | 0 (0) | 0 (0) | 2 (66.7) | 0 (0) | 0 (0) |
| Clinically healthya (41) | 0 (0) | 1 (2.4) | 15 (36.6) | 1 (2.4) | 0 (0) |
| Total noninfected (44) | 0 (0) | 1 (2.3) | 17 (38.6) | 1 (2.3) | 0 (0) |
Three of the noninfected serum samples were collected from dogs prior to being infected experimentally with E. canis; the others were from dogs that were brought to a clinic for a well-patient checkup.
TABLE 2.
Comparison of the accuracies, sensitivities, and specificities of four serodiagnostic tests with IFA as the gold standard
| Diagnostic test | % Accuracy | % Sensitivity (confidence limits)a | % Specificity (confidence limits)a |
|---|---|---|---|
| rMAP2 ELISA | 97.0 | 96.2b,c (85.3-99.1) | 97.7b (86.0-99.7) |
| Dip-S-Ticks | 85.1 | 100b (91.3-100) | 61.4c (44.6-75.8) |
| Snap Combo | 82.9 | 64.2d (48.8-77.1) | 97.7b (86-99.7) |
| Snap3Dx | 89.8 | 79.2c,d (64.5-88.9) | 100b (89.8-100) |
Lower and upper 95% confidence limits of sensitivity and specificity are shown. Data, within each respective sensitivity or specificity category, having different superscript letters differ significantly (P ≤ 0.0083) on the basis of the McNemar paired χ2 test (2).
Dip-S-Ticks are semiquantitative assays. On each stick, two windows containing different dilutions of E. canis antigen were present. One window corresponded to E. canis IFA titers of approximately 1:40 to 1:80, whereas the other window represented IFA titers of 1:5,000 to 1:10,000. All of the infected serum samples tested with IFA titers of 1:40 to 1:80 were positive in the 1:40-to-1:80 Dip-S-Ticks window. We observed that 73.9% of the serum samples having IFA titers of ≥5,000 were positive in the 1:5,000-to-1:10,000 Dip-S-Ticks window. No false positives were recorded for the second test window. Twenty-eight percent of the serum samples within an IFA titer range of 1:320 to 1:2,560 were reactive in the 1:5,000-to-1:10,000 Dip-S-Tick window.
One of the strengths of this study was that it used duplicate sera from the same dogs to compare different serodiagnostic tests. The assays tested in this study varied in the ability to detect E. canis antibodies. The Dip-S-Ticks assay was 100% sensitive in detecting sera from infected dogs but lacked specificity, as a high percentage of false positives was reported. This may be due to the fact that whole cells from E. canis (Jake strain) were used as antigens causing cross-reactivities and thus false-positive reactions. Semiquantitative results can be obtained with the Dip-S-Ticks assay; however, the Dip-S-Ticks titers did not always correspond to the reported IFA titers. Reading of the assay strips was difficult when recording borderline reactions and may have led to misleading interpretations and to the high percentage of false positives recorded. Also, the range between the upper and lower confidence limits was wide because of the limited number of samples available within each of the infected or uninfected groups.
Previously, we demonstrated 97.2% overall agreement between the IFA and the rMAP2 ELISA for E. canis antibody detection (1). In this study, 96.2% sensitivity and 97.7% specificity were obtained with the E. canis rMAP2 ELISA. The rMAP2 ELISA has the advantage of being a quantitative test. Therefore, detection of recent exposure and active infection can be done by comparing paired titers.
