Abstract
Haemophilus ducreyi is the etiologic agent of chancroid, a sexually transmitted disease that increases the rate of transmission of human immunodeficiency virus. Chancroid ulcerations are difficult to distinguish from those produced by syphilis and herpes. Diagnosis based solely on clinical grounds is inaccurate, and culture is insensitive. Highly sensitive PCR has largely superseded culture as the preferred method of laboratory diagnosis; however, neither culture nor PCR is feasible where chancroid is endemic. We developed a rapid (15-min) diagnostic test based on monoclonal antibodies (MAbs) to the hemoglobin receptor of H. ducreyi, HgbA. This outer membrane protein is conserved in all strains of H. ducreyi tested and is required for the establishment of experimental human infection. MAbs to HgbA were generated and tested for cross-reactivity against a panel of geographically diverse strains. Three MAbs were found to be unique and noncompetitive and bound to all strains of H. ducreyi tested. Using an immunochromatography format, we evaluated the sensitivity and specificity of the test using geographically diverse strains of H. ducreyi, other Haemophilus strains, and other bacteria known to superinfect genital ulcers. All H. ducreyi strains were positive, and all other bacteria were negative, resulting in a specificity of 100%. The minimum number of CFU of H. ducreyi detected was 2 × 106 CFU, and the minimum amount of purified HgbA protein detected was 8.5 ng. Although this level of sensitivity may not be sufficient to detect H. ducreyi in all clinical specimens, further work to increase the sensitivity could potentially make this a valuable bedside tool in areas where chancroid is endemic.
Chancroid, caused by the gram-negative bacterium Haemophilus ducreyi (for reviews, see references 2, 47, and 60), is one of the sexually transmitted genital ulcer diseases. Although outbreaks are more prevalent in developing countries, sporadic outbreaks have occurred in the United States. Up to 50% of patients visiting sexually transmitted disease clinics in sub-Saharan Africa may have chancroid (12, 23). Additionally, it is an independent risk factor for the heterosexual transmission of human immunodeficiency virus (HIV) (34; F. A. Plummer, M. A. Wainberg, P. Plourde, P. Jessamine, L. J. DCosta, I. A. Wamola, and A. R. Ronald, Letter, J. Infect. Dis. 161:810-811, 1990); therefore, the interest in chancroid has recently intensified. In the last 10 years, several laboratories have begun to define the molecular biology of this pathogen. The study of virulence factors, the body's immune response to infection, and potential vaccines for H. ducreyi have all made significant contributions to the understanding of this bacterium (3-10, 13-16, 22, 24, 26-28, 32, 33, 36, 38, 39, 46, 50, 53, 54, 59, 61, 64). With the genome sequence completed, the process of annotation will, undoubtedly, further accelerate progress on this strictly human pathogen (www.microbial-pathogenesis.org).
At present, there are several laboratory methods for the diagnosis of chancroid, including Gram stain, culture, and PCR. Various rates of sensitivity have been reported for the Gram stain, all approximating 50% (51, 57). The presence of other organisms in the polymicrobial chancroid ulcer reduces the specificity of the Gram stain, as these bacteria may be confused with H. ducreyi morphologically, especially by inexperienced personnel. Thus, the Gram stain has little clinical utility in the diagnosis of chancroid.
Before the advent of reliable PCR methods, culture was the widely accepted standard of laboratory diagnosis (43, 57). H. ducreyi is a fastidious bacterium that has an absolute requirement for heme because it lacks a heme biosynthetic pathway (60); therefore, use of a special medium is required. Many strains require the addition of fetal bovine serum for growth. Furthermore, H. ducreyi requires a CO2 or microaerophilic atmosphere, and its optimal growth temperature (33°C) is lower than those of most human pathogens, mandating an additional dedicated incubator if it is to be routinely cultured. On primary isolation, small colonies of H. ducreyi usually appear after 2 to 3 days of incubation. Because ulcers are often secondarily contaminated with other more rapidly growing bacteria, cultures can be lost due to contamination (41). Culture is relatively insensitive, with the sensitivity ranging between 56 and 84% (25). The use of more than one primary isolation medium improves isolation rates, but not substantially (25). Culture is thought to be 100% specific; however, traditional biochemical identification of a presumptive H. ducreyi isolate is problematic due to its relative biochemical inertness. Furthermore, H. ducreyi fails to grow on standard biochemical media used to test other bacteria. Thus, for culturing, optimum sensitivity and specificity require microbiologists with specific training and experience with H. ducreyi. Such personnel are lacking both in underdeveloped areas where the organism is endemic and in developed countries such as the United States where it is not endemic. Two advantages that culture offers are the abilities to perform sensitivity testing of isolates and strain typing for epidemiological studies. Despite its limitations, culture will remain a valuable tool in the future.
