Abstract
A collection of 119 strains of Mycobacterium tuberculosis isolated from patients with pulmonary tuberculosis in the Archangel Oblast, Russia, in 1998 and 1999 were studied by using restriction fragment length polymorphism (RFLP) analysis with the IS6110 probe and spoligotyping. Resistance of the strains to antituberculosis drugs was analyzed by the BACTEC method, and mutations associated with rifampin resistance were detected by using the Inno-LiPA Rif. TB test. RFLP analysis and spoligotyping demonstrated that 53 (44.5%) of the strains belonged to the Beijing genotype. These strains showed a significantly higher rate of resistance than M. tuberculosis strains of other genotypes circulating in the region. In particular, 43.4% of the strains of the Beijing genotype were multidrug resistant; in contrast, only 10.6% of the other strains were. Of the strains of the Beijing genotype, 92.5% were part of a cluster, while only 33.3% of the remaining strains were clustered. Analysis of the medical records of the patients demonstrated that individuals infected with a strain of the Beijing genotype were significantly more likely to be alcohol abusers and to have chronic obstructive pulmonary disease prior to the tuberculosis diagnosis. Multivariate analysis showed that both variables were independently associated with infection by strains belonging to the Beijing genotype. Our study demonstrated that strains of the Beijing genotype are an important cause of tuberculosis in the Archangel Oblast and that dissemination of these strains is associated with the high incidence of drug resistance.
A reversion of the previously declining rates of tuberculosis has been observed in Russia in the past decade (24, 26). This increase in tuberculosis incidence is attributed to socioeconomic changes and an unsatisfactory national program for control of the disease (13). Insufficient financial support provided to medical institutions for tuberculosis diagnosis and treatment, lack of availability of quality drugs, and irregular drug supply during the past 15 years have resulted in inadequate treatment of the disease (13).
The Archangel Oblast, situated in the northwestern part of the country, is no exception. The incidence rate in the general populatin increased from 20.0/100,000 in 1991 to 48.0/100,000 in 2000. When the prison system is included, the incidence in the Archangel Oblast in 2000 reached 104.0/100,000. The mortality rate also increased in that period, from 3.6/100,000 in 1991 to 16.5/100,000 in 2000.
The resurgence of tuberculosis has been accompanied by a rise in drug resistance. The Archangel Regional Tuberculosis Dispensary (ARTD), which has performed drug resistance testing by using the absolute concentration method for many years, reported that, in 1991, primary drug resistance to one or more antituberculosis drugs was 15% and acquired drug resistance was 60% (16). In 2000, these proportions were 33 and 85%, respectively (29).
Mycobacterium tuberculosis strains from various geographical origins have been characterized by using restriction fragment length polymorphism (RFLP) analysis with the IS6110 insertion element as a probe. The method is based on the detection of differences in the numbers and locations of the insertion element in the genomes of the bacteria. It has proved convenient and reliable and has been internationally standardized for the genotyping of M. tuberculosis strains (31). Molecular characterization of M. tuberculosis strains has provided answers to fundamental questions regarding the transmission of tuberculosis within defined geographical settings (6, 32).
Spoligotyping, a newer technique based on the detection of the spacer DNA sequences in between the direct repeats in the direct repeat locus of the chromosome of M. tuberculosis (12), is now often used in addition to IS6110 RFLP analysis. Spoligotyping is simple and highly reproducible and has been used to distinguish between strains with highly similar IS6110 RFLP patterns or between strains with fewer than five copies of IS6110.
In recent years, the rapid dissemination of M. tuberculosis strains belonging to one particular genetic lineage has been described. This family of strains, with highly similar IS6110 RFLP patterns, was first identified from tuberculosis patients in the Beijing province of China and was thus designated the Beijing genotype (32, 34). M. tuberculosis strains of the Beijing genotype have been dominant since the mid-1950s and have remained so in the 1990s in the countries of East Asia (27, 32, 34). Members of the Beijing genotype were recently found in countries outside Asia, e.g., in the United States and South Africa, where they have been implicated in several outbreaks (32). Spoligotyping has demonstrated that the multidrug-resistant (MDR) W strain family, which was responsible for a large outbreak in New York City in the 1990s, is a member of the Beijing genotype.
The objectives of our study were to analyze the level of active transmission of tuberculosis in the Archangel Oblast by using molecular methods and to study the resistance patterns of M. tuberculosis strains circulating in the region. Medical records of patients were reviewed retrospectively to identify possible factors associated with infection by strains of the Beijing genotype.
MATERIALS AND METHODS
Patients and bacterial strains.
The study population included patients with pulmonary tuberculosis diagnosed and treated in the ARTD, in Archangel Oblast, Russia. The ARTD provides services in the Oblast to all patients with tuberculosis and performs both smear microscopy and culturing for case findings.
A total of 151 M. tuberculosis strains isolated from patients with pulmonary tuberculosis in the ARTD in 1998 and 1999 were cultivated on Lowenstein-Jensen medium and forwarded to the Reference Laboratory for Tuberculosis, Norwegian Institute of Public Health (NIPH), Oslo, Norway, for further analyses. Strains from patients in the prison system were not available. Forty-eight strains were collected from consecutive new and previously treated patients during a 3-month period (June, July, and August) in 1998. The remaining 103 strains were collected consecutively during the third and fourth quarters of 1999. According to the quarterly reports of the ARTD, 283 patients were diagnosed with tuberculosis during the second half of 1999. These included 240 new patients, 31 previously treated patients declared cured prior to again becoming sputum positive (relapses), 6 patients who had treatment interrupted for more than 2 months before returning to the health facilities and being found sputum positive (defaults), and 6 patients who remained smear positive after 5 months of treatment or longer (treatment failures) (9). Of the 283 registered patients, 148 (52.3%) had culture-positive samples. There were no significant differences in age, sex, or case category (new patients or previously treated patients) between the 103 patients from whom strains were forwarded to the NIPH and the remaining 45 patients whose samples were found culture positive in the second half of 1999.
