Abstract
Methane trapped in the 3,053-m-deep Greenland Ice Sheet Project 2 ice core provides an important record of millennial-scale climate change over the last 110,000 yr. However, at several depths in the lowest 90 m of the ice core, the methane concentration is up to an order of magnitude higher than at other depths. At those depths we have discovered methanogenic archaea, the in situ metabolism of which accounts for the excess methane. The total concentration of all types of microbes we measured with direct counts of Syto-23-stained cells tracks the excess of methanogens that we identified by their F420 autofluorescence and provides independent evidence for anomalous layers. The metabolic rate we estimated for microbes at those depths is consistent with the Arrhenius relation for rates found earlier for microbes imprisoned in rock, sediment, and ice. It is roughly the same as the rate of spontaneous macromolecular damage inferred from laboratory data, suggesting that microbes imprisoned in ice expend metabolic energy mainly to repair damage to DNA and amino acids rather than to grow. Equating the loss rate of methane recently discovered in the Martian atmosphere to the production rate by possible methanogens, we estimate that a possible Martian habitat would be at a temperature of ≈0°C and that the concentration, if uniformly distributed in a 10-m-thick layer, would be ≈1 cell per ml.
Keywords: metabolism by methanogenic archaea, methane in glacial ice, methanogens on Mars, origin of microbes in glacial ice
The record of atmospheric methane (CH4) concentration trapped in the Greenland Ice Sheet Project 2 (GISP2) ice core serves as a climate proxy, showing that climate during the last glacial period oscillated rapidly between cold and warm states that lasted for several thousand years (1). The source is believed to be wetland methane emissions that depend on temperature, precipitation, net ecosystem production, and oxidation by tropospheric OH. Because of its short atmospheric mixing time relative to its lifetime, variations of methane recorded in ice cores are believed to reflect global changes in the methane budget.
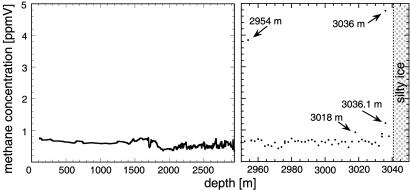
Fig. 1 shows measurements of methane in the GISP2 ice core as a function of depth by Ed Brook [National Oceanic and Atmospheric Administration Geophysical Data Center (www.ngdc.noaa.gov/paleo/paleo.html); and E. Brook, additional data for depths of 2,806–3,038 m, personal communication] down to 3,038 m, just 3 m above the silt-laden basal ice. All but four of his methane values range between 337 and 880 parts per billion by volume (ppbV) and correlate with other climate proxies such as δ18O and CO2 (1). The intervals between his samples were >10 m at depths <2,000 m and 2–6 m at depths of ≈2,000–3,038 m. Our study focuses on the origin of the four values marked with arrows in Fig. 1, which stand out above the 99% that are related to climate. We report here our discovery that the excess methane values are produced by methanogens (microbes that metabolize with emission of methane) that were metabolizing while frozen within the ice at those depths. We also show that their average in situ metabolic rates correspond to a cellular carbon turnover time of ≈105 yr at an ice temperature of –11°C.
Fig. 1.
Methane concentration as a function of depth in the GISP2 ice core (ref. 1; E. Brook, personal communication). Below 2,950 m, the scale is expanded to show the anomalously high methane values at 2,954, 3018, and 3,036 m.
Bacteria and archaea have been found in all subfreezing terrestrial environments (2–6). Studies of terrestrial psychrophiles and psychrotrophs have involved extraction and examination of cells from cold water, ice, or permafrost. Studies of microbial life in cold environments on Earth help us to understand how life could have arisen on cold planets such as Mars. Instruments to look for chemical evidence of extant or extinct life in the Martian subsurface are being developed for future missions, and Mars samples eventually will be returned to Earth for study. Ideally, remote selection of such samples can be expedited by equipping rovers with instruments that exploit molecular signatures of microbial life rather than just by photography of Martian surface morphology or rock type.