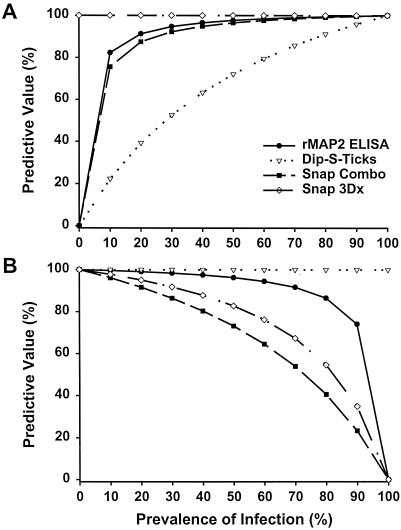
The use of recombinant proteins, such as p30 and p43, to increase the quality of the test antigen and to eliminate test subjectivity in Western immunoblot and dot blot assays was demonstrated previously (8, 9). The Snap Canine Combo from IDEXX, which uses whole cells of E. canis strain Oklahoma as the antigen, is being replaced on the market by the new Snap3Dx assay, which uses recombinant analogs of the major outer membrane proteins (9, 10), specifically, p30 and p30-1. According to our study, the sensitivity and specificity of the Snap3Dx assay were 15 and 2.3% higher, respectively, when recombinant proteins were used as antigens instead of E. canis whole cells. However, these differences were not statistically significant within the limited sample size. The Snap3Dx assay combines specificity and rapidity and can be used in any clinic. The rMAP2 ELISA, even though it is more time consuming and is not applicable to all laboratories, provides high sensitivity, high specificity, quantitative results, and the convenience of the ability to test many samples in a short time. Its accuracy, which reflects the chance of correctly identifying the infection status of an animal (6), was the highest among the assays tested. In choosing a serodiagnostic test for a laboratory, one should take into consideration many factors, including the cost of the test, its rapidity, its convenience of use, the sample load, and the predictive value of a positive or negative result. The seroprevalence of E. canis infection within different canine populations may vary dramatically. For example, in a recent survey of sick dogs from North Carolina and Virginia, the seroprevalence of E. canis was estimated to be 2.5% (13). On the basis of our study and an E. canis prevalence of 2.5%, the calculated predictive values of positive and negative results were both 100% for the Snap3Dx test (Fig. 1). Therefore, if the prevalence of E. canis infection is expected to be low in the population tested, the Snap3Dx test would be an excellent test to help in the identification of such infections.
FIG. 1.
Predictive values of positive (A) and negative (B) rMAP2 ELISA, Dip-S-Ticks, Snap Combo, and Snap3DX results at prevalence rates ranging from 0 to 100% with IFA as the reference standard test.
Alternatively, for serodiagnosis during an outbreak or when testing dogs with clinical and/or laboratory findings consistent with canine ehrlichiosis, the chances of finding more E. canis infections are higher and the predictive values of the results obtained in such an environment would be different. Kordick and colleagues (7) reported an E. canis prevalence of 55.6% in a Walker Hound kennel in North Carolina during an outbreak. In this case, the rMAP2 ELISA would be a good choice as its predictive value of positive and negative results would be 98.1 and 95.4%, respectively (Fig. 1). It must be emphasized that serodiagnostic assays do not distinguish between current infection and prior exposure.
In this study, specificity was determined in relation to that of IFA, the gold standard. The IFA and most of the rapid serodiagnostic tests do not differentiate among E. canis, E. chaffeensis, and E. ewingii infections in dogs, as these species are closely related and have some cross-reacting antigens (5, 11). It has been observed that the clinical disease, prognosis, and potential drug efficacy associated with E. canis or E. chaffeensis infection may differ (3). Therefore, further development of tests for canine monocytic ehrlichiosis should address this important problem and try to eliminate cross-reactivities to provide a more accurate diagnosis.
Acknowledgments
This work was supported by grants from the University of Florida Division of Sponsored Research, projects UPN #00030864 and UPN #98062369. M. Bowie was the recipient of National Institute of Allergy and Infectious Diseases fellowship AI45580-01S1.
We are in debt to PanBio InDx, Inc., for supplying the InDx canine multitest Dip-S-Ticks necessary for this study. We thank S. Giguère and C. H. Courtney for helpful discussions and advice.
Footnotes
College of Veterinary Medicine Journal Series publication 610.
REFERENCES
- 1.Alleman, A. R., L. J. McSherry, A. F. Barbet, E. B. Breitschwerdt, H. L. Sorenson, M. V. Bowie, and M. Bélanger. 2001. Recombinant major antigenic protein 2 of Ehrlichia canis: a potential diagnostic tool. J. Clin. Microbiol. 39:2494-2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Armitage, P. 1971. Statistical methods in medical research. Blackwell Scientific Publications, London, United Kingdom.