Of the total of 151 strains forwarded to the NIPH in 1988 and 1999, 18 strains were either heavily contaminated or did not survive transport. Among the remaining 133 strains, 1 strain (the first one isolated) per patient was selected. Thus, the final number of M. tuberculosis strains studied was 119: 43 strains from 1998 and 76 strains from 1999.
Medical records for the 119 patients were reviewed retrospectively to identify factors that might be associated with infection from particular strains. Information collected by the doctor in charge of treatment and included in medical records at the ARTD served as a basis for the data collection (29). Variables with missing values were excluded. The following variables were analyzed: age; gender; social factors, including smoking habits, alcohol abuse, and having been in prison; and medical factors, such as body mass index, presence of diabetes and chronic obstructive pulmonary disease (COPD) prior to tuberculosis, human immunodeficiency virus (HIV) status, possible contacts with another tuberculosis patient, and whether or not the patient completed the treatment. Medical information about diseases prior to tuberculosis was obtained from general practitioners. The presence of COPD and diabetes was defined according to standard diagnostic criteria. Possible contacts with other tuberculosis patients were evaluated during special home and office visits performed by the doctor and nurse in charge of treatment. Testing for HIV infection is performed routinely for all patients diagnosed and treated for tuberculosis at the ARTD. Blood samples collected at the ARTD were sent for analysis to the Center for HIV Testing in Archangel. These data are routinely recorded for all tuberculosis patients treated at the ARTD.
Identification of strains.
Isolates were confirmed as M. tuberculosis at the NIPH by using a 16S rRNA gene hybridization technique (AccuProbe; GenProbe Inc., San Diego, Calif.) and standard microbiological tests (niacin accumulation test and nitrate reduction test).
Drug susceptibility testing.
Susceptibility testing with the antituberculosis drugs isoniazid, rifampin, ethambutol, and streptomycin was performed at the NIPH by using the radiometric broth method (BACTEC; Becton Dickinson Diagnostic Systems, Sparks, Md.) (11, 19, 25). The concentrations of the antituberculosis drugs used were as follows: isoniazid, 0.2 μg/ml; rifampin, 2.0 μg/ml; ethambutol, 7.5 μg/ml; and streptomycin, 6.0 μg/ml.
To identify the mutations associated with rifampin resistance in the gene encoding the β subunit of RNA polymerase (rpoB), the Inno-LiPA Rif. TB test (Innogenetics N.V., Ghent, Belgium) was performed according to the instructions of the manufacturer (18, 28) for all strains found resistant to rifampin by the BACTEC method.
RFLP analysis.
RFLP analysis of M. tuberculosis DNA was performed according to internationally standardized methodology (20, 31, 33). Briefly, cells were harvested by centrifugation and chromosomal DNA was isolated as described by Van Soolingen et al. (33). DNA was restricted with PvuII (Boehringer, Mannheim, Germany) according to the manufacturer's instructions, and fragments were separated by electrophoresis in an 0.8% agarose gel. After transfer of the DNA to a GeneScreen Plus membrane (DuPont, Boston, Mass.) by the alkaline transfer procedure, hybridization was performed with a 245-bp PCR-amplified probe directed against the right arm of IS6110 (33). The probe was labeled by using a digoxigenin-dUTP labeling and detection kit (Boehringer). To facilitate the comparison of the IS6110 RFLP patterns, a 1-kb DNA ladder (Gibco BRL, Gaithersburg, Md.) was deposited on the first, middle, and last lanes of each gel. The ladder, labeled by using the digoxigenin-dUTP labeling and detection kit, was mixed with the IS6110 probe in hybridization buffer (5× SSC [1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate], 0.1% Sarkosyl, 0.02% sodium dodecyl sulfate) to allow visualization.
The IS6110 RFLP patterns were inspected visually, scanned, and analyzed by using the computer software GelCompar, version 4.1 (Applied Maths, Sint-Martens-Latem, Belgium). The unweighted pair-group method of arithmetic averaging with the Dice coefficient as a similarity measure was used. Band position tolerance was set to 1.20%, and optimization was 0.50%.
Spoligotyping.
Spoligotyping was performed by using a commercially available kit (Isogen Bioscience BV, Maarssen, The Netherlands) according to the instructions supplied by the manufacturer as previously described (12).
Definitions.
M. tuberculosis strains of the Beijing genotype were defined as a group of genetically closely related strains which showed the following characteristics: the strains harbored a high number of IS6110 copies, reaching up to 17 in our study, and more than two-thirds of them were present at the same genomic sites; they clustered within 60% similarity; and they had identical spoligotyping results, showing hybridization only with the last 9 of the 43 possible spacers (32, 34).
A cluster of isolates was defined as two or more isolates exhibiting 100% identical IS6110 RFLP patterns (8, 10, 32).
Statistical analyses.
Epi Info, version 6.04b (Centers for Disease Control and Prevention, Atlanta, Ga., and World Health Organization, Geneva, Switzerland), and SPSS for Windows, version 9.0.1 (SPSS Inc., Chicago, Ill.), were used for the statistical analyses. Associations between categorical variables were assessed by the χ2 test and Fisher's exact test for values of less than five. Differences between groups were tested by univariate and multivariate analyses and expressed as odds ratios (OR) with 95% confidence intervals (95% CI). Student's t test was used to test differences in means of continuous variables. A P value of <0.05 was considered significant.
RESULTS
RFLP analysis of the M. tuberculosis strains.