Hints of microbial production of excess greenhouse gases in glacial ice have been reported. In a study of N2O in portions of the Vostok ice core, Sowers (7) found a 30% excess at a depth corresponding to the penultimate glacial maximum [≈135,000 yr (135 kyr) ago], at which excess bacterial counts and dust had been found, and he suggested that the N2O had been produced in situ by nitrifying bacteria. Flückiger et al. (8, 9) reported occasional spikes of excess N2O at several depths in the Greenland Ice Core Project (GRIP) and North GRIP ice cores and referred to them as N2O “artifacts.” They found no spikes of excess CH4 at those depths. Flückiger listed microbial activity in glacial ice as one of four possible explanations for the N2O artifacts (10). Campen et al. (11) reported very large excesses of CO2, CH4, and N2O (≈32, 8, and up to 240 times greater than the tropospheric values, respectively) at two depths in air extracted from the Sajama (Bolivia) glacier. They concluded that the best explanation for the excesses of these gases was that they were products of in situ microbial metabolism. None of the authors noted above searched for microbes in their ice samples.
Since those studies, following up on a report of huge excesses of CO2 and CH4 in silt-laden basal ice at the GRIP site (12), two groups discovered very high concentrations of microbes in silt-laden basal ice at the nearby GISP2 site (5, 6, 13, 14). Price and Sowers (13) measured both CH4 and microbial concentrations in the GISP2 basal ice and concluded that in situ metabolism at –9°C accounted quantitatively for the excess CH4. The silty, basal ice probably originated in flow-induced mixing of glacial ice with a frozen wetland soil (12) some 3 × 105 yr ago (14).
Materials and Methods
Ice Samples. From the National Ice Core Laboratory we obtained samples of GISP2 ice at and just above and just below the depths (labeled in Fig. 1) at which excess methane had been found. The samples were located at 2953.5, 2954, 2954.5, 3017, 3018, 3018.6, 3034.7, 3035.6, and 3036.7 m. In addition, we obtained ice samples at eight other depths from 500 to 3,000 m as controls.
Sample Preparation. We sterilized our equipment by baking at 600°C for up to 5 h. In a sterile laminar flow hood, we removed >30% of the exterior of each GISP2 ice sample by rinsing it sequentially with 10 M HCl, DNase- and RNase-free sterile water, and 10 M NaOH and a final rinse in sterile water to eliminate any contamination that might have been introduced during the drilling process or subsequent handling of the samples. This very stringent treatment was designed to remove and destroy cells, bacterial spores, and nucleic acid from the sample exterior. To test our methods, we made numerous ice samples, coated them with various types of bacteria (including Escherichia coli and Micrococcus luteus) and with Bacillus subtilis spores at concentrations of at least 108 cells per ml, subjected them to the same sterilizing procedure that we used with the GISP2 sample, and verified, with direct counting methods and culturing, that none of the cells and spores coated on the ice surfaces made it into the final test sample. We processed the GISP2 ice samples together with control samples in a double-blind manner.
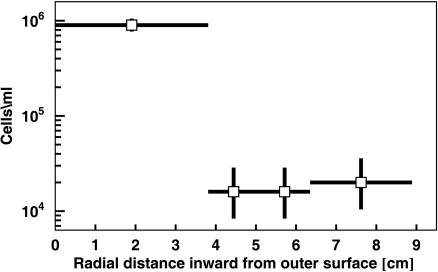
Removal of Ice Contaminated by Drill Fluid. Microbes in the fluid used for drilling ice cores have been shown to contaminate the outer portions of cores on which biological studies have been done. Christner et al. (15) evaluated microbial contamination of the accreted lake ice from Lake Vostok in the Vostok ice core by measuring the radial gradient of microbial concentration in a core section. After their procedure, we measured the microbial concentration as a function of radial distance from the outer surface in a GISP2 sample from a depth of 500 m. Our direct cell counts, plotted in Fig. 2, showed that some of the outermost 3.8-cm portion had been contaminated. Consequently, we took all of our ice samples from the innermost 2.5 cm extending to the center of the core, at which the microbial concentration was independent of radial distance.
Fig. 2.