As technology has improved, PCR has become the most sensitive method for the diagnosis of chancroid (19, 40, 44, 58; K. A. Orle et al., Abstr. 94th Gen. Meet. Am. Soc. Microbiol. 1995, abstr. C-247, p. 43, 1995). It has a sensitivity approaching 100%. Although some surmise that false-positive results occur, this has been very difficult to prove. To date, PCR studies are done in laboratories remote from the clinic setting and therefore are not useful for establishing a diagnosis in a time frame that would benefit patient treatment. At present, multiplex PCR is not available for commercial use; rather, it is used for limited research purposes only.
The need to maintain trained personnel to perform and interpret cultures and PCR in the resource-poor settings where chancroid is endemic makes these tests economically prohibitive. Furthermore, the costs of media, equipment, and reagents for culture and PCR are considerable. Because of these limitations, culture and PCR in their current formats are not suitable for on-site, immediate detection of H. ducreyi in clinical specimens in areas where the organism is endemic. Therefore, a stable, inexpensive, and rapid test that is simple to perform and interpret at the bedside would be a valuable tool in chancroid control, so long as it maintains its sensitivity and specificity.
The immunochromatography (IC) test described in this report is based on novel monoclonal antibodies (MAbs) to the hemoglobin receptor, HgbA, which is an abundant outer membrane protein (OMP) that is required for the acquisition of heme from hemoglobin (26, 27; C. Elkins, unpublished data, 1999). The ability of H. ducreyi to obtain heme from hemoglobin is required for pustule formation. An isogenic hgbA mutant which is unable to utilize hemoglobin was unable to form pustules in a human experimental model of infection (5). As HgbA is conserved in geographically diverse isolates, we developed an IC test based on these MAbs to this protein. In the present report, we describe progress in the initial development of a rapid diagnostic test for H. ducreyi.
MATERIALS AND METHODS
Strains and media.
All strains are described in Table 1. We used the extensively characterized strain H. ducreyi 35000 for most of these studies. For routine daily growth, H. ducreyi was maintained on chocolate agar plates prepared by the University of North Carolina Hospitals Media Laboratory. The basal medium for chocolate agar was gonococcal medium base containing 1% IsoVitaleX and 1% autoclaved hemoglobin. Outer membranes were isolated as described previously (26). For growth of H. ducreyi under heme-limiting conditions, we used gonococcal medium broth with IsoVitaleX and 1 μg of hemin per ml for all strains except hgbA mutant FX504 (27). For FX504 grown under heme-limiting conditions, we used 5 μg of hemin per ml. Neisseria gonorrhoeae (17) and Haemophilus influenzae (21) (including typeable, nontypeable, and genital isolates of biotype IV) were grown on gonococcal medium base agar plates with IsoVitaleX, 50 μM desferal, and 100 μg of human hemoglobin per ml (18) under heme- and iron-limiting conditions. Hemoglobin receptors of N. gonorrhoeae and H. influenzae are subject to on-off phase variation. In order to select for the hemoglobin receptor on-phase variants, these were grown on hemoglobin plates containing the iron chelator desferal. Since this medium contains hemoglobin (heme) as the sole source of iron, hemoglobin receptor off-phase variants do not grow on it, thus ensuring that these bacteria would express a hemoglobin receptor. Staphylococcus aureus ATCC 25923, Enterococcus faecalis ATCC 29212, Escherichia coli ATCC 29522, Klebsiella pneumoniae ATCC 13883, Enterobacter aerogenes ATCC 13048, and Bacteroides fragilis ATCC 25283 were all grown on 5% tryptic soy sheep blood agar plates.
TABLE 1.
