Abstract
Human immunodeficiency virus type 2 (HIV-2) is much less pathogenic than HIV-1, and HIV-2 infection is associated with plasma viral loads significantly lower than those found in HIV-1 infection. We have developed a real-time quantitative PCR method for measuring the HIV-2 RNA load that covers the range of genetic diversity of HIV-2 isolates and that detects extremely low viral loads. Samples from 49 patients were studied. Proviral DNA was first detected and quantified. The strains that were detected were then genotyped: 21 patients were infected with HIV-2 subtype A and 15 patients were infected with HIV-2 subtype B; 1 patient was infected with a highly divergent strain. Env PCR failed for the remaining 12 patients, so subtypes could not be determined. For viral RNA quantification, a stock of HIV-2 strain NIHZ, which was counted by electron microscopy, was used as the standard. Several primer sets targeting the highly conserved gag region were evaluated. Various primer combinations failed to amplify subtype B strains. With the final primer pair selected, which detected both subtype A and subtype B strains, the sensitivity of the assay was 100% at a viral load of 250 copies/ml and 66% at a viral load of 125 copies/ml. We found a correlation between the CD4+-cell count, the clinical stage, and the plasma HIV-2 RNA level. The median plasma HIV-2 RNA value for the 33 asymptomatic patients was 2.14 log10, whereas it was 3.1 log10 for the 16 patients with AIDS (P < 0.01). Proviral DNA was detectable in 18 symptom-free patients with high CD4+-cell counts, in whom viral RNA was undetectable.
Human immunodeficiency virus (HIV) type 2 (HIV-2) differs from HIV-1 by its lower pathogenicity and higher level of intrasubtype strain diversity. In addition, the incubation period for HIV-2 infection is longer, the time to progression to AIDS is slower (12), and the survival time with AIDS is longer (19). Similarly, the heterosexual and vertical transmission rates are lower with HIV-2 than with HIV-1. HIV-2 remains restricted to West Africa, where its prevalence is lower than that of HIV-1 (15-18). Thus, if HIV-2 is not less pathogenic, it is less virulent than HIV-1. No clear explanation for this difference has been found. The main hypothesis is that HIV-2 replication is less efficient than HIV-1 replication in vivo. This is based on the HIV-2 cellular load in quantitative culture and on a lower rate of plasma RNA positivity in vivo (4, 25). The first quantitative data on plasma HIV-2 RNA confirmed these differences in replication efficiency (23). In contrast, the HIV-2 proviral load is relatively high, with values similar to the HIV-1 proviral load (2).
The second major characteristic of HIV-2 is its high degree of genetic diversity (7, 8, 13, 21). The genetic distance between different HIV-2 subtypes is greater than that between the HIV-1 subtypes (9). It is thought that the HIV-2 subtypes identified so far correspond to separate cross-species transmission events between mangabeys (Cercocebus atys) and humans and thus are analogous to the HIV-1 groups, which are the result of separate cross-species transmission between chimpanzees (Pan troglodytes) and humans. Only HIV-2 subtype A and HIV-2 subtype B are prevalent and have been found outside West Africa (7, 8, 9, 13). The other subtypes (subtypes C to G) correspond to strains that are seemingly epidemiological dead ends (7). This high level of genetic distance between the two major subtypes, subtypes A and B, makes it difficult to develop a single tool to detect their genomes. The extremely low viral load in most patients further hinders development of a quantitative assay for HIV-2 RNA in plasma. Real-time PCR provides a new approach to viral quantification. Its rapid execution permits large numbers of probes and primers to be tested. In the absence of a commercial PCR method for the follow-up of HIV-2-infected patients, we have developed a real-time PCR-based quantitative assay for HIV-2 RNA in plasma. This assay is not limited by the high degree of diversity of HIV-2 strains and permits a highly sensitive quantification of HIV-2 subtype A and subtype B.
MATERIALS AND METHODS
Patients and samples.
