Abstract
Nosocomial oxacillin-resistant Staphylococcus aureus (ORSA) bloodstream isolates were tested to determine the prevalence of vancomycin heteroresistance. We screened 619 ORSA nosocomial bloodstream isolates from 36 hospitals between 1997 and 2000. Only one isolate exhibiting heterotypic resistance was detected. Thus, vancomycin heteroresistance in clinical bloodstream isolates remains rare in the United States.
Staphylococcus aureus is responsible for 15% of all bacteremias (14) and is the second most common cause of nosocomial bloodstream infections (5). In 1999, 54% of S. aureus strains causing nosocomial infections in the critical care setting were resistant to oxacillin, reflecting a 40% increase in resistance compared to the previous 5-year period (3). Much emphasis has been placed on identifying oxacillin-resistant S. aureus (ORSA) isolates with reduced vancomycin susceptibility since the first isolate was detected in 1995 (10, 11). Although early studies reported high rates of vancomycin-intermediate S. aureus strains in Japanese hospitals (10), subsequent studies have not confirmed this (9).
The ORSA isolates tested in our study were obtained from the Surveillance and Control of Pathogens of Epidemiological Importance (SCOPE) project, a prospective national surveillance system (5). Thirty-six U.S. sentinel hospitals participated in the project described herein. We chose the agar dilution method to screen ORSA isolates with subsequent determination of vancomycin population analysis profiles to ascertain the prevalence of heteroresistance among U.S. isolates.
Local infection control practitioners utilized a standardized definition to identify nosocomial bacteremias (5). All organisms were forwarded to Virginia Commonwealth University and stored at −70°C in tryptic soy broth with glycerol following identification and susceptibility testing at the local laboratory. The organisms chosen for this study were obtained from bloodstream infections from 1 January 1997 to 31 December 2000. Identification of ORSA isolates was confirmed via subculture to mannitol salt agar containing 4 mg of oxacillin (Remel, Lenexa, Kans.) per liter and incubation for 24 to 48 h at 37°C (15). ORSA isolates were plated to tryptic soy agar with 5% sheep blood (Remel) and incubated for 18 to 24 h at 37°C. Isolated colonies were transferred to brain heart infusion (BHI) broth (Remel) and incubated overnight on an orbital shaker at 37°C. All isolates were subjected to a population analysis method as previously described (11) with the following modifications: Mueller-Hinton (MH) agar with various concentrations of vancomycin ranging from 0.5 to 5.0 mg/liter was inoculated with 0.001 ml (104 CFU/ml) of a saline suspension equivalent to a McFarland 1.0 standard, and plates were incubated for 72 h at 37°C and inspected for growth at 24-h intervals. Control organisms plated with patient isolates included Michigan-963sm (MIC, 8 mg/liter) and Japanese Mu3 (MIC, 2 mg/liter) (11).
The definitions of vancomycin susceptibility described by Fridkin (8) were applied. Vancomycin-intermediate S. aureus (VISA) was defined by MICs of 8 to 16 mg/liter, vancomycin-resistant S. aureus (VRSA) was defined by MICs of ≥32 mg/liter, and heteroresistant VRSA (hetero-VRSA) was defined by MICs of 1 to 4 mg/liter with resistant subpopulations (8). Susceptibility to vancomycin was determined by the agar dilution method in accordance with NCCLS guidelines (13).
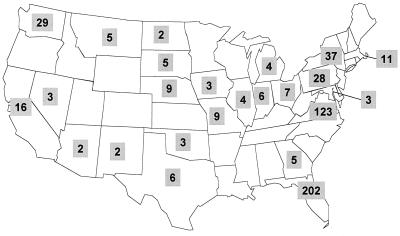
The patient population studied included 55% men and 45% women with a mean age of 62 years. The geographic distribution of the isolates studied is shown in Fig. 1. The primary diagnoses of these patients included cardiac disease (19%), pulmonary disease (14%), gastrointestinal disease (14%), cancer (10%), and trauma (10%). Primary clinical services included internal medicine (51%), general surgery (22%), cardiothoracic surgery (10%), and adult hematology/oncology (6%). Forty-four percent of the ORSA bloodstream infections occurred in an intensive care unit. On average, these infections occurred on hospital day 20.