Microbial concentration as a function of radial distance from the surface to the center of the GISP2 ice core at a depth of 500 m. Some of the outermost 3.8 cm of ice had clearly been contaminated by either the drilling fluid used to cut the core or subsequent handling.
Enumeration of All Cells and of Methanogens. To count concentrations of all cells, we melted 4–7 ml of an interior sample at each depth, immediately stained the cells in the melted ice with up to 20 μM Syto-23, and filtered a known volume of the melted ice through a small, circular region on a 0.015-μm Nuclepore filter. We used a Zeiss Axiovert epifluorescence microscope with an ×100 objective at room temperature to count all of the fluorescing cells on the region of the filter through which the solution had passed. For Syto-23-stained cells, the volume per droplet was 0.25–1.5 μl.
Using the same microscope, we counted unstained cells from the sample interior by viewing the blue-green autofluorescence of the F420 coenzyme, accepted as a unique signature for methanogens (16). To examine the cells without introducing chemical artifacts, we omitted the usual fixation process. To increase counting statistics for methanogens, we used a volume of 20 μl per droplet on a 0.015-μm filter. Using excitation at 420 nm and a long-pass filter at 460 nm, we observed this fluorescence in a small fraction of cells in the band at ≈460–490 nm. We were able to account for possible interference caused by autofluorescence of micrometer-sized mineral grains that might accompany the microbes by taking advantage of the relatively high photobleaching rate and narrow excitation spectrum of F420. By photobleaching our samples after counting cells and then counting those cells with multiple excitation/emission wavelengths, we were able to subtract the mineral background. We tested these methods by using a laboratory culture of the methanogen Methanococcus jannaschii and mineral grains filtered from melted GISP2 ice. The results showed that mineral grains from GISP2 were far more resistant to photobleaching than were methanogens. Moreover, the minerals tended to fluoresce over a broad spectrum of excitation wavelengths, whereas F420 fluoresced only at 420-nm excitation. Although F420 has been detected in cells of several members of all three domains (17), in which it serves a variety of roles, it is abundant only in methanogens. In nonmethanogens, its concentration is typically lower by several orders of magnitude (18), and its detection requires purification.
Results
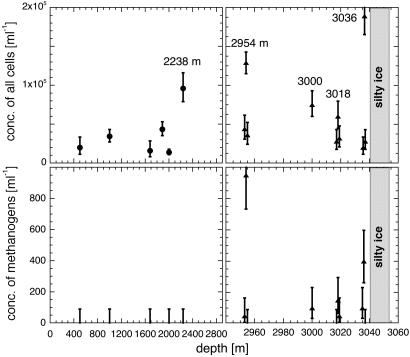
Excess Cell Concentrations Correlated with Excess Methane. Fig. 3 Upper shows the concentrations of all cells as a function of depth, determined by scanning and counting Syto-23-stained cells, observed by their fluorescence, and Fig. 3 Lower shows the concentrations of unstained cells detected by their F420 autofluorescence. The number of cells found at a given depth ranged from 4 to 90 for Syto-23 and from 0 to 19 for F420 autofluorescence. The error bars shown in Fig. 3, resulting from application of Poisson statistics, are for an 84% confidence level; the upper limits of 90 cells per ml on F420 fluorescence at depths of 500 to 2,238 m correspond to a null count for a volume of 20 μl. On average, the ratio of concentrations of methanogens to all cells was ≈1 in 300, which is almost an order of magnitude lower than the ratio of ≈1 in 40 found earlier in the GISP2 silty ice (14).
Fig. 3.
Concentrations of all cells (Upper) and of methanogens (Lower). Syto-23 stain was used to visualize fluorescence of all cells, and F420 autofluorescence was used to image methanogenic cells. Measurements were made with both Syto-23 and F420 on the same samples at all depths except at 1,887 m. Error bars indicate counting statistics. Upper limits at 90 cells per ml correspond to a null count at an 84% confidence level. At depths ≈1 m above and below the values at 2,954, 3,018, and 3,036 m, the concentrations drop to background values, just as was the case for methane in Fig. 1.
