Abstract
Through the use of photobleaching techniques, we examined the dynamic interaction of three members of the transcrsiption apparatus with a target promoter in living cells. The glucocorticoid receptor (GR) interacting protein 1 (GRIP-1) exhibits a half maximal time for fluorescent recovery (τR) of 5 s, reflecting the same rapid exchange as observed for GR. In contrast, the large subunit (RPB1) of RNA polymerase II (pol II) required 13 min for complete fluorescence recovery, consistent with its function as a processive enzyme. We also observe a complex induction profile for the kinetics of GR-stimulated transcription. Our results indicate that GR and GRIP-1 as components of the activating complex are in a dynamic equilibrium with the promoter, and must return to the template many times during the course of transcriptional activation.
Introduction
In the continued presence of ligand, steroid receptors have been thought to remain statically bound to regulatory sites in hormone-responsive promoters (Becker et al., 1986; McKenna et al. 2002). Even in the absence of ligand, some members of the nuclear receptor family are implicated as constitutively bound to chromatin (Horlein et al., 1995). The events initiated by steroid receptor binding at the promoter include chromatin remodeling processes, recruitment of secondary factors with sequencespecific DNA-binding activity, recruitment of factors exclusively by protein–protein interactions (coactivators, corepressors), and finally loading of the basal (or general) transcription apparatus. Added to this complexity is our recent observation that in the continued presence of ligand the glucocorticoid receptor (GR) exchanges rapidly with regulatory elements in living cells (McNally et al., 2000).
To investigate whether other factors interact with the template in a similarly dynamic manner, we have examined exchange of a GR co-activator, glucocorticoid receptor interacting protein 1 (GRIP-1), at specific promoters in live cells. We have also analyzed RNA polymerase II (pol II) exchange at these sites, providing the first direct observation of actively transcribing pol II on a specific template in living cells. We find that GRIP-1 exhibits a rapid exchange comparable to GR, whereas pol II, although dynamic, is resident on the template for extended periods. Experiments with pol II inhibitors provide evidence that the observed pol II exchange rate results primarily from complexes engaged in transcription elongation. The markedly different exchange rates of pol II and GRIP-1 underscore the highly dynamic nature of the initial events leading to transcription, and challenge the concept that a long-lived complex exists on the template.
Results
We utilized for these experiments a cell line (3134) that contains a tandem array of the mouse mammary tumor virus (MMTV) promoter, in this report referred to as the MMTV array (Walker et al., 1999; McNally et al., 2000). The response of the promoters within this array to hormone induction is identical to that of single copy genes, as judged by several criteria (Fragoso et al., 1998), including nucleosome positions on the promoter, the position and extent of the receptor-induced hypersensitive transition, and the kinetics of RNA accumulation.
GRIP-1 (Hong et al., 1997) [also known as Tif-2 (Voegel et al., 1996)] belongs to the p160 class of nuclear receptor coactivators (Xu et al., 1999). To examine the dynamic behavior of GRIP-1 on an actual promoter, stable subclones of the 3134 cell line were constructed with an EGFP-tagged version of the protein (Baumann et al., 2001) expressed from a chromosomal locus. This protein adopts a diffuse nuclear distribution, with some evidence of localized accumulations (Figure 1A). Within 15 min after application of the GR ligand, a single, 0.5–2 μm diameter, fluorescent structure appears in the nucleus. The example in Figure 1D shows an array after 60 min of dexamethasone (dex) treatment. To test whether the structure stained by GFP–GRIP-1 represented transcriptionally active MMTV arrays, we performed an RNA FISH analysis, using a Cy5 labeled probe specific for array transcripts (Figure 1E and F).
Figure 1.
