Abstract
This report describes the first isolation of Schizophyllum commune from a granulomatous lesion on the neck of a dog. The biopsy specimen from the lesion disclosed granulomatous inflammation with branching fungal hyphae without clamp connections. The clinical isolate was identified as S. commune by mycological examination and analysis of ribosomal DNA sequences.
CASE REPORT
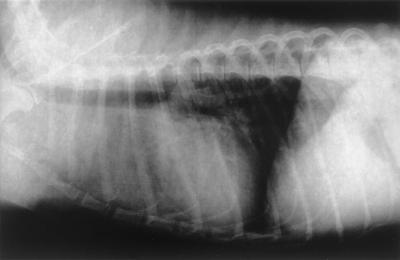
A 3-year-old male mongrel dog weighing 10 kg was referred to the Minami Animal Hospital in Mie, Japan, with the chief complaint of a subcutaneous nodule on the ventral surface of the neck. This subcutaneous nodule with no fistula and discharge was round, elastic, unfixed to the subcutis, and 6 by 11 cm in size. The dog was depressed and showed dyspnea. Furthermore, chest radiographs revealed a nodule (5 by 5 cm in size) at the anterior mediastinum and a diffuse interstitial pattern in the cranial lobes (Fig. 1).
FIG. 1.
Chest radiograph revealing the nodule at the anterior mediastinum and a diffuse interstitial pattern in the cranial lobes.
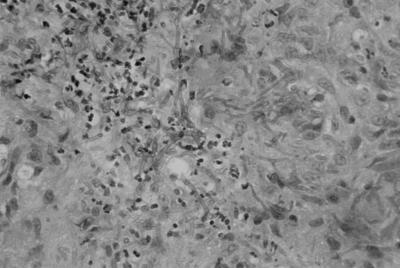
Microscopic examination of a biopsy specimen from the subcutaneous nodule disclosed (i) granulomatous inflammation with branching fungal hyphae without clamp connections and (ii) many granules (Fig. 2).
FIG. 2.
Microscopic view of a biopsy specimen from a nodule on the neck disclosing granulomatous inflammation with fungal branching hyphae.
Because the owner refused surgery or other invasive procedures, the dog was treated with ketoconazole at 10 mg/kg administered orally once a day for 3 months. This regimen was chosen because ketoconazole is an antifungal drug with few adverse effects and is reasonably priced. However, nodules did not diminish in size. Three months later, the dog died. No autopsy was performed.
The basidiomycetous fungus Schizophyllum commune is emerging as one of the important agents of sinusitis and pneumonia in humans (4, 5); however, reports of its infection of animals have been lacking. The diagnosis of this infection is sometimes difficult, and the identification of the isolate is uncertain. Clamp connections on hyphae in tissues are often missing, and fruiting bodies are usually not formed in vitro. The fungus is identified only when it is a dikaryon capable of basidiocarp production (5).
In recent years, molecular techniques have greatly improved the identification of S. commune. Analysis of the internal transcribed spacer region of the rRNA genes has been utilized for identification of the species (2, 5). The present report describes the first isolation of S. commune from a dog.
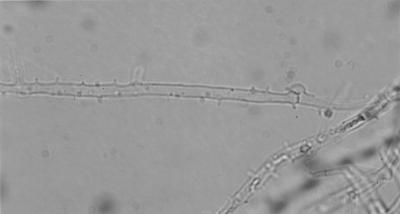
The clinical isolate grew on Sabouraud's glucose agar at 24°C for 1 week, and its colony was white and cottony (Fig. 3). Microscopic examination of the isolate revealed hyaline hyphae 2.5 to 3.0 μm in diameter and confirmed the presence of clamp connections and tubercles (Fig. 4), indicating that the isolate was a basidiomycete.
FIG. 3.
White, cottony colony of the clinical isolate.
FIG. 4.
Microscopic view of the clinical isolate revealing clamp connections and tubercles.
To identify the species of the clinical isolate, molecular analysis of its 25S large-subunit ribosomal DNA was carried out. A mycelial sample (about 10 mg) of the isolate was lysed in lysis buffer consisting of 0.1% Zymolyase 100T (Takara, Kyoto, Japan), 0.1 mM EDTA, 1% sodium dodecyl sulfate, 10 mM Tris hydrochloride (pH 8.0) and 0.3% 2-mercaptoethanol at 37°C for 16 h. High-molecular-weight DNA was obtained from this mycelial sample by phenol and chloroform extraction. A 100-ng DNA sample dissolved in TE buffer (10 mM Tris-hydrochloride [pH 8.0], 1 mM EDTA) was used for PCR amplification. The genomic DNA sample was amplified by PCR in a reaction mixture (30 μl) containing 10 mM Tris-HCl (pH 8.3), 50 mM KCl, 1.5 mM MgCl, 0.001% gelatin, 200 mM each deoxynucleoside triphosphate, 1.0 U of Taq polymerase (Takara), and 0.5 μg of a pair of primers.