FIG.1.
Geographic distribution of the isolates studied.
A total of 626 (58%) of the 1,079 ORSA isolates in the SCOPE collection were available for screening. From this sample, 89 (14.2%) of the isolates were misidentified as ORSA: 85 (13.6%) were oxacillin-susceptible staphylococci, 3 (0.5%) were enterococci, and 1 (0.2%) was a gram-negative rod. The percent errors were similar in all four quadrants of the United States: northwest, 8 (13%) of 62 isolates; northeast, 18 (15%) of 119 isolates; southwest, 8 (16%) of 49 isolates; southeast, 54 (14%) of 389 isolates. An additional 13 isolates (2%) were nonviable. The remaining 524 isolates (83.7%) were screened for decreased vancomycin susceptibility and heteroresistant subpopulations.
The vancomycin MICs at which 50 and 90% of the isolates were inhibited were the same at ≤0.5 mg/liter. Three hundred ninety-seven (75.8%) of the isolates did not grow at vancomycin concentrations of ≤0.5 mg/liter, 115 (21.9%) grew at a vancomycin level of 0.5 mg/liter, and 11 (2.1%) tolerated a vancomycin level of 1.0 mg/liter. For one isolate (0.2%), the vancomycin MIC was determined to be 1.5 mg/liter. Vancomycin population analysis profiles of this isolate were completed, and vancomycin-resistant subpopulations were able to grow at a vancomycin concentration of 4.0 mg/liter. Michael Climo, from a collaborating laboratory, confirmed these results. This isolate was obtained from a patient who had had a 6-week course of vancomycin therapy while undergoing dialysis treatments.
In this large collection of nosocomial ORSA bloodstream infections identified via prospective surveillance from geographically dispersed hospitals across the United States over a 4-year period, no organism demonstrated reduced susceptibility to vancomycin. Furthermore, only one isolate demonstrated heteroresistant subpopulations. Thus, VISA and hetero-VRSA strains continue to be uncommon causes of nosocomial bacteremias in the Unites States.
In 1997, Hubert et al. screened more than 630 clinical ORSA isolates from Project ICARE (Intensive Care Antimicrobial Resistance Epidemiology) and found only 2 hetero-VRSA isolates and no VISA isolates (11). Our group has also previously demonstrated no evidence of hetero-VRSA isolates among a population of 261 hospitalized patients with vancomycin-resistant enterococci in a single hospital (7). Additional studies done outside the United States have shown conflicting results, with widely varying rates of hetero-VRSA (1, 4, 9, 10, 12). Many of these discrepancies may be due to the wide variation in the screening methods employed, including the media, inocula, and test conditions used. In this study, we chose MH-based agar supplemented with vancomycin with a low inoculum concentration (104 CFU/ml). Several studies with higher reported rates of hetero-VRSA detection have employed BHI agar and higher inoculum concentrations, up to 106 to 108 CFU/ml. Although Hubert et al. found no difference between MH and BHI agars in the detection of VISA isolates (11), several investigators have described better growth and detection of hetero-VRSA on BHI-based agar (2, 6).
Identification errors were broadly distributed across the 36 hospitals, with the exception of one outlier. The inaccurate reporting serves as an alert for laboratories to review their procedures and raises concern regarding the unnecessary use of vancomycin.
Acknowledgments
We thank the infection control practitioners and microbiologists at the SCOPE hospitals. We thank Gordon Archer for contributing the two control isolates.