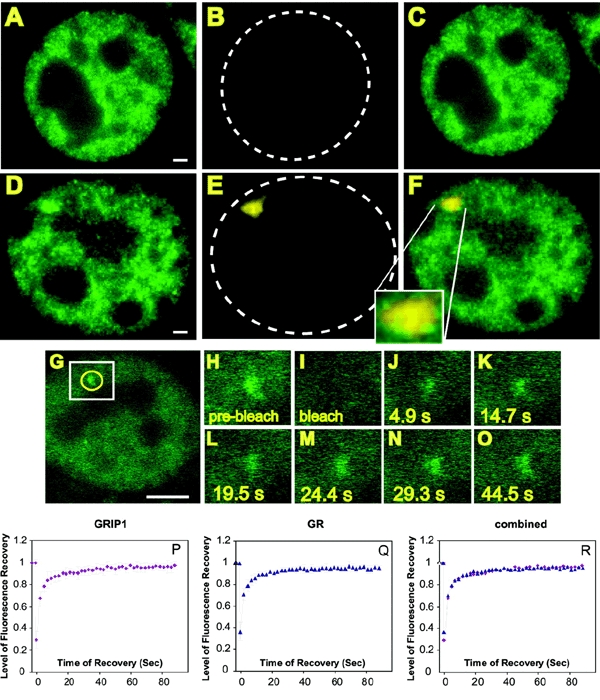
GFP–GRIP-1 colocalizes with nascent MMTV transcripts detected by RNA FISH and undergoes the same rapid exchange with the LTR as the GR in the continuous presence of ligand. (A–F) GFP–GRIP-1 Cells were subjected to RNA FISH analysis. Carrier [ethanol; (A–C)] or dex [100 nM; (D–F)] was added for 1 h prior to fixation. Cells were fixed and processed for RNA FISH. GFP fluorescence alone is shown in (A) and (D). The RNA FISH staining (Cy5 dye) is shown in (B) and (E), the hatched lines outline the nuclei. (C and F) Overlays of (A) and (B) and (D) and (E), respectively, the segment magnification in (F) shows the colocalization of GFP–GRIP-1 and the RNA FISH signal on the array. Scale bars: 1 μm. (G–R) FRAP analysis of GFP–GRIP-1 at the MMTV array. GFP–GRIP-1 images show single z-sections. Dex was added at 100 nM for 1 h, cells were imaged before and during fluorescence recovery (H) pre-bleach image. The first post bleach image (I) was collected ∼0.1 s after the bleach followed by images collected at 4.9 s intervals. he 4.9 s (J), 14.7 s (K), 19.5 s (L) 24.4 s (M), 29.3 s (N) and 44.5 s (O) images are shown. (H–O) Represent segment magnifications and correspond to the white box in (G). The yellow circle in (G) marks the position and size of the bleach spot. In this experiment the recovery of fluorescence at the array was completed by 29.3 s and did not change after prolonged time of observation. Note that due to bleaching during imaging the same level of fluorescence as seen in the pre-bleach image is not reached after complete recovery. Scale bar: 5 μm. (P–R) Recovery curves from GFP–GRIP-1 (P), and GFP–GR expressing 3617 cells (Q), treated with dex (100 nM) for 15–90 min. To reach higher resolution of the fluorescence recovery process, the cells were imaged with an interval of 2.3 s. The graphs represent averages of nine separate FRAP data sets. The two proteins show almost identical dynamic properties at the array as shown in the overlay in (R).
As seen in Figure 1 G–O, FRAP analysis indicates that this protein also exchanges rapidly with the MMTV promoter. The half maximal time for fluorescent recovery (τR) for GFP–GRIP-1 on the MMTV array is 5 s, representing an exchange rate that is essentially the same as that found with GR [compare, GR recovery curve (Figure 1Q) and GRIP-1 recovery curve (Figure 1P)]. We conclude that the interaction of GRIP-1 with the MMTV template is virtually indistinguishable from the rapid exchange observed for GR. This finding indicates that the highly dynamic behavior is not limited to the initiating protein, in this case the GR, but extends to other members of the coactivator/coregulator group.
For comparison, we then examined the dynamic behavior of RNA pol II. RNA pol II is composed of 12 highly conserved subunits, including the largest subunit, RPB1(Woychik et al. 2002). We stably introduced an EGFP tagged, α-amanitin resistant, version of RPB1 [GFP-pol II (Sugaya et al., 2000)] into 3134 cells. As cells were selected for resistance to α-amanitin, and maintained under α-amanitin selection during the course of the experiments, the only functional polymerase in these cells is GFP tagged. In the absence of dex GFP–pol II distributes in the nucleus in a distinct reticular pattern (Figure 2A). Within 15 min after hormone treatment the formation of bright GFP–pol II stained structures could be observed in the nucleus of these cells. The representative example in Figure 2D shows an array after 30 min of hormone treatment. RNA FISH analysis with a probe specific for array transcripts confirmed that these structures represent the MMTV array (Figure 2D–F). As indicated in Figure 2G–R, FRAP analysis shows a very slow fluorescence recovery of almost 900 s for the GFP–pol II associated with the MMTV array. The early part of this curve appears to show a biphasic component that may be associated with abortive initiation events; further analysis will be needed to clarify this point. The rate for complete recovery, however, is in stark contrast to the kinetics of GFP–GR and GFP–GRIP-1, and consistent with the interpretation that all elongating polymerases must finish transcripts and clear the template for complete recovery of fluorescence.