We reiterate that although F420 is present at some level in certain members of all three domains, only in methanogens is its autofluorescence visible. In Fig. 3 Upper we see that, at depths of <2,238 m and just above and below the peaks at 2,954, 3,018, and 3,036 m, the concentration of Syto-23-stained cells was typically ≈2 × 104/ml, and we take that as the average microbial concentration resulting from aeolian deposition. In Fig. 3 Lower we see that at depths <2,950 m there was no indication of F420 autofluorescence, from which we set an upper limit of <90 cells per ml (84% confidence level) on concentration of methanogens. We found both methanogens and large excesses of Syto-23-stained cells at 2,954, 3,000, 3,018, and 3,036 m. In addition, at 2,238 m we found a high concentration (1 × 105 cells per ml) of Syto-23-stained cells but no methanogens. Because Brook did not measure methane within 3 m of 2,238 m, the null result for methanogens is not significant. Brook measured methane at ≈3,000 m but did not find an excess.
The correspondence of our high concentrations of microbes, and especially of methanogens, with Brook's high methane concentrations at depths of 2,954, 3,018, and 3,036 m strongly supports our hypothesis that methanogenic metabolism accounts for the anomalously high methane in GISP2 ice. The large excess of Syto-23-stained cells at those three depths shows that those layers were also enriched in nonmethanogenic microbes. The absence of excess methane where we saw methanogens at ≈3,000 m suggests that the excess methane and methanogens may have been localized in a layer, probably no thicker than ≈1 m, that did not exactly correspond with the depth of Brook's sample.
To see whether the concentration of cells, including methanogens, was enhanced in the deep, disturbed regions of the GISP2 ice where no excess methane was found, we made measurements not only at 2,954, 3,018, and 3,036 m but also at ≈1 m above and below each of those depths. From Fig. 3 we see that ≈1 m above and below those depths, the concentrations of all cells (Upper) and of methanogens (Lower) drop to low values consistent with background. The sharp drops are consistent with Brook's results: 932 parts per billion by volume (ppbV) at 3,018 m and 632 and 633 ppbV at depths 2 m above and below it.
Metabolic Rates in GISP2 Ice. Price and Sowers (13) recently showed that the average metabolic rates of communities of microbes imprisoned in ice and other solid media can be calculated from the concentrations of trapped gas that they produced during a time t at an absolute temperature T. The metabolic rate R(T), defined as the fractional rate of turnover of carbon per cell per year, is given by
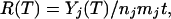 |
[1] |
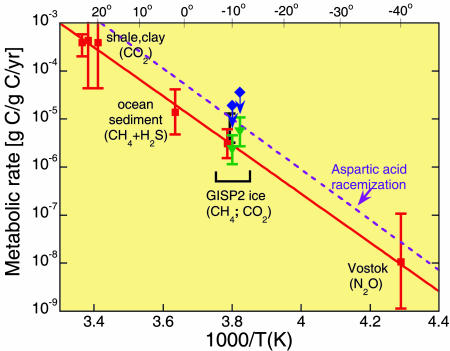
where Yj(T) is the concentration of biogenic gas of type j at ice temperature T, with T taken from ref. 19; nj is the concentration of microbial cells of type j; mj is the mean carbon mass per cell, with mj taken to be 19 fg based on measurements of cell size in the silty ice (14); and t is the retention time of the gas in the ice. For depths below ≈2,700 m (including 2,954, 3,018, and 3,036 m) in both GISP2 and GRIP ice, comparisons of δ18O of O2 (20, 21) and methane (22), electrical conductivity (23), and stratigraphy (24) have shown that either one or both of the records was sufficiently disturbed that the age vs. depth record does not increase monotonically but falls within the range ≈100 to 250 kyr. Adopting the middle value, we take for retention time, t, the value 180 ± 80 kyr. We used Eq. 1 to estimate metabolic rates for microbial production of CH4 and CO2. Fig. 4 shows our results, together with results from Price and Sowers (13), on an Arrhenius plot of rate versus 1/T. In calculating rates for CO2 production (solid-green triangles), we assumed that cells imaged with Syto-23 fluorescence were mainly anaerobic CO2 producers such as Fe reducers and sulfate reducers and that the ratio of CO2 to CH4 concentrations was 20, as was found in the basal ice at the nearby GRIP (12). Rates for cells imaged in F420 autofluorescence are shown with solid-blue diamonds. The arrows indicate that those two rates might be overestimated if the scanning efficiency for detecting methanogens were <100% because of weak F420 fluorescence of some species. Such would be the case if some methanogens had a lower-than-typical concentration of F420 molecules or if the F420 were reduced (16) or had been in a low-pH environment (16) such as acidic veins (25). The carbon turnover times for the cluster of points reported here are inferred to be at least 105 yr.