We selected and tested 49 random plasma samples from patients living in France (24 men and 25 women). Most of the patients were of West African origin and were included in the French National Agency for AIDS Research HIV-2 national cohort. According to the Centers for Disease Control and Prevention (CDC) classification (6), 32 patients were at stage A, 1 patient was at stage B, and 16 patients were at stage C. Twenty-seven patients had CD4+-cell counts above 300/μl (mean ± standard deviation [SD], 548 ± 220/μl; median, 529/μl; range, 302 to 1,161/μl) and 22 had values below 300/μl (mean ± SD, 118 ± 88/μl; median, 81/μl; range, 7 to 283/μl). Only seven patients had received antiretroviral treatment by the date of sampling: three had received long-term treatment (10 months to 6 years) with zidovudine, and the remaining four had received short-term treatment (less than 1 year) with a three-drug regimen.
HIV-2 infection was diagnosed by Western blotting (New Lav Blot II; Bio-Rad-Pasteur, Marnes la Coquette, France), and HIV-1 infection was ruled out by an HIV-1-specific enzyme-linked immunosorbent assay (Wellcozyme, HIV recombinant; Murex Biotech Limited, Rungis, France). Plasma collected in tubes containing EDTA was centrifuged less than 3 h after sampling and stored at −80°C. Mononuclear cells were harvested on Ficoll (25), washed twice in phosphate-buffered saline, and stored as a dry pellet at −80°C.
HIV-2 subtype determination.
The viral genotype was determined by nucleotide sequencing of the V3 loop and gp41 envelope region as described previously (11). Briefly, DNA was amplified by a first-round long (XL) PCR procedure with primers whose sequences are specific for sequences located in the pol and long terminal repeat regions. This was followed by nested PCR with primer pairs whose sequences correspond to the sequences of the V3 domain and the gp36 region of the HIV-2 env gene (9). The env sequences were compared to multiple env HIV-2 sequence alignments obtained from the Los Alamos National Laboratory HIV sequence database (http://hiv-web.lanl.gov) (20).
Proviral DNA quantification.
Proviral DNA was quantified by a recently described real-time PCR method with primers whose sequences are specific for the sequence in the gag region. The real-time PCR method detects HIV-2 subtypes A and B (10). The linear range of our assay was 5 to 500,000 copies/105 peripheral blood mononuclear cells (PBMCs).
Preparation of standards for RNA quantification.
A stock of HIV-2 strain NIHZ, which was counted by electron microscopy, was used as the standard (Advanced Biotechnology Incorporated, Columbia, Md.). Prior to lysis, the stock solution contained 1.84 × 1010 virus particles/ml. The HIV-2 NIHZ stock solution was diluted in HIV-negative plasma to obtain 500,000, 50,000, 5,000, 500, and 250 copies in 140 μl. RNA was extracted with the Qiagen viral RNA minikit. Standards and samples were treated under the same conditions.
PCR primers and hybridization probe for HIV-2 RNA.
In order to avoid major mismatches due to HIV-2 variability, the primers and probe were designed on the basis of published HIV-2 subtype A and B sequences (20). The first step was to select a highly conserved probe. The central part of the gag gene was chosen on the basis of its strong intersubtype homology. The sequence of the probe, designated S65GAG2, was 5′-R-TAGGTTACGGCCCGGCGGAAAGA-Q-3′, where R indicates the reporter dye 6-carboxyfluorescein and Q indicates the quencher dye 6-carboxytetramethylrhodamine, as described previously (27).
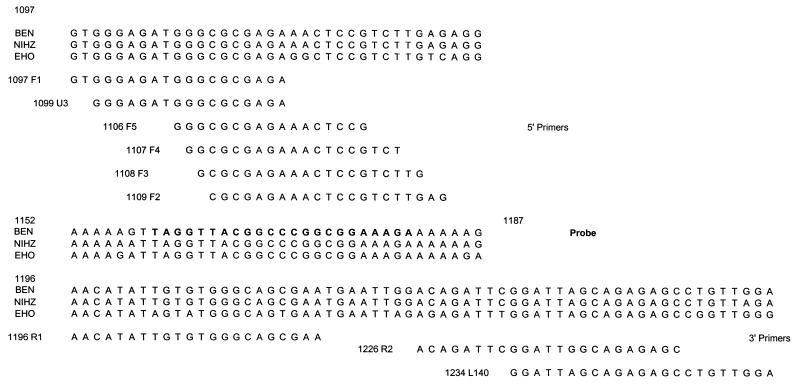
We then selected as potential primers all the sequences in the region surrounding the probe for use in the real-time PCR. Six 5′-3′ and three 3′-5′ primers were designed. Figure 1 shows the sequences selected. Quantitative PCR was performed by using real-time technology in the LightCycler System (Roche Molecular, Indianapolis, Ind.).