Figure 2.
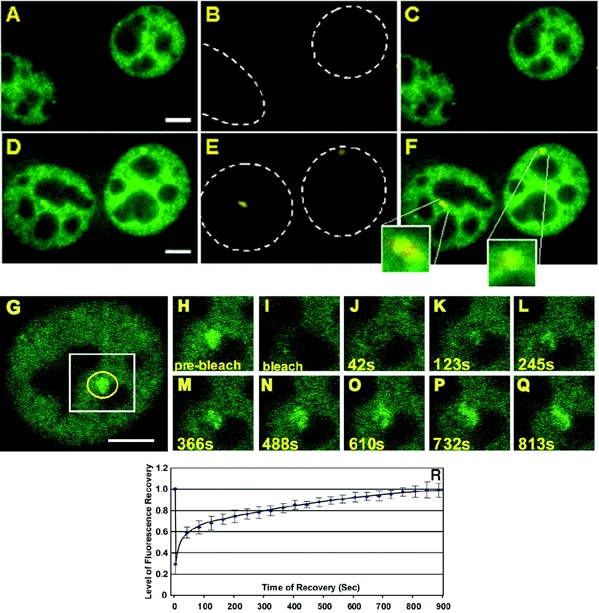
GFP–Pol II co-localizes with nascent MMTV transcripts detected by RNA FISH and undergoes very slow exchange with the LTR in the continuous presence of ligand. (A–F) Carrier [ethanol; (A–C)] or dex [100nM; (D–F)] was added for 1 h prior to fixation. Cells were fixed and processed for RNA FISH. GFP fluorescence alone is shown in (A) and (D). The RNA FISH staining (Cy5 dye) is shown in (B) and (E). The hatched lines outline the nucleus. (C and F) Overlays of (A) and (B) and (D) and (E), respectively. The segment magnifications in (F) show the co-localization of GFP–Pol II and the RNA FISH signal at the array. Scale bars: 5 μm. (G–R) FRAP analysis of GFP–Pol II expressing cells. Images show single z-sections. Dex was added at 100 nM for 15 min, cells were imaged before and during fluorescence recovery (G) pre-bleach image. The first post bleach image (H) was collected ∼0.1 s after the bleach followed by images collected at 40 s intervals. The 42 s (J), 123 s (K), 245 s (L) 366 s (M), 488 s (N) 610 s (O) 732 s (P) and 813 s (Q) images are shown. (H–Q) are segment magnifications and correspond to the white box in (G). The yellow circle in (G) marks the position and size of the bleach spot. In this experiment the recovery of fluorescence at the array was completed by 732 s. Note, that due to bleaching during imaging the same level of fluorescence as seen in the pre-bleach image is not reached after complete recovery. (R) Recovery curve from GFP–Pol II cells, treated with dex (100 nM) for 15–25 min. The graph represents the average of nine FRAP experiments. Scale bar: 5 μm.
To test this possibility further, we treated cells with 5,6-dichloro-1β-D-ribofuranosyl-benzimidzaole (DRB) or actinomycin D (AMD). DRB prevents transition from pol II initiation to elongation (Chodosh et al., 1989; Marshall et al. 1992). Thus in the continued presence of DRB only initiating polymerases should be visible at the template. We observed that GFP–pol II illuminated arrays did not form after cotreatment of cells with DRB and dex (results not shown), suggesting that under normal conditions predominantly elongating, as opposed to initiating/reinitiating, polymerase is visualized. In contrast, cotreatment of these cells with dex and AMD, which intercalates in the DNA and prevents polymerase progress (Puvion-Dutilleul et al., 1992), led to pol II array formation (Figure 3D). RNA FISH analysis confirmed that AMD leads to a strong inhibition of transcriptional activity from the array (compare FISH signals in Figure 3B and E). The arrays stained by GFP–pol II in the cotreated cells generally were smaller in size (compare Figure 3A and D). This phenomenon is likely due to the higher state of chromatin condensation for transcriptionally inactive arrays (Mueller et al., 2001).