Strains evaluated in the present study
| Species and isolate no. | Strain | Geographical site, yr of isolation or typea | Reference or sourceb |
|---|---|---|---|
| H. ducreyi | |||
| 8 | HD 105 | VDRL, Atlanta, Ga. 1962 | 26 |
| 18 | NOHD | New Orleans, La., 1989-1992 | 26 |
| 23 | NOHD | New Orleans, La., 1989-1992 | 26 |
| 26 | NOHD | New Orleans, La., 1989-1992 | 26 |
| 32 | NOHD | New Orleans, La., 1989-1992 | 26 |
| 34 | HMC49 | Jackson, Miss., 1994 or 1995 | 26 |
| 48 | HMC47 | Seattle, Wash., 1995 | 26 |
| 49 | HMC86 | Seattle, Wash., 1992 | 26 |
| 50 | HMC87 | Seattle, Wash., 1995 | 26 |
| 52 | HMC89 | Seattle, Wash., 1992 | 26 |
| 53 | LA225 | Los Angeles, Calif., 1982 | 26 |
| 54 | LA228R | Los Angeles, Calif., 1982 | 26 |
| 56 | V 180 | Rwanda, 1991 | 26 |
| 60 | HMC39 | England, 1982 | 26 |
| 62 | HMC54 | Dominican Republic, 1995 | 26 |
| 64 | HMC56 | Dominican Republic, 1995 | 26 |
| 65 | HMC91 | Raleigh, N.C., 1996 | 26 |
| 68 | HMC90 | Florida, 1989 | 26 |
| 71 | HMC65 | Kenya, 1984 | 26 |
| 73 | CH2 | Thailand, 1985 | 26 |
| 77 | CH5 | Thailand, 1985 | 26 |
| 81 | ML067 | Kenya, 1985 | 26 |
| 88 | 6V | Atlanta, Ga. | 26 |
| 112 | HD342 | CDC | 26 |
| H. influenzae | |||
| 1861 | Type A | Peter Gilligan | |
| Type B | Peter Gilligan | ||
| 1094 | Type C | Peter Gilligan | |
| 15992 | Untypeable | Peter Gilligan | |
| 12575 | Untypeable | Peter Gilligan | |
| 15504 | Untypeable | Peter Gilligan | |
| 15993 | Untypeable | Peter Gilligan | |
| 15991 | Untypeable | Peter Gilligan | |
| 597 | Type IV genital strain | Tim Murphy | |
| 756 | Type IV genital strain | Tim Murphy | |
| 799 | Type IV genital strain | Tim Murphy | |
| 1595 | Type IV genital strain | Tim Murphy | |
| 1610 | Type IV genital strain | Tim Murphy | |
| 6351 | Type IV genital strain | Tim Murphy | |
| S. aureus | ATCC 25923 | Peter Gilligan | |
| E. coli | ATCC 29522 | Peter Gilligan | |
| N. gonorrhoeae | |||
| FA1090 | P. Fred Sparling | ||
| FA19 | P. Fred Sparling | ||
| E. faecalis | ATCC 29212 | Peter Gilligan | |
| K. pneumoniae | ATCC 13883 | Peter Gilligan | |
| E. aerogenes | ATCC 13048 | Peter Gilligan | |
| B. fragilis | ATCC 25283 | Peter Gilligan |
VDRL, Venereal Disease Research Laboratory; CDC, Centers for Disease Control and Prevention.
Peter Gilligan, University of North Carolina; Tim Murphy, Buffalo, N.Y.; P. Fred Sparling, University of North Carolina.
Production of MAbs.
Female BALB/c mice (Charles River, Wilmington, Mass.) were used as spleen donors for all fusions. HgbA was purified by affinity purification on a hemoglobin agarose column as described previously (26), with the following modification: HgbA was eluted from the agarose column with a low-pH buffer in 1% octyl glucoside. HgbA liposomes were prepared as described previously (29). HgbA liposome immunogen (5 μg) was injected subcutaneously without adjuvant. Soluble HgbA immunogen (25 μg in 1% octyl glucoside) was emulsified in Titermax adjuvant (CytRx Corporation, Norcross, Ga.) and injected subcutaneously. Further intraperitoneal injections were given until hyperimmunization was achieved. Hybridoma 1.51 was generated from a fusion donor immunized with purified soluble H. ducreyi hemoglobin receptor in octyl glucoside. Three hybridomas (hybridomas 4.23, 4.65, and 6.18) were generated from a mouse immunized with liposomes containing HgbA. Sarkosyl-insoluble OMPs from strain 35000 grown under heme-limiting conditions were used to immunize the fusion mouse that generated hybridoma 9.76.