FIG. 1.
Positions of the sequences of the different primers and probes evaluated for use in the real-time PCR on the gag genes of two strains of HIV-2 subtype A (strains BEN and NIHZ) and one strain of HIV-2 subtype B (strain EHO).
Development of HIV-2 RNA quantitative assay.
RNA was extracted from plasma by using the Qiagen viral RNA minikit according to the instructions of the manufacturer. To improve the sensitivity, 1 ml of plasma was ultracentrifuged for 1 h at 16,500 × g at 4°C, and the pellet was resuspended in 140 μl of HIV-negative human plasma before extraction. RNA was eluted from the silica columns in 35 μl of elution buffer. Real-time reverse transcription-PCR was done with the LightCycler-RNA Master hybridization probes kit (Roche Molecular Biochemicals). The 20-μl reaction mixtures consisted of 7.5 μl of LightCycler RNA master hybridization mixture, 3.25 mM manganese acetate, 10 μM each primer and probe, and 6 μl of extracted RNA. The PCR conditions were as follows: (i) reverse transcription at 61°C for 20 min; (ii) denaturation at 95°C for 2 min; (iii) 45 cycles of denaturation at 95°C for 5 s, annealing at 60°C for 20 s, and elongation at 65°C for 50 s; and (iv) cooling to 40°C for 30 s.
Determination of specificity, sensitivity, and reproducibility of the RNA assay.
The specificity of our assay was determined by testing plasma obtained from 25 HIV-negative blood donors and 25 HIV-1-seropositive patients. The efficiency and sensitivity of the real-time reverse transcription PCR for HIV-2 RNA quantification were determined by testing the HIV-2 RNA standard. The standard was diluted in 140 μl of HIV-negative plasma to obtain 500, 250, and 125 copy equivalents (six replicates each). It was then extracted and treated as described above. To assess the reproducibility of the assay, a stock of HIV-2 strain NIHZ was diluted in HIV-negative plasma and tested in replicate at concentrations from 500,000 to 250 copy equivalents/ml (50,000, 5,000, and 500 copies). The standard was tested in five replicates to determine within-run variability and in seven separate runs to determine between-run variability.
RESULTS
Primer selection.
PCR with primers F4 and R2 was negative for subtype B strains. The detection limit was unsatisfactory with primers F1, F2, F5, U3, and L140 (cutoff above 1,000 copies/ml) and/or these primers did not have the melting temperature required for reverse transcription (Fig. 1). The F3-R1 primer pair amplified all the strains with an adequate sensitivity and was selected for further development of the quantitative assay.
Specificity, sensitivity, and reproducibility.
As expected because of the high degree of divergence between the HIV-1 and HIV-2 genomes, the HIV-2-specific primers did not hybridize to HIV-1 genes. The specificity of our assay was 100%; all HIV-negative and HIV-1-positive plasma samples had values below 250 copies/ml. The sensitivity of the assay, based on repeated testing (6 replicates each) of the lower standard concentrations, was 100% at 250 and 500 copies/ml and 66% at 125 copies/ml. The quantification cutoff for the assay was thus set at 250 copies/ml.
The LightCycler system offers good reproducibility for HIV-2 RNA quantification. For a theoretical virus concentration of 5.69 log10 copies/ml, we obtained a mean value of 5.67 log10 copies/ml, with a within-run coefficient of variation of 1.78%. A mean copy number of 5.73 log10 copies/ml was obtained in repeat assays, with a between-run coefficient of variation of 1.04%. At the lowest concentration of 2.39 log10 copies/ml, we found mean values of 2.39 and 2.43 log10 copies/ml for within- and between-run assays, respectively, with coefficients of variation of 1 and 4.4%, respectively.
HIV-2 subtype A and subtype B distribution in the patient population.