Figure 3.
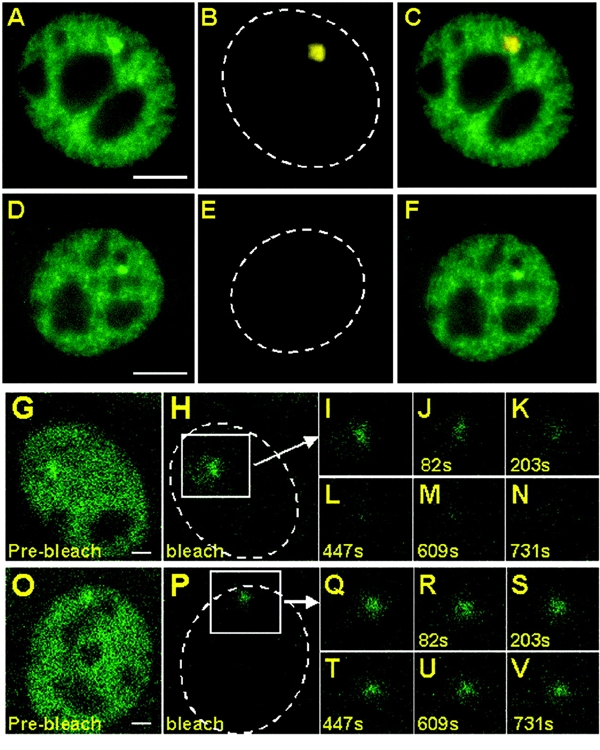
Inhibition of MMTV transcription by actinomycin D leads to the immobilization of GFP–Pol II on the array. Dex (100nM) alone (A–C) or dex (100 nM) and AMD (2.5 μg/ml) (D–F) was added for 20 min. Cells were fixed and processed for RNA FISH. GFP fluorescence alone is shown in (A) and (D). The RNA FISH staining (Cy5 dye) is shown in (B) and (E). The hatched lines outline the nucleus. (C) Composite image of (A) and (B) and (F) composite image of (D) and (E). Scale bars: 5 μm. (G–V) iFRAP analysis of cells treated with dex (100 nM) (G–N) or dex (100 nM) and AMD (O–V). (G) and (O) pre-bleach images. The first post bleach images (H) and (P) were collected ∼0.3 s after the bleach followed by images collected at 40 s intervals. (I–N) and (Q–V) are segment magnifications and correspond to the white boxes in (H) and (P), respectively. The hatched lines outline the nucleus. Scale bars: 2 μm.
To study the effect of AMD on the dynamic behavior of GFP–pol II, we used iFRAP [inverse FRAP (McNally and Smith, 2002)]. In this method, everything but the array is instantly bleached, and subsequent loss of pol II fluorescence from the array is measured. For GFP–pol II stained arrays in dex-treated cells, fluorescence loss from the array (Figure 3G–N) was comparable to the rate of fluorescence recovery in a FRAP experiment (Figure 2). Cotreatment of cells with AMD and dex, however, resulted in the nearly complete abrogation of fluorescence loss at the array (Figure 3O–V). This finding indicates that the polymerase is immobilized, consistent with blocked elongation of the GFP–pol II by AMD.
The visualization of actively elongating complexes in real time allows us to examine directly the kinetics of pol II recruitment to the transcription unit in individual cells. The number of active transcription complexes increases dramatically after addition of ligand, passes through a maximum at 25 min, then decreases rapidly again to a low steady-state level (Figure 4A). To confirm the rapid nature of the hormone response and its independence of pol II expression levels, we performed a 'nuclear run-on' transcription experiment using parental 3134 cells (Figure 4B). Previous analysis of expression rates by 'run-on' transcription had indicated an early spike of transcription activity (Archer et al., 1994; Sheldon et al., 2001), but not the very rapid kinetics of this process. Receptor activation clearly induces a multiphasic cascade of events. An initial rapid recruitment of active polymerases to the promoter is followed quickly by a period of rapid polymerase depletion.
Figure 4.