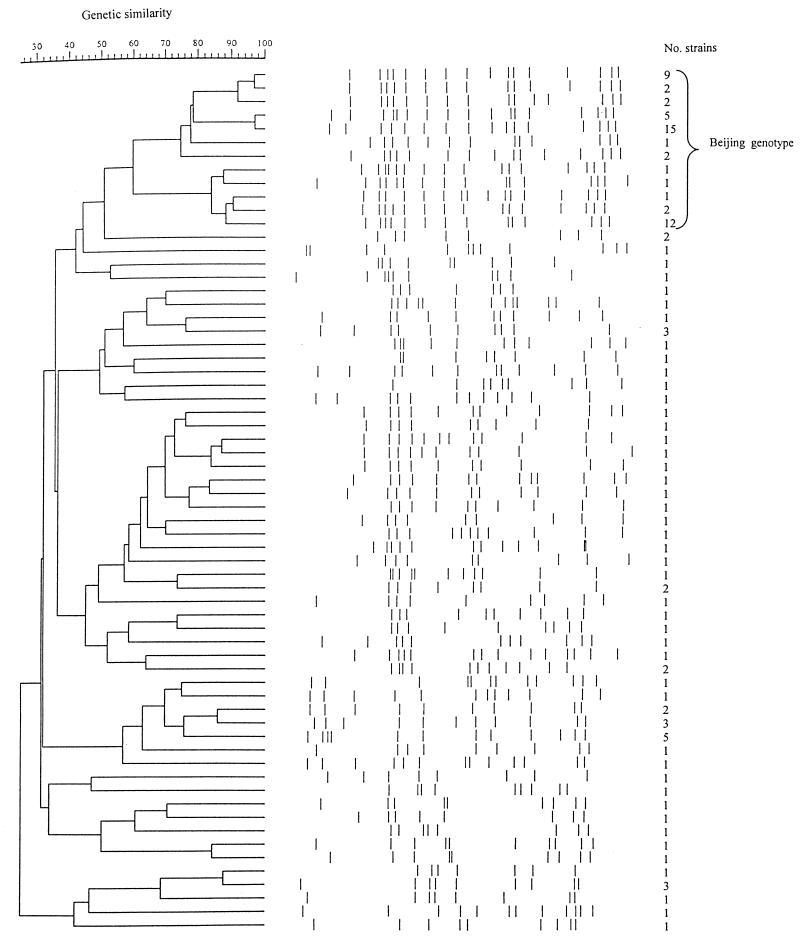
The 119 strains were analyzed by RFLP with IS6110 as a hybridization probe. The number of IS6110 copies varied between 7 and 17, with a mean number of 12.6 copies. Sixty-four distinct RFLP patterns were revealed (Fig. 1). Of these, 48 (75.0%) were unique and 16 (25.0%) were represented by 2 to 15 strains. Twelve RFLP patterns, representing 53 of the 119 strains (44.5%), shared the majority of the IS6110 copies and clustered together in the dendrogram (Fig. 1). By comparison with fingerprints described in the literature (2, 21, 23, 34), these RFLP patterns were found to correspond to the Beijing genotype. These strains harbored from 13 to 17 copies of IS6110 (mean, 15.4 copies), and their IS6110 RFLP patterns clustered at within 60% similarity. The remaining strains had a significantly lower number of IS6110 copies in their genomes (mean, 10.3 copies) (P < 0.001).
FIG. 1.
Dendrogram showing the genetic similarities of the 64 IS6110 RFLP patterns identified among the 119 M. tuberculosis strains. The RFLP patterns of the strains of the Beijing genotype are indicated. The number of strains in each RFLP is also indicated.
Spoligotyping.
The spoligotyping method was used as an additional tool to further determine the relationships among the strains and to confirm the identification of strains belonging to the Beijing genotype. All 53 strains identified as belonging to the Beijing genotype by their IS6110 RFLP patterns showed identical spoligotypes, lacking spacers 1 to 34, typical for M. tuberculosis strains of the Beijing genotype (32, 34). One additional strain in our collection harbored that same spoligotype. The IS6110 RFLP pattern of that strain showed less than 50% similarity to the Beijing genotype and had only 12 copies of IS6110. Thus, it was not considered to belong to the Beijing genotype.
For the remaining 65 strains, 35 different spoligotypes were identified. Twenty-four spoligotypes were represented by a single strain, and 11 were represented by from 2 to 10 strains. In most cases, strains with identical IS6110 RFLP patterns had identical spoligotypes.
The prevalences of the Beijing genotype were 41.9% among strains isolated in 1998 (18 of the 43 strains) and 46.1% among strains isolated in 1999 (35 of the 76 strains).
Clustering of strains.
Of the 119 patients, 48 (40.3%) were infected by a strain harboring a unique IS6110 RFLP pattern and 71 (59.7%) had strains identified in at least 1 other patient. These 71 patients had strains belonging to one of 16 clusters (Table 1) and were likely to be individuals among whom tuberculosis was recently transmitted.
TABLE 1.
Clustering and IS6110 copy numbers of M. tuberculosis strains with different genotypes isolated from patients with pulmonary tuberculosis in Archangel Oblast, Russia, in 1998 and 1999
| Genotype | No. of strains (% of total) | Mean no. of IS6110 copies | No. of clusters | No. of clustered strains (% of total) | Size of the largest cluster |
|---|---|---|---|---|---|
| Beijing | 53 (44.5) | 15.4 | 8 | 49 (92.5) | 15 |
| Non-Beijing | 66 (55.5) | 10.3 | 8 | 22 (33.3) | 3 |
| Total | 119 | 12.6 | 16 | 71 (59.7) | 15 |
Of the 53 patients infected with a strain of the Beijing genotype, 49 (92.5%) were part of a cluster. The largest cluster comprised 15 patients; other clusters included 12, 9, and 5 patients each, and four clusters comprised 2 patients. Of the 66 patients infected with a strain that did not belong to the Beijing genotype (designated non-Beijing strains), only 22 (33.3%) were part of a cluster. The largest cluster of non-Beijing strains consisted of five strains. Being infected by a strain of the Beijing genotype and being part of a cluster were significantly associated in the Archangel Oblast (OR, 18.0; 95% CI, 5.7 to 60.4; P < 0.001).
Susceptibility patterns.