Standard polyethylene glycol (American Type Culture Collection, Manassas, Va.)-mediated fusion techniques were used at the North Carolina State University Hybridoma Facility to generate the hybridomas used in this study. Briefly, equal numbers of spleen cells were fused with the P3X63-Ag8.653 myeloma by using 40% polyethylene glycol. Standard selection with hypoxanthine-aminopterin-thymidine (Sigma, St. Louis, Mo.) was used to generate hybridoma growth. Enzyme-linked immunosorbent assays (ELISAs) and dot blot screening assays were used to detect antigen-specific antibody during the fusion and cloning procedures. Each hybridoma used in this study was cloned by limiting dilution, which was repeated three times with one cell per well. During cloning procedures, wells with antigen-specific antibody and a single isolated colony were selected for further development. Static exhausted supernatant was generated from each hybridoma and used for the various procedures described herein. Commercially available radial immunodiffusion plates (Binding Site, San Diego, Calif.) were used to isotype and quantitate static exhausted supernatants from established murine hybridomas.
Purification of MAbs.
Ascites was produced by standard techniques (37). MAbs were purified on protein G columns (Pierce) according to the directions of the manufacturer, followed by dialysis against phosphate-buffered saline (PBS). Sodium dodecyl sulfate (SDS)-polyacrylamide gels stained with Coomassie blue indicated a single immunoglobulin G (IgG) band of approximately 150 kDa under nonreducing conditions (data not shown). The protein concentration was determined with a bicinchoninic acid assay kit from Pierce and was confirmed by Coomassie blue staining of SDS-polyacrylamide gels by comparison with bovine serum albumin (BSA) standards.
Immunoprecipitation.
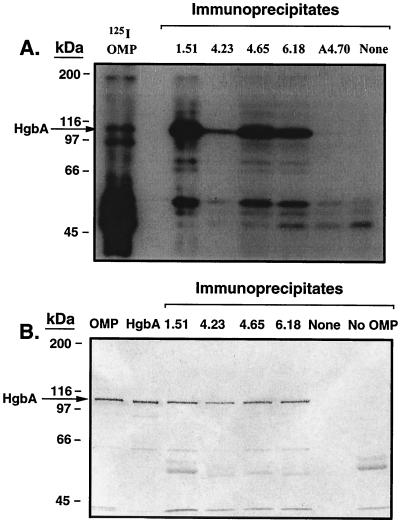
We followed the presolubilized immunoprecipitation method described by Gulig and Hansen (35), with the following modifications. OMPs were obtained from strain 35000 grown under heme-limiting conditions. For experiments with iodinated OMP, 100 μg of OMP was iodinated as described below for MAbs. OMP (100 μg of iodinated OMP or 250 μg of unlabeled OMP) was solubilized with 1 ml of 2% Zwittergent 3,14 (Zw 3,14; Calbiochem-Novabiochem, La Jolla, Calif.) in TEN (50 mM Tris, 100 mM NaCl, 5 mM EDTA [pH 8.0]) (Zw/TEN) for 2 h at 37°C. Insoluble material was removed by centrifugation at 14,000 × g for 10 min. Each MAb was immobilized by mixing 100 μl of a 50% protein G agarose slurry with 4 ml of each hybridoma supernatant, whose pH had been adjusted with 1 ml of 1 M sodium acetate (pH 5.0). After 1 h, the protein G agarose with the attached MAb was washed three times with 0.1 M sodium acetate (pH 5.0) and finally one time with Zw/TEN. A total of 100 μl (containing 10 μg of iodinated OMP or 25 μg of unlabeled OMP) of Zw/TEN-soluble OMP was diluted with 400 μl of TEN to reduce the detergent concentration. A total of 40 μl of a 50% slurry of each protein G-immobilized MAb was added and rocked overnight at 4°C. The slurry was washed three times in 0.1% Zw/TEN and finally one time in TEN, changing tubes midway. After 75 μl of reducing Laemmli sample buffer was added, each sample was boiled for 5 min and loaded onto SDS-polyacrylamide gels. For experiments in which iodinated OMP was immunoprecipitated (see Fig. 1A), the gel was stained with Coomassie blue, dried, and subjected to autoradiography at −20°C with an intensifying screen. For experiments with unlabeled OMPs (see Fig. 1B), SDS-polyacrylamide gels were transferred to nitrocellulose (NC) and Western blotting was performed with affinity-purified anti-HgbA synthetic peptide IgG as described previously (26).
FIG. 1.