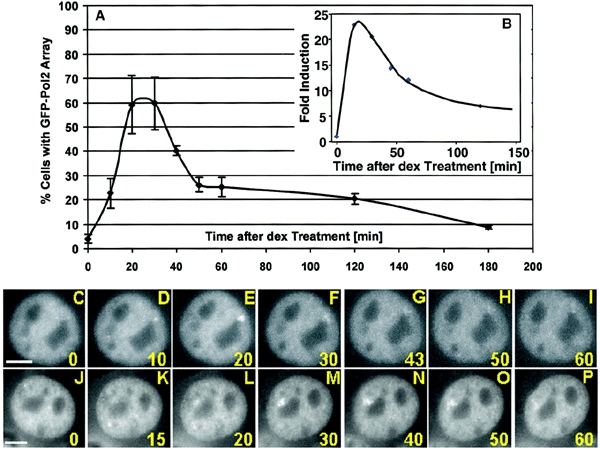
GFP–Pol II shows an early peak in array staining after dex treatment with heterogeneity between individual cells. (A) Appearance of GFP–Pol II stained arrays in a cell population after dex treatment. The graph shows the percentage of cells with a GFP–Pol II illuminated array over a time course of 180 min. For each time point the presence or absence of a GFP–Pol II stained array was determined in 120–180 cells. The graph represents the results of three individual experiments. (B) Nuclear run-on assays were done on nuclei isolated from 3134 cells treated for 0, 15, 30, 45, 60 and 120 min with 100 nM dex. Levels of MMTV transcription are expressed as fold induction with basal transcription (0 min) set to a value of 1. (C–P) Time-lapse study of individual cells after hormone treatment. Scale bars: 5 μm.
Single-cell analysis of transcription also reveals a considerable heterogeneity in the timing of activation. The maximum for GFP–pol II loading varies over the cell population (Figure 4C–P). For the cell shown in Figure 4C–I, the peak of pol II association occurs at 20 min, while the maximum level is detected at 30 min for the cell in Figure 4J–P. This variation could derive from several sources, including effects related to cell cycle, and clearly would not be detected in biochemical analyses applied to the complete cell population.
Discussion
Increasing evidence from photobleaching studies shows that proteins exchange rapidly at binding sites in the nucleus (for review see Hager et al., 2002). However, to date, most bleaching studies have been performed at random sites in the nucleoplasm, making it difficult to assess to what degree, if any, specific binding to a promoter contributes to the exchange rates measured. Using an estrogen receptor–lac repressor fusion and an array of lac operator elements, Stenoien and colleagues (2001) demonstrated rapid exchange between a truncated form of SRC1 and ER bound to the genomic array through the repressor binding sites. However, the relationship between ER/coativator function in this artificially tethered state and coactivator function at a true hormone response element is unclear. The MMTV array system utilized here contains an array of intact promoters that exhibit many features characteristic of single copy genes, including the well-characterized GR-dependent chromatin transition (Fragoso et al., 1998). Our measurements of GRIP-1 coactivator exchange reveal a rapid rate commensurate with GR dynamics. We show in addition that polymerase exchanges at this site at a rate consistent with elongation, with an early component in the bleaching curve that may indicate a fraction of abortive initiation events.
The consequences of this rapid exchange have major implications for our understanding of transcriptional regulation. The observed dynamic behavior of the GR and GRIP-1 implies that during the time course of transcriptional activation both the receptor and co-activator return continuously to regulatory elements in the promoter. The precise composition of the receptor/cofactor complex that interacts productively with the target gene at a given stage of the transcriptional program may be purely stochastic. However, it could also be impacted by the modification status of the receptor or the promoter at that time. Such a mechanism would then achieve an ordered recruitment of transcription factors to the promoter. In support of this, recent studies using chromatin immunoprecipitation (ChIP) assays have shown such an ordered composition of transcription factor complexes on ER responsive promoters over time (Shang et al., 2000). The observed dynamic behavior of these different transcription factor complexes is on the order of several minutes, in contrast to the rapid exchange of GR and its cofactor GRIP-1 reported here. This suggests that the variant organization of transcription factors detected by ChIP analysis on the promoters as a function of time likely reflects altered equilibrium states for the factors, and not the actual dynamic behavior of the proteins. In addition to the dynamic exchange of GRIP-1 and pol II, we also found that the actual number of pol II complexes resident on the gene is regulated in a complex fashion: an initial increase is followed quickly by an equally rapid depletion. These changes in pol II loading are consistent with nuclear run-on studies for this promoter (Archer et al., 1994; Sheldon et al., 2001), and thereby provide an in vivo system for study of transcriptional regulation. The system described here, as well as other amplified gene arrays (Stenoien et al., 2001), offer a direct approach to dissection of the rapid exchange mechanisms involved in transcription factor recruitment, as well as a real-time evaluation of promoter associated multiprotein complexes and template modifications that occur during the complex process of transcription activation.