The 119 strains were tested for susceptibility to the first-line antituberculosis drugs ethambutol, isoniazid, rifampin, and streptomycin by using the BACTEC method. A total of 52 isolates (43.7%) were fully susceptible. Nearly half of the strains were resistant to isoniazid (46.2%) and to streptomycin (47.1%). Thirty strains (25.2%) were MDR, that is, resistant to at least isoniazid and rifampin.
Of the 53 strains of the Beijing genotype, only 12 (22.6%) were susceptible to all four antituberculosis drugs (Table 2). The highest resistance rates among these strains were observed for streptomycin and isoniazid, 73.6 and 62.3%, respectively. MDR was observed in 43.4% of the strains belonging to the Beijing genotype. Non-Beijing strains were significantly less resistant to each drug and were less likely to show MDR (Table 2). The associations between resistance to different antituberculosis drugs and infection by a strain of the Beijing genotype were tested by using logistic regression. Multivariate analysis showed that resistance to rifampin (OR = 6.7; 95% CI 1.1 to 40.2; P = 0.04) and resistance to streptomycin (OR, 12.9; 95% CI, 2.6 to 63.8; P = 0.001) were independently associated with infection by a strain belonging to the Beijing genotype. On the other hand, resistance to ethambutol (OR, 0.3; 95% CI, 0.1 to 1.7; P = 0.18) and resistance to isoniazid (OR, 0.3; 95% CI, 0.1 to 1.8; P = 0.20) were not found to be independently associated with infection by a strain of the Beijing genotype by multivariate analysis, though they played the role of confounders.
TABLE 2.
Resistance to antituberculosis drugs of M. tuberculosis strains isolated from 119 patients in Archangels Oblast, Russia, in 1998 and 1999, according to genotype and clustering
| Resistance | Genotype
|
Clustering
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| No. (%) of strains
|
OR (95% CI) | P | No. (%) of strains that were:
|
OR (95% CI) | P | ||||
| Total | Of the following genotype:
|
Clustered (n = 71) | Nonclustered (n = 48) | ||||||
| Beijing (n = 53) | Non-Beijing (n = 66) | ||||||||
| Ethambutol | 38 (31.9) | 22 (41.5) | 16 (24.2) | 2.2 (1.0-5.3) | 0.04 | 28 (39.4) | 10 (20.8) | 2.5 (1.0-6.3) | 0.03 |
| Isoniazid | 55 (46.2) | 33 (62.3) | 22 (33.3) | 3.3 (1.4-7.6) | 0.001 | 42 (59.2) | 13 (27.1) | 3.9 (1.6-9.4) | 0.001 |
| Rifampin | 30 (25.2) | 23 (43.4) | 7 (10.6) | 6.5 (2.3-19.0) | <0.001 | 27 (38.0) | 3 (6.3) | 9.2 (2.4-41.7) | <0.001 |
| Streptomycin | 56 (47.1) | 39 (73.6) | 17 (25.8) | 8.0 (3.3-20.2) | <0.001 | 45 (63.4) | 11 (22.9) | 5.8 (2.4-14.7) | <0.001 |
| Any one drug | 67 (56.3) | 41 (77.4) | 26 (39.4) | 5.3 (2.2-13.0) | <0.001 | 50 (70.4) | 17 (35.4) | 4.3 (1.8-10.4) | <0.001 |
| MDR | 30 (25.2) | 23 (43.4) | 7 (10.6) | 6.5 (2.3-19.0) | <0.001 | 27 (38.0) | 3 (6.3) | 9.2 (2.4-41.7) | <0.001 |
In univariate analysis, M. tuberculosis resistance to ethambutol, isoniazid, rifampin, and streptomycin, resistance to any one of these four drugs, and MDR were significantly more commonly found in clustered strains than in nonclustered strains (Table 2). Multivariate analysis showed that only resistance to rifampin (OR, 3.2; 95% CI, 1.3 to 44.8; P = 0.03) was independently associated with clustering. Resistance to isoniazid (OR, 1.1; 95% CI, 0.3 to 4.2; P = 0.84), resistance to ethambutol (OR, 0.4; 95% CI, 0.1 to 1.7; P = 0.22), and resistance to streptomycin (OR, 3.2; 95% CI, 0.9 to 10.7; P = 0.07) were not independently associated with infection by a clustered strain.
The 30 rifampin-resistant strains identified by the BACTEC method were further investigated with the Inno-LiPA Rif. TB test to identify the mutations associated with rifampin resistance. Amplicons of the 69-bp polymorphic fragment of the rpoB gene obtained from the specimens were hybridized by using the Inno-LiPA kit. Resistance to rifampin was confirmed for all 30 strains, and seven different mutations were identified (Table 3). The 531 TCG→TTG mutation was predominant among strains of the Beijing genotype, with 18 (78.3%) of the 23 rifampin-resistant strains having this mutation. The same mutation was found in three (42.9%) of the seven rifampin-resistant non-Beijing strains. The difference between Beijing and non-Beijing strains, however, was not significant (OR, 4.8; 95% CI, 0.6 to 43.8; P = 0.09).
TABLE 3.
Mutations in the rpoB genes of 30 M. tuberculosis strains found resistant to rifampin by the BACTEC method
| Mutation | No. (%) of strains |
|---|---|
| 531 TCG→TTG | 21 (70.0) |
| 513 CAA→CTA | 3 (10.0) |
| 526 CAC→CTC | 2 (6.8) |
| 516 GAC→GTC | 1 (3.3) |
| 533 CTG→CCG | 1 (3.3) |
| 531 TCG→TGG | 1 (3.3) |
| 526 CAC→TAC | 1 (3.3)a |
Both the wild-type sequence and the mutant sequence were represented.
One non-Beijing strain showed the resistant Inno-LiPA pattern representing the 526 CAC→TAC mutation and had bands typical of the wild-type M. tuberculosis strain at the same time, suggesting a heterogeneous population.