Fig. 4.
Temperature dependence of metabolic rate of microbial communities imprisoned in ice, rock, and sea sediment. Metabolic products are shown in parentheses. Solid-red squares show the rates at temperatures from 24 to –40°C; the rate for cells in GISP2 silty ice is shown at –9.5°C (13). Solid-blue diamonds denote the present results for methanogens in GISP2 ice at 2,954 and 3,018 m, and solid-green triangles denote the present results for CO2 producers at the same depths.
The dashed purple line in Fig. 4 is an extrapolation of an Arrhenius line for spontaneous racemization of aspartic acid in microbes from Siberian permafrost, measured at three temperatures from 100 to 145°C (26). The line through the three points in figure 4 of ref. 26 seems to be an exact fit, and no error is quoted. We estimate that the error in racemization rate extrapolated into the interval of 30 to –40°C is less than a factor of two. Rates for racemization of the other amino acids and for depurination of DNA are an order of magnitude lower (27) and can be neglected.
Discussion
Spikes of High Microbial Concentrations from Above or Below? We attribute the background concentration of ≈2 × 104 cells per ml to normal aeolian deposition. In principle, an excess of microbes far above this background concentration in a narrow ice layer might have resulted from aeolian deposition during an unusually intense dust storm. However, the dust concentration in GISP2 ice was not noticeably enhanced in a narrow interval at that depth (28), which leaves us with no independent evidence for aeolian deposition. Searches for microbes “hitchhiking” aboard aerosolized dust transported from deserts in intense dust storms are underway by others.§ Methanogenic archaea are strict anaerobes, but many species have considerable variability in their sensitivity to oxygen. Methanogens have been shown to survive in oxic desert soils (29). Viable methanogens have been cultured from nominally aerobic environments in which anaerobic microenvironments or transient anaerobic conditions occur (30). Some methanogens contain superoxide dismutase and catalase, which act as contingency defenses against oxygen toxicity (31). Methanogens from the nominally aerobic meltwater below an Arctic glacier have been detected by enrichment techniques (32). One interpretation of our results is that a fraction of the methanogens deposited in snow at the GISP2 site might have survived and begun to metabolize in the ice at depths at which the oxygen concentration was greatly reduced. At shallow depths, air bubbles at the corners of ice crystals intersect the network of liquid veins at triple junctions of ice crystals, and methanogens and other strict anaerobes in the veins in diffusive contact with air in bubbles might survive but not metabolize. However, at depths below ≈1,600 m, the air trapped in GISP2 ice is in the form of air-hydrate crystals, which, in general, do not intersect the liquid veins in which microbes are trapped. Thus, in the anaerobic environment of the deep-ice methanogenic metabolism should be possible, as we have inferred from the methane and F420 data.
A more likely possibility is that meter-thick layers of ice were excavated from the basal, microbe-rich ice by turbulent glacial flow during buildup of the ice sheet. Visual inspection of the GRIP and GISP2 ice cores provided evidence that, at depths below ≈2,450 m, ice flow caused occasional layer overturning (24) or flow-induced mixing (12). The high concentrations of microbes localized at 2,954, 3,000, 3,018, and 3,036 m may have resulted from interleaving of thin layers of microbe-rich basal ice with clear ice. At those depths, where the oxygen is locked up in air clathrates, it is not surprising that methanogens should be capable of making methane.