REFERENCES
- 1.Bierbaum, G., K. Fuchs, W. Lenz, C. Szekat, and H. G. Sahl. 1999. Presence of Staphylococcus aureus with reduced susceptibility to vancomycin in Germany. Eur J. Clin. Microbiol. Infect. Dis. 18:691-696. [DOI] [PubMed] [Google Scholar]
- 2.Boyle-Vavra, S., H. Labischinski, C. C. Ebert, K. Ehlert, and R. S. Daum. 2001. A spectrum of changes occurs in peptidoglycan composition of glycopeptide-intermediate clinical Staphylococcus aureus isolates. Antimicrob. Agents Chemother. 45:280-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. 2000. National Nosocomial Infection Surveillance (NNIS) System report, data summary from January 1992-April 2000, issued June 2000. Am. J. Infect. Control 28:429-448. [DOI] [PubMed] [Google Scholar]
- 4.Chesneau, O., A. Morvan, and N. El Solh. 2000. Retrospective screening for heterogeneous vancomycin resistance in diverse Staphylococcus aureus clones disseminated in French hospitals. J. Antimicrob. Chemother. 45:887-890. [DOI] [PubMed] [Google Scholar]
- 5.Edmond, M. B., S. Wallace, D. McClish, M. Pfaller, R. Jones, and R. Wenzel. 1999. Nosocomial bloodstream infections in United States hospitals: a three-year analysis. Clin. Infect. Dis. 29:239-244. [DOI] [PubMed] [Google Scholar]
- 6.Finan, J. E., G. L. Archer, M. J. Pucci, and M. W. Climo. 2001. Role of penicillin-binding protein 4 in expression of vancomycin resistance among clinical isolates of oxacillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 45:3070-3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Franchi, D., M. W. Climo, A. H. Wong, M. B. Edmond, and R. P. Wenzel. 1999. Seeking vancomycin resistant Staphylococcus aureus among patients with vancomycin-resistant enterococci. Clin. Infect. Dis. 29:1566-1568. [DOI] [PubMed] [Google Scholar]
- 8.Fridkin, S. K. 2001. Vancomycin-intermediate and -resistant Staphylococcus aureus: what the infectious disease specialist needs to know. Clin. Infect. Dis. 32:108-115. [DOI] [PubMed] [Google Scholar]
- 9.Fujino, T., N. Mori, A. Kawana, H. Kawabata, T. Kuratsuji, K. Kudo, O. Kobori, Y. Yazaki, and T. Kirikae. 2001. Molecular epidemiology of methicillin-resistant Staphylococcus aureus in a Tokyo hospital in 2000. Jpn. J. Infect. Dis. 54:91-93. [PubMed] [Google Scholar]
- 10.Hiramatsu, K., N. Aritaka, H. Hanaki, S. Kawasaki, Y. Hosoda, S. Hori, Y. Fukuchi, and I. Kobayashi. 1997. Dissemination in Japanese hospitals of strains of Staphylococcus aureus heterogeneously resistant to vancomycin. Lancet 350:1670-1673. [DOI] [PubMed] [Google Scholar]
- 11.Hubert, S. K., J. M. Mohammed, S. K. Fridkin, R. P. Gaynes, J. E. McGowan, Jr., and F. C. Tenover. 1999. Glycopeptide-intermediate Staphylococcus aureus: evaluation of a novel screening method and results of a survey of selected U.S. hospitals. J. Clin. Microbiol. 37:3590-3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marchese, A., G. Balistreri, E. Tonoli, A. Debbia, and G. C. Schito. 2000. Heterogeneous vancomycin resistance in methicillin-resistant Staphylococcus aureus strains isolated in a large Italian hospital. J. Clin. Microbiol. 38:866-869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.NCCLS. 2000. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 5th ed., vol. 17, no. 2. Approved standard M7-A5. NCCLS, Wayne, Pa.
- 14.Rubin, R. J., C. A. Harrington, A. Poon, K. Dietrich, J. A. Greene, and A. Moiduddin. 1999. The economic impact of Staphylococcus aureus infection in New York City hospitals. Emerg. Infect. Dis. 5:9-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simor, A. E., J. Goodfellow, L. Louie, and M. Louie. 2001. Evaluation of a new medium, oxacillin resistance screening agar base, for the detection of methicillin-resistant Staphylococcus aureus from clinical specimens. J. Clin. Microbiol. 39:3422.. [DOI] [PMC free article] [PubMed] [Google Scholar]