M. tuberculosis strains included in the same cluster could show different susceptibility patterns, as demonstrated for the largest clusters of strains of the Beijing genotype (Table 4). Fully susceptible and MDR strains showed identical RFLP patterns. The four largest clusters of strains of the Beijing genotype included rifampin-resistant strains; in three of these, more than one mutation in the rpoB gene was detected (Table 4).
TABLE 4.
Resistance patterns in relation to clusters of M. tuberculosis strains belonging to the Beijing genotype
| Cluster size | Pattern fora:
|
No. of strains | Mutation in rpoB gene (no. of strains) | |||
|---|---|---|---|---|---|---|
| E | H | Rif | Str | |||
| 15 | R | R | R | R | 10 | 531 TCG→TTG (9) |
| 531 TCG→TGG (1) | ||||||
| S | R | S | R | 2 | ||
| S | S | S | S | 2 | ||
| R | R | S | R | 1 | ||
| 12 | S | S | S | R | 6 | |
| R | R | R | R | 4 | 513 CAA→CTA (3) | |
| 531 TCG→TTG (1) | ||||||
| R | S | S | S | 1 | ||
| S | R | S | S | 1 | ||
| 9 | S | R | R | R | 3 | 531 TCG→TTG (2) |
| 516 GAC→GTC (1) | ||||||
| S | R | S | R | 3 | ||
| R | R | R | R | 2 | 531 TCG→TTG (2) | |
| S | S | S | S | 1 | ||
| 5 | R | R | R | R | 5 | 531 TCG→TTG (5) |
| 2 | S | S | S | R | 1 | |
| S | S | S | S | 1 | ||
| 2 | S | R | S | R | 2 | |
| 2 | S | S | S | S | 2 | |
| 2 | S | S | S | S | 2 | |
E, ethambutol; H, isoniazid; Rif, rifampin; Str, streptomycin. R, resistant; S, susceptible.
Patients.
The case histories of the 119 patients with pulmonary tuberculosis were analyzed by using data from the medical records. Eighty-nine patients (74.8%) were new patients who had never been treated for tuberculosis, and 30 patients (25.2%) had been previously treated. All patients tested negative for HIV. Patients infected with M. tuberculosis strains of the Beijing genotype were compared to those infected with strains of other genotypes (Table 5).
TABLE 5.
Demographic and medical characteristics of 119 patients with pulmonary tuberculosis (TB) in relation to infection with M. tuberculosis strains belonging to the Beijing genotype
| Characteristic | Resulta in the presence of infection with strains of the following genotype:
|
OR (95% CI) | P | |
|---|---|---|---|---|
| Beijing (n = 53) | Non-Beijing (n = 66) | |||
| Mean age (yrs) | 37.8 | 39.9 | 0.8 | 0.36 |
| Male | 39 (73.6) | 50 (75.8) | 1.1 (0.5-2.8) | 0.78 |
| Smoking habit | 44 (83.0) | 48 (72.7) | 1.8 (0.7-5.0) | 0.18 |
| Alcohol abuse | 30 (56.6) | 24 (36.4) | 2.3 (1.0-5.2) | 0.03 |
| Having been in prison | 18 (34.0) | 18 (27.3) | 1.4 (0.6-3.3) | 0.42 |
| Body mass index | 21.3 | 21.2 | 0.1 | 0.95 |
| Diabetes | 4 (7.5) | 3 (4.5) | 1.7 (0.3-10.4) | 0.38 |
| COPD | 18 (34.0) | 9 (13.6) | 3.3 (1.2-9.0) | 0.01 |
| Previous TB treatment | 17 (32.1) | 13 (19.7) | 1.9 (0.8-4.9) | 0.12 |
| Contact with another TB patient | 15 (28.3) | 28 (42.4) | 0.5 (0.2-1.3) | 0.11 |
| Interruption of present TB treatment | 20 (37.7) | 16 (24.2) | 2.0 (0.8-4.7) | 0.09 |
Reported as number (percent) of patients, unless otherwise indicated.
Twenty-seven (22.7%) of the 119 patients suffered from COPD, that is, bronchial asthma, pneumonia more than two times, or chronic bronchitis, prior to the diagnosis of tuberculosis. Evidence of COPD in the medical histories of the patients was significantly associated with infection by strains of the Beijing genotype (OR, 3.3; 95% CI, 6 1.2 to 9.0; P = 0.01). Patients infected with M. tuberculosis strains of the Beijing genotype were more often alcohol abusers than were those infected with strains of other genotype (OR, 2.3; 95% CI, 1.0 to 5.2; P = 0.03). Multivariate analysis showed that both variables were independently associated with infection by strains of the Beijing genotype (for COPD: OR, 3.2; 95% CI, 1.3 to 8.0; P = 0.01; for alcohol abuse: OR, 2.2; 95% CI, 1.0 to 4.8; P = 0.04).
DISCUSSION
This is the first report describing the molecular epidemiology of M. tuberculosis strains isolated from patients with pulmonary tuberculosis in the Archangel Oblast, Russia. The resurgence of tuberculosis in the Oblast in the past 10 years has been accompanied by high rates of drug resistance. In our study, the overall rate of resistance to any one drug was found to be 56.3%, and in new cases, this rate was found to be 49.4%. These results provide evidence that drug resistance is an important problem in the Archangel Oblast.
Active transmission of M. tuberculosis-resistant strains in the community is another emerging problem. It is generally assumed that the proportion of clustered strains in a population reflects the amount of recent transmission (10, 32). Overall, 59.7% of the patients in our study were part of a cluster. In particular, individuals infected with strains belonging to the Beijing genotype were more likely to be part of a cluster (92.5%). It was not practically possible to establish connections between the patients included in the same cluster. Possible connections were investigated by using notes in the medical records describing home and office visits performed by the doctor and nurse in charge of treatment for contact identification. A connection was established only between two patients infected by non-Beijing strains. Both patients were living in flats located in the same house. They harbored M. tuberculosis strains with identical RFLP and susceptibility patterns (resistance to isoniazid and streptomycin). No epidemiological links were established between the other patients.