Because all of the microbe-rich layers except the one at 2,238 m were at depths within ≈90 m of the silty, basal ice, it seems likely that they resulted from flow-induced mixing rather than aeolian deposition. The situation may be different at depths as shallow as 2,238 m, at which there is no evidence at either GRIP or GISP2 for overturning. At 2,238 m, the gaseous products of microbial metabolism will build up too slowly to produce a detectable signal: the accumulation time at that depth is only approximately one fourth that at 2,954 m, and the temperature is much colder (–28°C at 2,238 m compared with –11.5°C at 2,954 m), which reduces the metabolic rate by a factor of ≈30, leading to a net concentration of metabolic products a factor ≈102 lower for a given microbial concentration than at 2,954 m. At even shallower depths, because of the younger ages and lower temperatures, the concentration of metabolic products from trapped microbes would be lower still. Thus, it is to be expected that no excess CO2 or other product of microbial metabolism would be detectable at 2,238 m or at shallower depths.
Relative Concentration of Methanogens. The fraction of cells that show F420 autofluorescence is much lower in the glacial ice than in the silty basal ice (1/300 compared with 1/40), which is not surprising: methanogens in the silty ice were probably frozen in situ and thus were always in an anaerobic environment, whereas methanogens in shallower depths, having been exhumed from the underlying anaerobic environment by turbulent overturning, may have been exposed to some atmospheric oxygen during that process. Thus, the survival rate of those methanogens may have been lower than for those in the basal ice.
Microbial Interference with Climate Signals. From Eq. 1 it is obvious that the production of a concentration of greenhouse gases (CH4, CO2, and N2O) sufficient to interfere with climate signals would be favored by a high microbial concentration in ice (or ocean sediment), a long accumulation time of the gas being trapped, and a relatively high microbial metabolic rate, which is a strongly increasing function of temperature. Fortunately for climatology, in most cases the contribution of microbial metabolism to greenhouse-gas production in ice is negligibly small. On those rare occasions when a microbial signal is detected, it enables one to infer a metabolic rate (by Eq. 1) that is far too low to measure in laboratory experiments.
Metabolism Only to Repair Macromolecular Damage. Assuming that the activation energy for damage repair is constant from 145 to –40°C and similar for ice and permafrost, the approximate agreement of the racemization rate (Fig. 4, dashed purple line) with the metabolic rate for survival of immobilized microbes in ice and shale suggests that the rate of repair of macromolecular damage in living microbes may be the same as the rate of spontaneous damage (13). If so, a fascinating picture emerges: a subset of microbes able to survive the harsh conditions of temperature, darkness, pH, pressure, redox value, nutrient source, and desiccation in the environment in which they are trapped may survive for millions of years by using the limited source of energy and nutrients not to grow but just to repair macromolecular damage at the same rate at which it occurs spontaneously. This strategy of “repair as you go” differs from that of endospores, which remain metabolically inactive and do not repair damage until after conditions improve enough for them to germinate.
Implications for Methanogens on Mars. Our measurements of the temperature-dependent metabolic rate of confined microbes have an interesting application to life on other planets. Krasnopolsky et al. (33) and Formisano et al. (34) recently detected ≈10 parts per billion of methane in the Martian atmosphere. Krasnopolsky et al. estimated a lifetime of ≈340 yr for destruction of methane in the atmosphere by solar UV photolysis and a corresponding loss rate of 270 tons of methane per year. They calculated that abiotic production mechanisms cannot readily offset such a loss rate, and they concluded that ongoing methanogenesis by subsurface methanogens is a possible source. Abiotic explanations for the atmospheric methane would require ongoing magmatism and hydrothermal alteration in the presence of aqueous CO2 and H2O (35, 36). A future measurement of the carbon isotopes in Martian methane could decide between a biotic and an abiotic origin.
Having measured the temperature-dependent metabolic rate for methanogenesis (Fig. 4), we can calculate the required number of Martian methanogens in the biotic scenario. Setting the steady-state metabolic rate of production of methane equal to the loss rate of 1/340 = 3 × 10–3/yr1, we find, by using the rate given by the solid diamonds in Fig. 4, that the temperature of a Martian methanogenic habitat would have to be ≥0°C. From ref. 37 we find that the Martian subsurface temperature reaches 0°C at a depth between 150 m and 8 km, depending on soil thermal conductivity, and that the time for the methane to diffuse to the surface would be 15 yr if from a depth of 150 m or 30 kyr if from 8 km.