The env nucleotide sequences of the clinical HIV-2 isolates indicated that 21 patients were infected with HIV-2 subtype A and 15 were infected with HIV-2 subtype B; 1 patient, originating from the Ivory Coast, was infected with a highly divergent strain closely related to HIV-2 subtype C. We observed a slight but nonsignificant difference in CDC stages according to the infecting subtypes, with 11 subtype A-infected patients (52%) and 12 subtype B- and subtype C-infected patient (75%) being symptom-free (P < 0.20).
Amplification of the env gene was negative for 12 patients, all but 1 of whom had more than 310 CD4+ cells/μl, and 6 patients (50%) had proviral DNA levels below 5 copies/105 PBMCs. Amplification failed in a previously untreated patient with 25 CD4+ cells/μl, even in assays with primers whose sequences were specific for sequences located in other regions of the gene (9).
The assay detects RNA of both HIV-2 subtypes.
The viral loads in samples from 22 of the 49 patients (45%) were above the detection limit of 250 copies/ml. Viral load values were between 250 and 500 copies/ml in seven patients, between 500 and 1,000 copies/ml in two patients, between 1,000 and 10,000 copies/ml in six patients, and above 10,000 copies/ml in seven patients. The frequency of plasma RNA positivity did not depend on the subtype, as 13 (62%) of the 21 patients with subtype A infection were positive, whereas 7 (43%) of the 16 patients with subtype B or “subtype C” infection were positive. However, the HIV-2 RNA viral load differed only slightly according to the subtype; the mean log10 values of the viral loads were 3.16 and 2.57 for subtype A and subtype B, respectively (P = 0.05).
The plasma RNA level correlates with clinical stage.
Twenty-seven patients had plasma viral loads below 250 copies/ml, and 24 of 27 (88%) of those patients have CD4-cell counts above 300/μl. Two of the three patients with undetectable RNA and CD4 counts below 300/μl had been treated with zidovudine at the time of measurement. The symptom-free patients had a median plasma RNA value of 2.14 log10, whereas the patients with AIDS had a median plasma RNA value of 3.1 log10 (P = 0.009). Only seven patients were receiving antiretroviral therapy, so we could not analyze the impact of treatment on the HIV-2 RNA viral load. However, the viral load was either undetectable or below 3 log10 in six of the seven treated patients.
Correlation between plasma RNA level and CD4+-cell count.
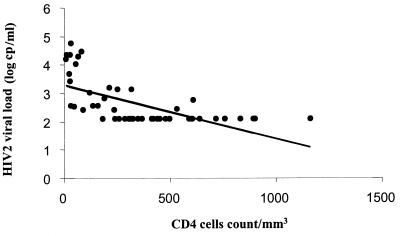
The median log10 RNA value was 3.0 in the patients with CD4+-cell counts of <300/μl and 2.1 in the patients with CD4+-cell counts of at least 300/μl (P < 10−4). The log10 plasma RNA value correlated with the CD4+-cell count, with an increase of 0.002 for the loss of 1 cell/μl (P < 10−4; r = −0.63) (Fig. 2).
FIG. 2.
Relation between plasma RNA viral load and CD4-cell counts. Points clustered at the bottom indicate undetectable HIV-2 viremia (limit of detection, 250 copies [cp]/ml). Log10 RNA = 3.29 − 0.0019 × CD4 count (P < 10−4); r = −0.6275.
Correlation between plasma RNA and proviral DNA levels.
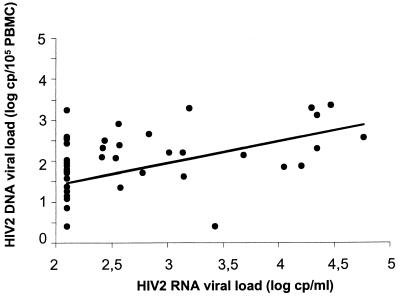
Proviral DNA was quantifiable in 39 of 49 (79.5%) patients, with a detection limit of 5 copies/105 cells. The mean ± SD log10 level of proviral DNA was 1.8 ± 0.9 copies per 105 PBMCs, with a median of 1.9 copies per 105 PBMCs (range, <0.7 to 3.4 copies per 105 PBMCs). The median log10 proviral DNA levels were 2.2 in the patients with CD4+-cell counts below 300/μl and 1.6 in the patients with CD4+-cell counts of at least 300/μl (P < 0.006). We found a correlation between plasma RNA and proviral DNA levels (P < 0.0006; r = 0.47): the log10 DNA value increased by 50% per 1 log10 increment in the RNA value (Table 1 and Fig. 3).