The largest cluster in our study comprised 15 patients. Studies performed in other parts of Russia revealed that most clusters were small, with only two to four patients per cluster (17, 22, 24). In a study conducted in the northwestern part of Russia, the largest cluster included 10 patients (21). The finding of relatively large clusters of resistant strains indicates the existence of active transmission of resistant strains in the Archangel Oblast, leading to high rates of resistance among new cases.
Our study showed that M. tuberculosis strains included in the same cluster had different susceptibility patterns and different types of mutations in the rpoB gene. The fact that the 531 TCG→TTG mutation was the most common is in agreement with results from other studies (17, 18). We identified one strain represented by two subpopulations of bacilli, one susceptible and one resistant to rifampin. Although the strain was isolated prior to the beginning of treatment, medical records showed that the patient had been previously treated for tuberculosis. Thus, it is likely that the mutation responsible for rifampin resistance occurred in the course of previous treatment, but the drug-resistant mutant did not completely substitute for the susceptible bacilli before the patient interrupted the previous treatment. A number of such cases have been described (28).
In the same cluster of strains of the Beijing genotype, strains susceptible to all tested drugs, strains resistant to rifampin and with different mutations in the rpoB gene, and strains resistant to rifampin in combination with resistance to various other drugs coexisted. This finding suggested that, when they were first introduced in the Archangel Oblast, strains of the Beijing genotype were susceptible to rifampin and to other drugs and later acquired resistance. We previously analyzed M. tuberculosis strains collected from 74 patients in the Archangel Oblast from 1995 to 1997 (P. Sandven, D. A. Caugant, A. Mariandyshev, N. Nizovtseva, and G. Litver, abstract from the 29th World Conference of the International Union Against Tuberculosis and Lung Disease, Int. J. Tuberc. Lung Dis. 2:S280, 1998). Only six of these patients (8.1%) were infected by a strain of the Beijing genotype. Thus, our data suggest that the introduction and high rate of transmission of the Beijing genotype in the Archangel Oblast are recent events.
Strains of the Beijing genotype were first described in China (34) and were already highly prevalent in 17 different areas around Beijing from 1956 to 1960 (27). Then, strains of the Beijing genotype were disseminated to the neighboring Asian countries, such as Mongolia (34), Thailand (23), South Korea (34), and Vietnam (2). Later, other continents were reached; strains of the Beijing genotype have been described in Iran (7), South Africa (34), Colombia (15), and Gran Canaria Island (5). A special member of the Beijing genotype, known as the W strain family, has been implicated in several large outbreaks of drug-resistant tuberculosis in the United States (1, 4). None of our strains of the Beijing genotype appeared to be identical to those of the W strain family identified in the United States, but several of them were similar to strains recently described as belonging to group B of the W strain family (3).
The spread of strains of the Beijing genotype to the former republics of the USSR and to Russia was recently described (14, 17, 21). In Estonia, 29.2% of the strains belonged to the Beijing genotype (14), and of 100 strains collected from newly diagnosed and chronically infected patients with pulmonary tuberculosis in the northwestern part of Russia, around St. Petersburg, 16 belonged to the Beijing genotype (21). In this study, we found that strains of the Beijing genotype were now present at a much higher rate in the Archangel Oblast, comprising 44.5% of the cases in 1998 and 1999.
The susceptibility to antituberculosis drugs of strains of the Beijing genotype has been studied in many countries (2, 7, 14, 15, 21). Infection by these strains was shown to be associated with resistance to antituberculosis drugs (2, 7) and with MDR (14, 15, 21). In the Archangel Oblast, we found that infection by strains of the Beijing genotype was significantly associated with resistance to ethambutol, isoniazid, rifampin, and streptomycin and with MDR in univariate analysis and was independently associated with resistance to rifampin and streptomycin in multivariate analysis.
The reasons for the selection and wide dissemination of strains of the Beijing genotype are not known. It has been suggested that BCG-vaccinated individuals may be more prone to infection by these strains (2). BCG vaccination has been compulsory in the Archangel Oblast for many decades and could have resulted in the dominance of the Beijing genotype in the region. In a study in Vietnam, strains of the Beijing genotype were more frequent in patients under the age of 25 years and in patients with a BCG scar (2). BCG vaccination coverage in Vietnam has increased during the past 2 decades, resulting in young people being more likely to be vaccinated than older ones. When adjusted for age, the occurrence of strains of the Beijing genotype in Vietnam was not associated with BCG vaccination (2).
Increased transmission is another hypothesis that could explain the rapid intercontinental spread of these strains. In our study, M. tuberculosis strains belonging to the Beijing genotype had a very high rate (92.5%) of clustering, suggesting a higher rate of transmission than for other strains. In addition, the prevalence of these strains among young patients (2, 32) indirectly supports a higher rate of transmission.
Strains of the Beijing genotype may be more virulent. A prospective study conducted in Indonesia revealed a transient febrile response, shortly after the start of treatment, induced by M. tuberculosis of the Beijing genotype (30). The presence of drug resistance and the severity of the disease could not account for the response. The increased risk of a febrile response suggests that these strains induce a different host response. Data on such occurrences have not been collected in the Archangel Oblast.
Our study showed that, in the Archangel Oblast, the presence of COPD prior to tuberculosis and alcohol abuse were associated with infection by a strain of the Beijing genotype. Such associations have not been identified in other investigations. A retrospective analysis of medical records compiled by different physicians certainly implies some degree of variation in the way in which the data were collected. There is no reason to believe, however, that it should introduce a bias in relation to the laboratory parameters analyzed here (e.g., the genotype and resistance pattern of the strains). Thus, it is likely that various factors play a role in favoring the spread of the Beijing genotype and that the most important ones may differ from one geographical area to another. Therefore, it is important to undertake studies to identify which factors are the most significant to consider for setting up a tuberculosis control program.