We used the 25S large-subunit ribosomal DNA-specific primer for typing (3). The sequences of the 25S large-subunit ribosomal DNAs of many species of basidiomycetes have been deposited in the DNA data bank of Japan (DDBJ), including those of the ribosomal DNA internal transcribed spacer region.
The sequence of forward primer S-1 was 5′-GCA TAT CAA TAA GCG GAG GAA AAG-3′, and that of reverse primer S-2 was 5′-GGT CCG TGT TTC AAG ACG-3′. With these primers, a 600-bp fragment containing the coding sequence of the 25S large-subunit ribosomal DNA was expected to be amplified. PCR amplification was carried out for 35 cycles consisting of template denaturation (1 min at 94°C), primer annealing (2 min at 55°C), and polymerization (3 min at 72°C). The PCR product was electrophoresed through a 2% agarose gel and then stained with ethidium bromide. The PCR product from the sample was sequenced by the dideoxy-chain termination method with an ABI PRISM 310 Genetic Analyzer (Perkin-Elmer Corp., Norwalk, Conn.). These procedures were carried out three times.
Amplification of the sample DNA with 25S large-subunit ribosomal DNA primers yielded a fragment of about 600 bp, consistent with the size of the 25S ribosomal DNA of fungal species reported previously (3). To examine homology relationships between the 25S large-subunit ribosomal DNA of a reference strain of S. commune and that of the clinical isolate, we used FASTA database analysis in DDBJ. The nucleotide sequences of the 25S large-subunit ribosomal DNAs of the clinical isolate and a reference strain of S. commune (DDBJ accession no. AF141873) showed 99% similarity (1). The results were obtained three times with this molecular system, and the isolate was identified as S. commune.
As stated previously, the lack of clamp connections in tissues is not an unusual finding (5) and the case reported herein is no exception. Moreover, morphological identification is made even more difficult when strains exhibit an inability to mate. For these reasons, we attempted to identify our isolate by comparing its 25S large-subunit rRNA gene sequence with that of a known strain of S. commune. Our results help to confirm observations by Sigler et al. (5) and Buzina et al. (2) indicating that this method has potential for the rapid identification of clinical isolates of S. commune.
The number of cases of S. commune infection is increasing in both immunocompetent and immunocompromised humans (4). In this case, no immunosuppressive condition or treatment was recognized in the canine patient. However, we feel that steroid treatment, chemotherapy, and immunosuppressive underlying diseases may facilitate subsequent infection with S. commune in dogs. Animal infection with S. commune has not been reported as far as we know, but with the aid of molecular analysis, additional animal cases can be found hereafter.
Nucleotide sequence accession number.
The partial sequence of the S. commune clinical isolate 25S large-subunit rRNA gene reported in this paper has been deposited in the GenBank database and assigned accession no. AB080723.
REFERENCES
- 1.Binder, M., D. S. Hibbett, and H. P. Molitoris. 2001. Phylogenetic relationships of the marine gasteromycete Nia vibrissa. Mycologia 93:679-688. [Google Scholar]
- 2.Buzina, W., D. Lang-Loidolt, B. Hannes, K. Freudenschuss, and H. Stammberger. 2001. Development of molecular methods for identification of Schizophyllum commune from clinical samples. J. Clin. Microbiol. 39:2391-2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fell, J. W., H. Roeijmans, and T. Boekhout. 1999. Cystofilobasidiales, a new order of basidiomycetous yeasts. Int. J. Syst. Bacteriol. 49:907-913. [DOI] [PubMed] [Google Scholar]
- 4.Rihs, J. D., A. A. Padhye, and C. Good. 1996. Brain abscess caused by Schizophyllum commune: an emerging basidiomycete pathogen. J. Clin. Microbiol. 34:1628-1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sigler, L., L. M. De La Maza, G. Tan, K. N. Egger, and R. K. Sherburne. 1995. Diagnosis difficulties caused by a nonclamped Schizophyllum commune isolate in a case of fungus ball of the lung. J. Clin. Microbiol. 33:1979-1983. [DOI] [PMC free article] [PubMed] [Google Scholar]