For a uniform distribution of methanogens over the Martian subsurface in a thickness interval δz, where the temperature is 0°C, and for a mass m of carbon per cell, we estimate that their concentration in the spherical subsurface shell would be
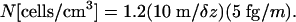 |
[2] |
By using an instrumented rover to find sites at which the methane concentration is greatly enhanced (34), one could make a meaningful search for methanogens with a bore-hole fluorimeter designed to detect F420 autofluorescence, provided it could be deployed at a suitable depth. A sensitivity to ≈1 cell per cm3 seems achievable. Such a device has been constructed in our laboratory (38).
Acknowledgments
We thank Ed Brook for providing his unpublished methane data; Boonchai Boonyaratanakornkit for providing the Methanococcus jannaschii cells; and Ed Brook, Jeff Severinghaus, Brent Christner, and Sydney Kustu for discussions. We also thank the National Science Foundation Office of Polar Programs for Grants OPP-0085400 and REU-0343999.
Author contributions: P.B.P. designed research; H.C.T. performed research; N.E.B. contributed new reagents/analytic tools; P.B.P. analyzed data; and P.B.P. wrote the paper.
Abbreviations: GISP2, Greenland Ice Sheet Project 2; GRIP, Greenland Ice Core Project.
Footnotes
Warren-Rhodes, K. A. & Griffin, D. W., American Geophysical Union Fall Meeting, Dec. 13–17, 2004, San Francisco (abstr. B33A-0250).
References
- 1.Blunier, T. & Brook, E. (2001) Science 291, 109–112. [DOI] [PubMed] [Google Scholar]
- 2.Priscu, J. C. & Christner, B. (2004) in Microbial Diversity and Bioprospecting, ed. Bull, A. T. (Am. Soc. Microbiol., Washington, DC), pp. 130–145.
- 3.Gaidos, E., Lanoil, B., Thorsteinsson, T., Graham, A., Skidmore, M., Han, S.-K., Rust, T. & Popp, B. (2004) Astrobiology 4, 327–344. [DOI] [PubMed] [Google Scholar]
- 4.Bulat, S. A., Alekhina, I. A., Blot, M., Petit, J.-R., de Angelis, M., Wagenbach, D., Lipenkov, V. Y., Vasilyeva, L. P., Wloch, D. M., Raynaud, D., et al. (2004) Int. J. Astrobiology 3, 1–12. [Google Scholar]
- 5.Sheridan, P. P., Miteva, V. I. & Brenchley, J. E. (2003) Appl. Environ. Microbiol. 69, 2153–2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miteva, V. I., Sheridan, P. P. & Brenchley, J. E. (2004) Appl. Environ. Microbiol. 70, 202–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sowers, T. (2001) J. Geophys. Res. Atmospheres 106, 31903–31914. [Google Scholar]
- 8.Flückiger, J., Dällenbach, A., Blunier, T., Stauffer, B., Stocker, T. F., Raynaud, D. & Barnola, J.-M. (1999) Science 285, 227–230. [DOI] [PubMed] [Google Scholar]
- 9.Flückiger, J., Blunier, T., Stauffer, B., Chappellaz, J., Spahni, R., Kawamura, K., Schwander, J., Stocker, T. F. & Dahl-Jensen, D. (2004) Global Biogeochem. Cycles 18, GB1020–GB1028. [Google Scholar]
- 10.Flückiger, J. (2003) Ph.D. thesis (Univ. of Bern, Bern, Switzerland).