Acknowledgments
We thank Elisabet Rønnild, Anne Klem, Solveig Undseth, and Kjersti Haugum for skillful technical assistance and Angelina Zemtsovskaya for organization of the strain collection.
Funding was provided by grant 49711 from the Norwegian Ministry of Health and Social Affairs to P.S. and by Norwegian Research Council grant 128083/730 to D.A.C.
REFERENCES
- 1.Agerton, T. B., S. E. Valway, R. J. Blinkhorn, K. L. Shilkret, R. Reves, W. W. Schluter, B. Core, C. J. Pozsik, B. B. Plikaytis, C. Woodley, and I. M. Onorato. 1999. Spread of strain W, a highly drug-resistant strain of Mycobacterium tuberculosis, across the United States. Clin. Infect. Dis. 29:85-92. [DOI] [PubMed] [Google Scholar]
- 2.Anh, D. D., M. W. Borgdorff, L. N. Van, N. T. N. Lan, T. van Gorkom, K. Kremer, and D. van Soolingen. 2000. Mycobacterium tuberculosis Beijing genotype emerging in Vietnam. Emerg. Infect. Dis. 6:302-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bifani, P. J., B. Mathema, Z. Liu, S. L. Moghazeh, B. Shopsin, B. Tempalski, J. Driscoll, R. Frothingham, J. M. Musser, P. Alcabes, and B. N. Kreiswirth. 1999. Identification of a W variant outbreak of Mycobacterium tuberculosis via population-based molecular epidemiology. JAMA 282:2321-2327. [DOI] [PubMed] [Google Scholar]
- 4.Bifani, P. J., B. B. Plikaytis, V. Kapur, K. Stockbauer, X. Pan, M. L. Lutfey, S. L. Moghazeh, W. Eisner, T. M. Daniel, M. H. Kaplan, J. T. Crawford, J. M. Musser, and B. N. Kreiswirth. 1996. Origin and interstate spread of a New York City multi-drug resistant Mycobacterium tuberculosis clone family. JAMA 275:452-457. [PubMed] [Google Scholar]
- 5.Caminero, J. A., M. J. Pena, M. I. Campos-Herrero, J. C. Rodriguez, I. Garcia, P. Cabrera, C. Lafoz, S. Samper, H. Takiff, O. Afonso, J. M. Pavon, M. J. Torres, D. van Soolingen, D. A. Enarson, and C. Martin. 2001. Epidemiological evidence of the spread of a Mycobacterium tuberculosis strain of the Beijing genotype on Gran Canaria Island. Am. J. Respir. Crit. Care Med. 164:1165-1170. [DOI] [PubMed] [Google Scholar]
- 6.Dahle, U. R., P. Sandven, E. Heidal, and D. A. Caugant. 2001. Molecular epidemiology of Mycobacterium tuberculosis in Norway. J. Clin. Microbiol. 39:1802-1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doroudchi, M., K. Kremer, E. A. Basiri, M. R. Kadivar, D. van Soolingen, and A. A. Ghaderi. 2000. IS6110-RFLP and spoligotyping of Mycobacterium tuberculosis isolates in Iran. Scand. J. Infect. Dis. 32:663-668. [DOI] [PubMed] [Google Scholar]
- 8.Dunlap, N. E. 2000. The use of RFLP as a tool for tuberculosis control: utility or futility? Int. J. Tuberc. Lung Dis. 4:S134-S138. [PubMed]
- 9.Enarson, D. A., H. L. Rieder, T. Arnadottir, and A. Trebucq. 1996. Tuberculosis guide for low income countries, 4th ed., p. 65. International Union Against Tuberculosis and Lung Diseases, Paris, France.
- 10.Glynn, J. R., J. Bauer, A. S. de Boer, M. W. Borgdorff, P. E. M. Fine, P. Godfrey-Faussett, E. Vynnycky, and European Concerted Action on Molecular Epidemiology and Control of Tuberculosis. 1999. Interpreting DNA fingerprint clusters of Mycobacterium tuberculosis. Int. J. Tuberc. Lung Dis. 3:1055-1060. [PubMed] [Google Scholar]
- 11.Inderlied, C. B., and M. Salfinger. 1999. Antimicrobial agents and susceptibility tests, p. 1601-1623. In P. R. Murray, E. J. Baron, M. A. Pfaller, F. C. Tenover, and R. H. Yolken (ed.), Manual of clinical microbiology, 7th ed. American Society for Microbiology, Washington, D.C.
- 12.Kamerbeek, J., L. Schouls, A. Kolk, M. van Agterveld, D. van Soolingen, S. Kuijper, A. Bunschoten, H. Molhuizen, R. Shaw, M. Goyal, and J. van Embden. 1997. Simultaneous detection and strain differentiation of Mycobacterium tuberculosis for diagnosis and epidemiology. J. Clin. Microbiol. 35:907-914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kimerling, M. E. 2000. The Russian equation: an evolving paradigm in tuberculosis control. Int. J. Tuberc. Lung Dis. 4:S160-S167. [PubMed]
- 14.Kruuner, A., S. E. Hoffner, H. Sillastu, M. Danilovits, K. Levina, S. B. Svenson, S. Ghebremichael, T. Koivula, and G. Kallenius. 2001. Spread of drug-resistant pulmonary tuberculosis in Estonia. J. Clin. Microbiol. 39:3339-3345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laserson, K. F., L. Osorio, J. D. Sheppard, H. Hernandez, A. M. Benitez, S. Brim, C. L. Woodley, M. H. Hazbon, M. V. Villegas, M. C. Castano, N. Henriquez, E. Rodriguez, B. Metchock, and N. J. Binkin. 2000. Clinical and programmatic mismanagement rather than community outbreak as the cause of chronic, drug-resistant tuberculosis in Buenaventura, Colombia, 1998. Int. J. Tuberc. Lung Dis. 4:673-683. [PubMed] [Google Scholar]
- 16.Mariandyshev, A. O., D. Caugant, and P. Sandven. 1999. Molecular epidemiology and drug resistance of Mycobacterium tuberculosis in the Barents region of Russia and Norway. Hum. Ecol. 4:30-32. (In Russian.) [Google Scholar]
- 17.Marttila, H. J., H. Soini, E. Eerola, E. Vyshnevskaya, B. I. Vyshnevskiy, T. F. Otten, A. V. Vasilyev, and M. K. Viljanen. 1998. A Ser315Thr substitution in KatG is predominant in genetically heterogeneous multidrug resistant Mycobacterium tuberculosis isolates originating from the St. Petersburg area in Russia. Antimicrob. Agents Chemother. 42:2443-2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marttila, H. J., H. Soini, B. I. Vyshnevskiy, T. F. Otten, A. V. Vasilyev, P. Huovinen, and M. K. Vijanen. 1998. Rapid detection of rifampin-resistant Mycobacterium tuberculosis by sequencing and line probe assay. Scand. J. Infect. Dis. 30:129-132. [DOI] [PubMed] [Google Scholar]
- 19.Middlebrook, G., Z. Reggiardo, and W. D. Tigerit. 1977. Automatable radiometric detection of growth of Mycobacterium tuberculosis in selective media. Am. Rev. Respir. Dis. 115:1066-1069. [DOI] [PubMed] [Google Scholar]
- 20.Mitchell, A. Y. 1999. DNA fingerprinting: RFLP analysis, p. 131-151. In R. A. Ollar and N. D. Connell (ed.), Molecular mycobacteriology. Year Book, New York, N.Y.