TABLE 1.
Plasma RNA titers and proviral DNA values
| RNA viral load (no. of copies/ml) | No. of patients with the following DNA viral loads (no. of copies/105 PBMCs):
|
||||
|---|---|---|---|---|---|
| <5 | 5-100 | 100-1,000 | >1,000 | Total | |
| <250 | 9 | 12 | 5 | 1 | 27 |
| 250-1,000 | 0 | 2 | 7 | 0 | 9 |
| 1,000-10,000 | 1 | 2 | 3 | 1 | 6 |
| >10,000 | 0 | 1 | 2 | 3 | 7 |
| Total | 10 | 17 | 17 | 5 | 49 |
FIG. 3.
Relation between plasma RNA and proviral DNA viral loads. Points clustered on the left indicate undetectable HIV-2 viremia (limit of detection, 250 copies [cp]/ml). Log10 DNA = 0.349 + 0.533 × log10 RNA (P < 0.0006; r = 0.473).
Viral RNA was undetectable and proviral DNA was detectable in 18 patients, of whom 13 (72%) were at CDC stage A and had more than 300 CD4+ cells/μl. Among the patients with CD4+ cell counts below 300, 18 of 21 (81%) were dually positive for viral RNA and DNA. Among the patients at CDC stage C, 10 of 16 (63%) were dually positive for RNA and DNA, and only 1 was dually negative (Table 2).
TABLE 2.
Plasma RNA positivity and proviral DNA positivity according to clinical stage and CD4+-cell countsa
| CDC stage | No. of CD4 cells/μla | No. of patients:
|
||||
|---|---|---|---|---|---|---|
| RNA negative, DNA negative | RNA positive, DNA negative | RNA negative, DNA positive | RNA positive, DNA positive | Total | ||
| C | <300 | 0 | 1 | 1 | 10 | 12 |
| ≥300 | 1 | 0 | 3 | 0 | 4 | |
| A | <300 | 2 | 0 | 1 | 8 | 11 |
| ≥300 | 6 | 0 | 13 | 3 | 22 | |
| Total | 9 | 1 | 18 | 21 | 49 | |
RNA positivity, ≥250 copies/ml; proviral DNA positivity, ≥5 copies/105 PBMCs.
DISCUSSION
We have developed a real-time PCR method for quantification of the RNA of HIV-2 subtype A and subtype B strains in plasma. The detection limit is 250 copy equivalents/ml, and the reproducibility of the assay is satisfactory. The genetic diversity of HIV-2 was a major obstacle to the development of the assay, as HIV-2 subtypes A and B are more distant from each other phylogenetically than HIV-1 subtypes are to each other (9). One of the clinical isolates tested in the present study was closely related to HIV-2 subtype C, but more sequences are needed to establish its true phylogenetic position. The plasma RNA of this variant was detected by our real-time PCR method, showing that our assay encompasses the large range of genetic diversity of HIV-2 strains. Previous amplification-based methods for HIV-2 quantification have been assessed only locally, generally in West African countries, where only subtype A is prevalent (1, 2, 5, 14, 22), but they have also been assessed in Europe (24, 26). The strategy that we adopted ensures that both subtype A and subtype B strains are detected, as we targeted a conserved region in the central part of the gag gene for probe hybridization and then selected potential primers according to the position of the probe.
We observed a difference between subtype A and subtype B RNA levels in plasma that was not explained by antiretroviral treatments. This may have been due to a population bias, as the patients infected with subtype B, who mainly originated from the Ivory Coast, may have been infected more recently. This is supported by the different distribution of clinical stages between patients infected with the two subtypes. Even if the RNA detection rate is the same for the two subtypes, less efficient amplification due to genomic diversity in subtype B cannot be ruled out. Indeed, only three HIV-2 subtype B gag sequences are available. An international effort is badly needed to sequence more HIV-2 strains.