- 3.Breitschwerdt, E. B., B. C. Hegarty, and S. J. Hancock. 1998. Sequential evaluation of dogs naturally infected with Ehrlichia canis, Ehrlichia chaffeensis, Ehrlichia equi, Ehrlichia ewingii, or Bartonella vinsonii. J. Clin. Microbiol. 36:2645-2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Breitschwerdt, E. B., B. C. Hegarty, and S. I. Hancock. 1998. Doxycycline hyclate treatment of experimental canine ehrlichiosis followed by challenge inoculation with two Ehrlichia canis strains. Antimicrob. Agents Chemother. 42:362-368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen, S. M., L. C. Cullman, and D. H. Walker. 1997. Western immunoblotting analysis of antibody responses of patients with human monocytotrophic ehrlichiosis to different strains of Ehrlichia chaffeensis and Ehrlichia canis. Clin. Diagn. Lab. Immunol. 4:731-735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Courtney, C. H., and J. A. Cornell. 1990. Evaluation of heartworm immunodiagnostic tests. J. Am. Vet. Med. Assoc. 197:724-729. [PubMed] [Google Scholar]
- 7.Kordick, S. K., E. B. Breitschwerdt, B. C. Hegarty, K. L. Southwick, C. M. Colitz, S. I. Hancock, J. M. Bradley, R. Rumbough, J. T. McPherson, and J. N. MacCormack. 1999. Coinfection with multiple tick-borne pathogens in a Walker Hound kennel in North Carolina. J. Clin. Microbiol. 37:2631-2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McBride, J. W., R. E. Corstvet, E. D. Breitschwerdt, and D. H. Walker. 2001. Immunodiagnosis of Ehrlichia canis infection with recombinant proteins. J. Clin. Microbiol. 39:315-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ohashi, N., A. Unver, N. Zhi, and Y. Rikihisa. 1998. Cloning and characterization of multigenes encoding the immunodominant 30-kilodalton major outer membrane proteins of Ehrlichia canis and application of the recombinant protein for serodiagnosis. J. Clin. Microbiol. 36:2671-2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reddy, G. R., C. R. Sulsona, A. F. Barbet, S. M. Mahan, M. J. Burridge, and A. R. Alleman. 1998. Molecular characterization of a 28 kDa surface antigen gene family of the tribe Ehrlichiae. Biochem. Biophys. Res. Commun. 247:636-643. [DOI] [PubMed] [Google Scholar]
- 11.Rikihisa, Y., S. A. Ewing, and J. C. Fox. 1994. Western immunoblot analysis of Ehrlichia chaffeensis, E. canis, or E. ewingii infections in dogs and humans. J. Clin. Microbiol. 32:2107-2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rikihisa, Y. 1999. Ehrlichiae of veterinary importance, p. 393-405. In D. Raoult and P. Brouqui (ed.), Rickettsiae and rickettsial diseases at the turn of the third millennium. Rickettsioses in animals. Elsevier, Paris, France.
- 13.Suksawat, J., B. C. Hegarty, and E. B. Breitschwerdt. 2000. Seroprevalence of Ehrlichia canis, Ehrlichia equi, and Ehrlichia risticii in sick dogs from North Carolina and Virginia. J. Vet. Intern. Med. 14:50-55. [DOI] [PubMed] [Google Scholar]
- 14.Waner, T., C. Strenger, and A. Keysary. 2000. Comparison of a clinical-based ELISA test kit with the immunofluorescence test for the assay of Ehrlichia canis antibodies in dogs. J. Vet. Diagn. Investig. 12:240-244. [DOI] [PubMed] [Google Scholar]
- 15.Waner, T., S. Harrus, F. Jongejan, H. Bark, A. Keysary, and A. W. C. A. Cornelissen. 2001. Significance of serological testing for ehrlichial diseases in dogs with special emphasis on the diagnosis of canine monocytic ehrlichiosis caused by Ehrlichia canis. Vet. Parasitol. 95:1-15. [DOI] [PubMed] [Google Scholar]