- 21.Narvskaya, O. V., I. V. Mokrousov, E. V. Limechenko, L. N. Steklova, T. F. Otten, O. V. Grashenkova, and B. I. Vyshnevskiy. 2000. Molecular epidemiology of tuberculosis. Bolshoi Celevoi J. Tuberk. 7-8:4-6. (In Russian.) [Google Scholar]
- 22.Narvskaya, O. V., I. V. Mokrousov, T. F. Otten, and B. I. Vyshnevskyi. 1999. Genetic marking of Mycobacterium tuberculosis polyresistant strains isolated in the north-west part of Russia. Probl. Tuberk. 3:39-41. (In Russian.) [PubMed] [Google Scholar]
- 23.Palittapongarnpim, P., P. Luangsook, S. Tansuphaswadikul, C. Chuchottaworn, R. Prachaktam, and B. Sathapatayavongs. 1997. Restriction fragment length polymorphism study of Mycobacterium tuberculosis in Thailand using IS6110 as probe. Int. J. Tuberc. Lung Dis. 1:370-376. [PubMed] [Google Scholar]
- 24.Perelman, M. I. 2000. Tuberculosis in Russia. Int. J. Tuberc. Lung Dis. 4:1097-1103. [PubMed] [Google Scholar]
- 25.Pfaller, M. A. 1994. Application of new technology to the detection, identification, and antimicrobial susceptibility testing of mycobacteria. Am. J. Clin. Pathol. 101:329-337. [DOI] [PubMed] [Google Scholar]
- 26.Portaels, F., L. Rigouts, and I. Bastian. 1999. Addressing multi-drug resistant tuberculosis in penitentiary hospitals and in the general population of the former Soviet Union. Int. J. Tuberc. Lung Dis. 3:582-588. [PubMed] [Google Scholar]
- 27.Qian, L., J. D. A. van Embden, A. G. M. van der Zanden, E. F. Weltevreden, H. Duanmu, and J. T. Douglas. 1999. Retrospective analysis of the Beijing family of Mycobacterium tuberculosis in preserved lung tissues. J. Clin. Microbiol. 37:471-474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rossau, R., H. Traore, H. de Beenhouwer, W. Mijs, G. Jannes, P. de Rijk, and F. Portaels. 1997. Evaluation of the Inno-LiPA Rif. TB assay, a reverse hybridization assay for the simultaneous detection of Mycobacterium tuberculosis complex and its resistance to rifampin. Antimicrob. Agents Chemother. 41:2093-2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Toungoussova, O. S., D. A. Caugant, P. Sandven, A. O. Mariandyshev, and G. Bjune. Drug resistance of Mycobacterium tuberculosis strains isolated from patients with pulmonary tuberculosis in Arkangelsk, Russia. Int. J. Tuberc. Lung Dis., in press [PubMed]
- 30.Van Crevel, R., R. H. H. Nelwan, W. de Lenne, Y. Veeraragu, A. G. van der Zanden, Z. Amin, J. W. M. van der Meer, and D. van Soolingen. 2001. Mycobacterium tuberculosis Beijing genotype strains associated with febrile response to treatment. Emerg. Infect. Dis. 7:880-883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van Embden, J. D. A., M. D. Cave, J. T. Crawford, J. W. Dale, K. D. Eisenach, B. Giequel, P. Hermans, C. Martin, R. McAdam, and T. M. Shinnick. 1993. Strain identification of Mycobacterium tuberculosis by DNA fingerprinting: recommendations for a standardized methodology. J. Clin. Microbiol. 31:406-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Van Soolingen, D. 2001. Molecular epidemiology of tuberculosis and other mycobacterial infections: main methodologies and achievements. J. Intern. Med. 249:1-26. [DOI] [PubMed] [Google Scholar]
- 33.Van Soolingen, D., P. E. W. de Haas, and K. Kremer. 1999. Restriction fragment length polymorphism (RFLP) typing of mycobacteria. National Institute of Public Health and Environmental Protection, Bilthoven, The Netherlands.
- 34.Van Soolingen, D., L. Qian, P. E. W. de Haas, J. T. Douglas, H. Traore, F. Portaels, H. Z. Qing, D. Enkhasaikan, and J. D. A. van Embden. 1995. Predominance of a single genotype of Mycobacterium tuberculosis in countries of East Asia. J. Clin. Microbiol. 33:3234-3238. [DOI] [PMC free article] [PubMed] [Google Scholar]