The technique described here is the first technique to quantify subtype A and subtype B HIV-2 RNA by real-time PCR on the LightCycler system. The detection limit of 250 copies/ml is high, as half the samples tested had RNA at levels below this value. Others studies with different PCR systems have reported similar results, with cutoffs between 100 and 500 copies/ml and rates of positivity ranging between 56 and 62%. (3, 22, 24, 26). As reported with other techniques, the HIV-2 RNA levels obtained by this real-time PCR method were lower than those observed in patients with HIV-1 infection (1, 23). Low levels of HIV-2 RNA corresponded to high CD4+-cell counts. Conversely, values above 3.5 log10 were found exclusively in patients with less than 100 CD4+ cells/μl. Anderson et al. (1) reported that the set point was 28 times lower after primary infection with HIV-2 than after primary infection with HIV-1. Popper et al. (23) observed a similar difference in a sex worker cohort in Dakar, Senegal, in which the differences in viral loads between HIV-1- and HIV-2-infected individuals persisted throughout the infection. Likewise, in our patients with AIDS, the HIV-2 viral load was lower than the HIV-1 viral load, in keeping with the different rates of clinical progression (19). The first longitudinal cohort studies indicated that the baseline HIV-2 RNA level and the CD4+-cell count could be predictive of the decline in the numbers of these cells (3).
We observed a wide range of proviral DNA values. Viral RNA and DNA were both detected in patients at CDC stage C, and the RNA and DNA loads correlated with each other. Discordant results on the correlation between RNA and DNA viral loads have been reported previously (3, 4, 22). The value of the HIV-2 proviral DNA load as a clinical marker remains to be demonstrated, but its correlation with the plasma RNA load indicates that it might be useful when the latter is undetectable. As Popper et al. (22) reported previously, the lack of a strong correlation between the HIV-2 proviral load and the different clinical stages indicates that the increase in replication is linked to an increase in provirus expression.
The primers and probes used for the real-time PCR can be adapted to all potential situations encountered in the field. The moderate cost and feasibility of this approach make it suitable for use in both industrialized and developing countries. As in HIV-1 infection, we found a correlation between the CD4+-cell count, the clinical stage, and the plasma RNA level. The last factor seems to be a good indicator of clinical status, reflecting both immune status and clinical stage, making it the marker of choice for prospective follow-up and monitoring of treatment. In contrast, while the proviral DNA level correlates with the CD4+-cell count, it does not correlate with the clinical status. Longitudinal studies of prospective cohorts are required to determine the predictive values of these two markers.
Acknowledgments
This work was supported by the French National Agency for AIDS Research.
REFERENCES
- 1.Anderson, S., H. Norrgren, Z. Da Silva, A. Biague, S. Bamba, S. Kwok, C. Christopherson, G. Biberfeld, and J. Albert. 2000. Plasma viral load in HIV-1 and HIV-2 singly and dually infected individuals in Guinea-Bissau, West Africa. Arch. Intern. Med. 160:3286-3293. [DOI] [PubMed] [Google Scholar]
- 2.Ariyoshi, K., N. Berry, A. Wilkins, D. Ricard, P. Aaby, A. Naucler, P. T. Ngom, O. Jobe, S. Jaffar, F. Dias, R. S. Tedder, and H. A. Whittle. 1996. Community-based study of human immunodeficiency virus type 2 provirus load in rural village in West Africa. J. Infect. Dis. 173:245-248. [DOI] [PubMed] [Google Scholar]