Immunoprecipitation of HgbA by anti-HgbA MAbs. (A) Zwittergent-solubilized iodinated OMP was incubated with protein G agarose containing immobilized MAb. Unbound material was removed by washing. The samples were boiled in Laemmli sample buffer and subjected to SDS-PAGE and Coomassie blue staining. The gel was then dried and subjected to autoradiography. Lanes, from left to right: 125I OMP, iodinated OMPs from strain 35000 (100 μg); empty lane; 1.51, 4.23, 4.65, and 6.18, anti-HgbA MAb immunoprecipitates; A4.70, negative control MAb immunoprecipitate to RTX toxin (repeat toxin) of N. meningitidis; None, immunoprecipitation performed in the absence of MAb (negative control). (B). Zwittergent-solubilized unlabeled OMP was subjected to immunoprecipitation as described above and in the text. After SDS-PAGE the gel was subjected to Western blotting with affinity-purified anti-HgbA synthetic peptide IgG. Anti-rabbit alkaline phosphatase was used as the secondary antibody. 5-Bromo-4-chloro-3-indolylphosphate-nitroblue tetrazolium was used for detection. Lanes: OMP, strain 35000 OMP (5 μg); purified native HgbA from strain 35000 (200 ng); 1.51, 4.23, 4.65, and 6.18, MAb immunoprecipitates obtained with the OMP antigen; None, immunoprecipitation done in the absence of an MAb; No OMP, MAb 1.51 immunoprecipitation done in the absence of OMP antigen. Immunoprecipitate lanes contained 20 μl (20% of total) per lane.
Capture ELISA.
None of the MAbs recognized HgbA under the denaturing conditions used for Western blotting (data not shown). In order to evaluate cross-reactivity, we developed a capture ELISA with the MAbs and a panel of H. ducreyi strains. Each MAb (purified IgG, 400 ng/well in 0.1 M carbonate buffer [pH 9.6]) was coated onto Microtiter ProBond ELISA plates (Falcon), and the plates were incubated at 4°C overnight. The plates were blocked for 1 h with 2% BSA in PBS. Total cellular proteins from Zw 3,14-solubilized H. ducreyi strains grown under heme-limiting conditions (108 CFU, about 25 μg of protein) were added to each well, and the plates were incubated overnight at 4°C. The plates were washed three times with PBS-0.05% Tween, an affinity-purified anti-HgbA peptide IgG (1:1,000) developed in rabbits (26) was added to each well, and the plates were incubated for 2 h at room temperature (RT). The plates were washed, and an anti-rabbit alkaline phosphatase-conjugated secondary antibody (A8702 [1:2,000]; Sigma) was added and allowed to incubate for 1 h at RT. The plates were washed and 100 μl of 1 mg of para-nitrophenol phosphate (N-2765; Sigma) per ml was added for detection. The plates were read after a 1-h incubation at 37°C. The positive control was H. ducreyi strain 35000, and the negative control was hgbA mutant FX504. The ELISA plates were blanked against no-antigen control wells.
Competition between MAbs.
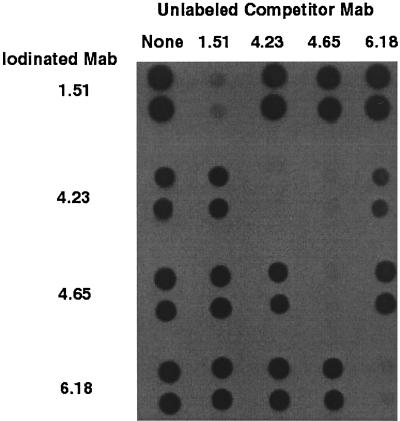
IgG was purified from hybridoma supernatants with protein G according to the instructions of the manufacturer (Pierce). MAbs were iodinated with Iodogen tubes (Pierce) according to the directions of the manufacturers. A total of 2 mCi of sodium iodide (20 μl of IMS-30 [Amersham] diluted in 200 μl of PBS) was activated for 6 min at RT. A total of 50 μl of activated iodine (0.5 mCi) was added to 100 μg of each purified MAb (100 μl of each purified MAb at 1 mg/ml in PBS), and the mixture was incubated for 7 min. The reaction was quenched with 50 μl of saturated tyrosine for 5 min. Each MAb was then desalted on a Bio-Gel P6 column (Bio-Rad). For competition experiments, purified native HgbA (nHgbA) (200 ng/dot) was immobilized onto NC membranes with a dot blot apparatus (Schleicher & Schuell). The membranes were blocked with 2% BSA for 30 min. Dots were cut out and placed into a 24-well tissue culture dish. A total of 2.5 ml of 2% BSA in PBS containing iodinated MAb, with or without a cold MAb competitor, was added, and the mixture was gently rotated for 2 h. The molar ratio of unlabeled competitor MAb with iodinated MAb was 50:1. The antibodies were removed, and the dots were washed four times with Tris-buffered saline with 0.05% Tween. The dots were taped to paper and exposed overnight to film by using an intensifying screen at −70°C. Each assay was done in duplicate on 2 separate days.