- 11.Campen, R. K., Sowers, T. & Alley, R. B. (2003) Geology 31, 231–234. [Google Scholar]
- 12.Souchez, R., Lemmens, M. & Chappellaz, J. (1995) Geophys. Res. Lett. 22, 41–44. [Google Scholar]
- 13.Price, P. B. & Sowers, T. (2004) Proc. Natl. Acad. Sci. USA 101, 4631–4636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tung, C., Price, P. B., Bramall, N. & Vrdoljak, G. (2005) Astrobiology, in press. [DOI] [PubMed]
- 15.Christner, B. C., Mikucki, J. A., Foreman, C. M., Denson, J. & Priscu, J. C. (2005) Icarus 174, 572–584. [Google Scholar]
- 16.DiMarco, A. A., Bobik, T. A. & Wolfe, R. S. (1990) Annu. Rev. Biochem. 59, 355–394. [DOI] [PubMed] [Google Scholar]
- 17.Purwantini, E. & Daniels, L. (1998) J. Bacteriol. 180, 2212–2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin, X. L. & White, R. H. (1986) J. Bacteriol. 168, 444–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alley, R. B. (2004) IGBP PAGES (NOAA/NGDC Paleoclimatology Program, Boulder, CO), World Data Center for Paleoclimatology Data Contribution Series 2004-013.
- 20.Grootes, P. M., Stuiver, M., White, J. W. C., Johnsen, S. & Jouzel, J. (1993) Nature 366, 552–554. [Google Scholar]
- 21.Bender, M., Sowers, T., Dickson, M.-L., Orchardo, J., Grootes, P., Mayewski, P. A. & Meese, D. A. (1994) Nature 372, 663–666. [Google Scholar]
- 22.Chappellaz, J., Brook, E., Blunier, T. & Malaizé, B. (1997) J. Geophys. Res. Oceans 102, 26547–26557. [Google Scholar]
- 23.Taylor, K. C., Hammer, C. U., Alley, R. B., Clausen, H. B., Dahl-Jensen, D., Gow, A. J., Gundestrup, N. S., Kipfstuhl, J., Moore, J. C. & Waddington, E. D. (1993) Nature 366, 549–552. [Google Scholar]
- 24.Alley, R. B., Gow, A. J., Johnsen, S. J., Kipfstuhl, J., Meese, D. A. & Thorsteinsson, Th. (1995) Nature 373, 393–394.7830789 [Google Scholar]
- 25.Price, P. B. (2000) Proc. Natl. Acad. Sci. USA 97, 1247–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brinton, K. L. F., Tsapin, A. I., Gilichinsky, D. & McDonald, G. D. (2002) Astrobiology 2, 77–82. [DOI] [PubMed] [Google Scholar]
- 27.Lindahl, T. & Nyberg, N. (1972) Biochemistry 11, 3610–3618. [DOI] [PubMed] [Google Scholar]
- 28.Bay, R. C., Bramall, N. & Price, P. B. (2004) Proc. Natl. Acad. Sci. USA 101, 6341–6345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peters, V. & Conrad, R. (1995) Appl. Environ. Microbiol. 61, 1673–1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zinder, S. H. (1993) in Methanogenesis, ed. Ferry, J. G. (Chapman and Hall, New York), pp. 128–206.
- 31.Kirby, T. W., Lancaster, J. R. & Fridovich, I. (1981) Arch. Biochem. Biophys. 210, 140–148. [DOI] [PubMed] [Google Scholar]
- 32.Skidmore, M. L., Foght, J. M. & Sharp, M. J. (2000) Appl. Environ. Microbiol. 66, 3214–3220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krasnopolsky, V. A., Maillard, J. P. & Owen, T. C. (2004) Icarus 172, 537–547. [Google Scholar]
- 34.Formisano, V., Atreya, S., Encrenaz, T., Ignatiev, N. & Giuranna, M. (2004) Science 306, 1758–1761. [DOI] [PubMed] [Google Scholar]
- 35.Lyons, J. R., Manning, C. & Nimmo, F. (2005) Geophys. Res. Lett. 32, L13201. [Google Scholar]
- 36.Oze, C. & Sharma, M. (2005) Geophys. Res. Lett. 32, L10203. [Google Scholar]
- 37.Mellon, M. T. & Phillips, R. J. (2001) J. Geophys. Res. Planets 106, 23165–23179. [Google Scholar]
- 38.Bay, R., Bramall, N. & Price, P. B. (2005) in Life in Ancient Ice, eds. Castello, J. D. & Rogers, S. O. (Princeton Univ. Press, Princeton), pp. 268–276.