Methods
Cell culture.
For the generation of cells expressing EGFP–GRIP-1 or EGFP–Pol II, 10 μg of either the pEGFP–GRIP-1 (Baumann et al., 2001) or the GFP–RPB1 (Sugaya et al., 2000) plasmid were introduced into 3134 cells by electroporation as described (Walker et al., 1999). The EGFP–GRIP-1 cell line was generated by selecting clones able to grow at a concentration of 1 mg/ml G418 in Dulbecco's modified Eagle's medium (DMEM, Gibco BRL, Grand Island, NY) supplemented with 10% fetal bovine serum (FBS) (Hyclone, Logan, UT). After selection, cells were maintained in the presence of 1 mg/ml G 418 (Gibco BRL). The GFP–Pol II cell line was obtained by selecting for clones able to grow at 25 μg/μl α-amanitin. GFP–GR expressing 3617 cells were obtained as described previously (Walker et al., 1999) and grown in DMEM, 10% FBS in the presence of 5mg/ml tetracycline to suppress GFP–GR expression.
Prior to live cell imaging, FRAP and iFRAP analysis, cells were transferred to 35 mm glass bottom dishes (MatTek Corporation, Ashland, MA) at a density of 2 x 105 in Phenol Red-free DMEM containing 3% charcoal stripped medium (HyClone) at least 16 h prior to imaging. For culture of the GFP–Pol II cells the medium was additionally supplemented with 10 μg/ml α-amanitin. For RNA FISH experiments, the cells were grown on 22-mm square coverslips each deposited on the bottom of a 6-well plate. Culture conditions were as described above.
RNA FISH and image analysis.
The RNA FISH procedure was carried out as described (Mueller et al., 2001). Live cell imaging and FRAP analysis was performed as previously discussed (McNally et al., 2000; Mueller et al., 2001). Details of the time-lapse microscopy and quantitative analysis of the FRAP data are presented in the Supplementary data (available at EMBO reports Online).
Nuclear run-on assay.
Nuclear run-ons were done as previously described in Sheldon et al. (2001).
Supplementary Material
Supplementary data
Acknowledgments
The authors would like to thank Terace Fletcher for critical reading of the manuscript, Tom Misteli for discussions and Mary Winters for development of the GRIP-1 cell lines. M.V. acknowledges the Association pour la Recherche sur Le Cancer (ARC 4521) for support.
References
- Archer T.K., Lee H.-L., Cordingley M.G., Mymryk J.S., Fragoso G., Berard D.S. and Hager G.L. (1994) Differential steroid hormone induction of transcription from the mouse mammary tumor virus promoter. Mol. Endocrinol., 8, 568–576. [DOI] [PubMed] [Google Scholar]
- Baumann C.T., Ma H., Wolford R.G., Reyes J.C., Maruvada P., Lim C.S., Yen P.M., Stallcup M.R. and Hager G.L. (2001) The glucocorticoid receptor interacting protein 1 (GRIP-1) localizes in discrete nuclear foci that associate with ND10 nuclear bodies and are enriched in components of the 26S proteasome. Mol. Endocrinol., 15, 485–500. [DOI] [PubMed] [Google Scholar]
- Becker P.B., Gloss B., Schmid W., Strahle U. and Schutz G. (1986) In vivo protein–DNA interactions in a glucocorticoid response element require the presence of the hormone. Nature, 324, 686–688. [DOI] [PubMed] [Google Scholar]
- Chodosh L.A., Fire A., Samuels M. and Sharp P.A. (1989) 5,6-dichloro-1-β-D-ribofuranosylbenzimidazole inhibits transcription elongation by RNA polymerase II in vitro. J. Biol. Chem., 264, 2250–2257. [PubMed] [Google Scholar]
- Fragoso G., Pennie W.D., John S. and Hager G.L. (1998) The position and length of the steroid-dependent hypersensitive region in the mouse mammary tumor virus long terminal repeat are is invariant despite multiple nucleosome B frames. Mol. Cell. Biol., 18, 3633–3644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hager G.L., Elbi C.C. and Becker M. (2002) Protein dynamics in the nuclear compartment. Curr. Opin. Genet. Dev., 12, 137–141. [DOI] [PubMed] [Google Scholar]