- 3.Ariyoshi, K., S. Jaffar, A. S. Alabi, N. Berry, M. Schim van der Loeff, S. Sabally, P. T. N Gom, T. Corrah, R. Tedder, and H. Whittle. 2000. Plasma RNA viral load predicts the rate of CD4 cell decline and death in HIV-2-infected patients in West Africa. AIDS 14:339-344. [DOI] [PubMed] [Google Scholar]
- 4.Berry, N., K. Ariyoshi, S. Jaffar, S. Sabally, T. Corrah, R. S. Tedder, and H. A. Whittle. 1998. Low peripheral blood viral HIV-2 RNA in individuals with high CD4+ percentage differentiates HIV-2 from HIV-1 infection. J. Hum. Virol. 1:457-468. [PubMed] [Google Scholar]
- 5.Berry, N., K. Aryoshi, O. Jobe, P. T. Ngum, T. Corrah, A. Wilkins, H. Whittle, and R. Tedder. 1994. HIV type 2 proviral load measured by quantitative polymerase chain reaction correlates with CD4+ lymphopenia in HIV type 2 infected individuals. AIDS Res. Hum. Retrovir. 10:1031-1037. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control. 1992. 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescent and adults. Morb. Mortal. Wkly. Rep. 41:1-19. [PubMed] [Google Scholar]
- 7.Chen, Z., A. Luckay, D. L. Sodora, P. Telfer, P. Reed, A. Gettie, J. M. Kanu, R. F. Sadek, J. Yee, D. D. Ho, L. Zhang, and P. A. Marx. 1997. Human immunodeficiency virus type 2 (HIV-2) seroprevalence and characterization of a distinct HIV-2 genetic subtype from the natural range of simian immunodeficiency virus-infected sooty mangabeys. J. Virol. 71:3953-3960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen, Z., P. Telfer, A. Gettie, P. Reed, L. Zhang, D. D. Ho, and P. A. Marx. 1996. Genetic characterization of new West African simian immunodeficiency virus SIVsm: geographic clustering of household-derived SIV strains with human immunodeficiency virus type 2 subtypes and genetically diverse viruses from a single feral sooty mangabey troop. J. Virol. 70:3617-3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Damond, F., C. Apetrei, D. L. Robertson, S. Souquière, A. Leprêtre, S. Matheron, F. Brun-Vézinet, and F. Simon. 2001. Variability of human immunodeficiency virus type 2 infecting patients living in France. Virology 280:19-30. [DOI] [PubMed] [Google Scholar]
- 10.Damond, F., D. Descamps, I. Farfara, J. N. Telles, S. Pueyo, P. Campa, A. Lepretre, S. Matheron, F. Brun-Vézinet, and F. Simon. 2001. Quantification of HIV-2 subtype A and B proviral load using real-time PCR. J. Clin. Microbiol. 39:4264-4268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Damond, F., I. Loussert-Ajaka, C. Apetrei, D. Descamps, S. Souquière, A. Leprêtre, S. Matheron, F. Brun-Vézinet, and F. Simon. 1998. Highly sensitive method for amplification of human immunodeficiency virus type 2 DNA. J. Clin. Microbiol. 36:809-811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Cock, K., and F. Brun-Vézinet. 1989. Epidemiology of HIV-2 infection. AIDS 3(Suppl. 1):S89-S95. [DOI] [PubMed] [Google Scholar]
- 13.Gao, F., L. Yue, D. L. Robertson, S. C. Hill, H. Hui, R. J. Biggar, A. E. Neequaye, T. M. Whelan, D. D. Ho, G. M. Shaw, P. M. Sharp, and B. H. Hahn. 1994. Genetic diversity of human immunodeficiency virus type 2: evidence for distinct sequence subtypes with differences in virus biology. J. Virol. 68:7433-7447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gomes, P., N. C. Taveira, J. M. Pereira, F. Antunes, M. O. Ferreira, and M. H. Lourenco. 1999. Quantitation of human immunodeficiency virus type 2 DNA in peripheral blood mononuclear cells by using a quantitative-competitive PCR assay. J. Clin. Microbiol. 37:453-456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kanki, P. J., K. U. Travers, S. Mboup, C. C. Hsieh, R. G. Marlink, A. Guèye-Ndiaye, T. Siby, I. Thior, M. Hernandezavila. J. L. Sankale, I. Ndoye, and M. E. Essex. 1994. Slower heterosexual spread of HIV-2 than HIV-1. Lancet 343:943-946. [DOI] [PubMed] [Google Scholar]