- Hong H., Kohli K., Garabedian M.J. and Stallcup M.R. (1997) GRIP1, a transcriptional coactivator for the AF-2 transactivation domain of steroid, thyroid, retinoid and vitamin D receptors. Mol. Cell. Biol., 17, 2735–2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horlein A.J. et al. (1995) Ligand-independent repression by the thyroid hormone receptor mediated by a nuclear receptor co-repressor. Nature, 377, 397–404. [DOI] [PubMed] [Google Scholar]
- Marshall N.F. and Price D.H. (1992) Control of formation of two distinct classes of RNA polymerase II elongation complexes. Mol. Cell Biol., 12, 2078–2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna N.J. and O'Malley B.W. (2002) Combinatorial control of gene expression by nuclear receptors and coregulators. Cell, 108, 465–474. [DOI] [PubMed] [Google Scholar]
- McNally J.G. and Smith C.L. (2002) Photobleaching by confocal microscopy. In Diaspro, A. (ed.), Confocal and Two-Photon Microscopy: Foundations, Applications, and Advances. Wiley-Liss, Inc., New York, NY, pp.525–538. [Google Scholar]
- McNally J.G., Mueller W.G., Walker D., Wolford R.G. and Hager G.L. (2000) The glucocorticoid receptor: Rapid exchange with regulatory sites in living cells. Science, 287, 1262–1265. [DOI] [PubMed] [Google Scholar]
- Mueller W.G., Walker D., Hager G.L. and McNally J.G. (2001) Large scale chromatin decondensation and recondensation in living cells and the role of transcription. J. Cell Biol., 154, 33–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puvion-Dutilleul F., Mazan S., Nicoloso M., Pichard E., Bachellerie J.P. and Puvion E. (1992) Alterations of nucleolar ultrastructure and ribosome biogenesis by actinomycin D. Implications for U3 snRNP function. Eur. J. Cell Biol., 58, 149–162. [PubMed] [Google Scholar]
- Shang Y., Hu X., DiRenzo J., Lazar M.A. and Brown M. (2000) Cofactor dynamics and sufficiency in estrogen receptor-regulated transcription. Cell, 103, 843–852. [DOI] [PubMed] [Google Scholar]
- Sheldon L.A., Becker M. and Smith C.L. (2001) Steroid hormone receptor-mediated histone deacetylation and transcription at the mouse mammary tumor virus promoter. J. Biol. Chem., 276, 32423–32426. [DOI] [PubMed] [Google Scholar]
- Stenoien D.L., Nye A.C., Mancini M.G., Patel K., Dutertre M., O'Malley B.W., Smith C.L., Belmont A.S. and Mancini M.A. (2001) Ligand-mediated assembly and real-time cellular dynamics of estrogen receptor α-coactivator complexes in living cells. Mol. Cell Biol., 21, 4404–4412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugaya K., Vigneron M. and Cook P.R. (2000) Mammalian cell lines expressing functional RNA polymerase II tagged with the green fluorescent protein. J. Cell Sci., 113, 2679–2683. [DOI] [PubMed] [Google Scholar]
- Voegel J.J., Heine M.J., Zechel C., Chambon P. and Gronemeyer H. (1996) TIF2, a 160 kDa transcriptional mediator for the ligand-dependent activation function AF-2 of nuclear receptors. EMBO J., 15, 3667–3675. [PMC free article] [PubMed] [Google Scholar]
- Walker D., Htun H. and Hager G.L. (1999) Using inducible vectors to study intracellular trafficking of GFP-tagged steroid/nuclear receptors in living cells. Methods (Companion to Methods Enzymol.), 19, 386–393. [DOI] [PubMed] [Google Scholar]
- Woychik N.A. and Hampsey M. (2002) The RNA polymerase II machinery: structure illuminates function. Cell, 108, 453–463. [DOI] [PubMed] [Google Scholar]
- Xu L., Glass C.K. and Rosenfeld M.G. (1999) Coactivator and corepressor complexes in nuclear receptor function. Curr. Opin. Genet. Dev., 9, 140–147. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data