- 16.Marlink, R. 1996. Lessons from the second virus, HIV-2. AIDS 10:689-699. [DOI] [PubMed] [Google Scholar]
- 17.Marlink, R. G., D. Ricard, S. Mboup, P. J. Kanki, J. L. Romet-Lemonne, I. N. Doye, K. Diop, M. A. Simpson, F. Greco, M. J. Chou, V. Degruttola, C. C. Hsieh, C. Boye, F. Barin, F. Denis, M. F. McLane, and M. Essex. 1988. Clinical, hematologic and immunologic cross-sectionnal evaluation of individuals exposed to human immunodeficiency virus type 2. AIDS Res. Hum. Retrovir. 2:137-148. [DOI] [PubMed] [Google Scholar]
- 18.Matheron, S., C. Courpotin, F. Simon, H. Di Maria, S. Balloul, S. Bartzack, D. Dormont, F. Brun-Vézinet, A. G. Saimot, and J. P. Coulaud. 1990. Vertical transmission of HIV-2. Lancet 335:1103-1104. [DOI] [PubMed] [Google Scholar]
- 19.Matheron, S., G. Sassi, F. Simon, R. Olivares, J. P. Coulaud, and F. Brun-Vézinet. 1997. HIV-2 versus HIV-1 AIDS in African patients living in Paris. AIDS 11:934-936. [PubMed] [Google Scholar]
- 20.Myers, G., B. Foley, J. W. Mellors, B. Korber, K. T. Jeang, and S. Wain-Hobson. 1996. Human retroviruses and AIDS 1996. A compilation and analysis of nucleic acid and amino-acid sequences. Los Alamos National Laboratory, Los Alamos, N.M.
- 21.Norrgren, H., S. Marquina, T. Leitner, P. Aaby, M. Melbye, A. G. Poulsen, O. Larsen, F. Dias, D. Escanilla, S. Andersson, J. Albert, and A. Naucler. 1997. HIV-2 genetic variation and DNA load in asymptomatic carriers and AIDS cases in Guinea-Bissau. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 16:31-38. [DOI] [PubMed] [Google Scholar]
- 22.Popper, S. J., A. D. Sarr, A. Gueye-Ndiaye, S. Mboup, M. E. Essex, and P. J. Kanki. 2000. Low plasma human immunodeficiency virus type 2 viral load is independent of proviral load: low virus production in vivo. J. Virol. 74:1554-1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Popper, S. J., A. D. Sarr, K. U. Travers, A. Gueye-Ndiaye, S. Mboup, M. E. Essex, and P. J. Kanki. 1999. Lower human immunodeficiency virus (HIV) type 2 viral load reflects the difference in pathogenicity of HIV-1 and HIV-2. J. Infect. Dis. 180:1116-1121. [DOI] [PubMed] [Google Scholar]
- 24.Schutten, M., B. Van den Hoogen, M. E van der Ende, R. A. Gruters, A. D. M. E. Osterhaus, and H. G. M. Niesters. 2000. Development of a real-time quantitative RT-PCR for the detection of HIV-2 RNA in plasma. J. Virol. Methods 88:81-87. [DOI] [PubMed] [Google Scholar]
- 25.Simon, F., S. Matheron, C. Tamalet, I. Loussert-Ajaka, S. Bartzack, J. M. Pépin, C. Dever, E. Gamba, C. Elbim, J. A. Gastaut, A. G. Saimot, and F. Brun-Vézinet. 1993. Cellular and plasma viral load in patients infected with HIV-2. AIDS 7:1411-1417. [DOI] [PubMed] [Google Scholar]
- 26.Soriano, V., P. Gomes, W. Heneine, A. Holguin, M. Doruana, R. Antunes, K. Mansinho, W. M. Switzer, C. Araujo, V. Shanmugam, H. Lourenço, J. Gonzalez-Lahoz, and F. Antunes. 2000. Human immunodeficiency virus type 2 in Portugal: clinical spectrum, circulating subtypes, virus isolation and plasma viral load. J. Med. Virol. 6:111-116. [PubMed] [Google Scholar]
- 27.Wittwer, C. T., K. M. Ririe, R. V. Andrew, D. A. David, R. A. Gundry, and U. J. Balis. 1997. The Light Cycler: a microvolume multisample fluorimeter with rapid temperature control. BioTechniques 22:176-181. [DOI] [PubMed] [Google Scholar]