Preparation of IC test strips.
MAbs 1.51 and 4.65 were purified from ascitic fluid by standard affinity chromatography as described above. To develop the rapid assay, 40 nm colloidal gold particles were generated by the reduction of gold chloride with citric acid. After the particles were stabilized, the MAbs were optimally conjugated and assessed as signal reagents. The unlabeled MAbs were also immobilized to NC membranes (Millipore Corporation, Bedford, Mass.) for assessment as capture reagents. They were then compared for their reaction intensities with H. ducreyi and relative lack of reactivity with the H. influenzae controls. The best combination was MAb 1.51 conjugated to colloidal gold and MAb 4.65 immobilized to the NC membrane. An optimal concentration of MAb 4.65 was then applied in thin lines onto strips of NC (25 by 300 mm). A second line of goat anti-mouse IgG (Dako, Carpinteria, Calif.) was sprayed onto the NC at a 5-mm distance from the first line to serve as a procedural control. To assemble the strips, materials including the NC membrane, sample application pad (Ahlstrom Technical Specialties, Inc., Mt. Holly Park, Pa.), and sample absorption pad (Whatman, Newton, Mass.) were attached as slightly overlapping strips (25 by 300 mm) to plastic cards (75 by 300 mm; G&L Precision Diecutting, San Jose, Calif.), from which 5-mm strips were cut. The strips are relatively stable if they remain thoroughly desiccated and can be stored with dehumidification in cylinders or in foil pouches at ambient temperatures until use.
Testing of IC test strips.
All 26 H. ducreyi strains were grown overnight on chocolate agar and suspended in PBS to an optical density at 600 nm of 0.2 (approximately 108 CFU/ml). A total of 100 μl of this suspension was added to 100 μl of detergent buffer (2% Triton X-100, 0.1% SDS, and 200 mM NaCl in Tris-EDTA [pH 8.0]). The use of Zw 3,14 in this system produced false-positive results, precluding its use. Triton X-100, like Zw 3,14, preserves the native structure of HgbA (data not shown). Test strips were added, and chromatography was allowed to proceed for 15 min at RT. The test strips were observed for the presence of pink or purple lines. S. aureus, E. coli, E. faecalis, K. pneumoniae, E. aerogenes, B. fragilis, and H. influenzae (including typeable, nontypeable, and biotype IV genital isolates) were also tested, as were the two laboratory strains of N. gonorrhoeae, FA19 and FA1090. A total of 14 strains of H. influenzae were used, including 3 typeable, 5 untypeable, and 6 biotype IV genital strains. All the bacteria, including the H. ducreyi laboratory strains and the non-H. ducreyi bacteria, were tested once. The sensitivity of the strips was determined by using fivefold dilutions of H. ducreyi strain 35000. The result was determined qualitatively after the assay was allowed to run for 15 min at RT. The appearance of two lines was interpreted as a positive result, whereas the appearance of only one line (the procedural control) was interpreted as a negative yet valid test result.
RESULTS
Background.
Previously, we showed that we could solubilize and purify HgbA antigen from H. ducreyi under nondenaturing conditions using the detergent Zw 3,14 and hemoglobin agarose, respectively (26). Ulcer samples are generally obtained with swabs, and this suggested that we could release the HgbA antigen from swabs using a detergent. Therefore, we used native HgbA (nHgbA), purified under nondenaturing conditions, as both the immunogen and the antigen in a screening ELISA for the development of MAbs. We hypothesized that any MAbs to native conformational epitopes on HgbA would recognize HgbA released from swabs under nondenaturing conditions and could then be detected by an IC format.
Development and confirmation of MAbs.
A total of 2,400 wells from three fusions were screened by ELISA with purified HgbA as the antigen. After subcloning and limiting dilutions, five stable clones were obtained. Four MAb clones (clones 1.51, 4.23, 4.65, and 6.18) were studied in detail. MAb 1.51 was of isotype IgG2b, and MAbs 4.23, 4.65, and 6.18 were of isotype IgG1. One clone (clone 9.76) was an IgM isotype and was not further studied. After limiting dilution, we confirmed the specificity of each MAb beyond the previous ELISA results, in case the MAbs were reacting to a minor contaminant in the HgbA preparation used to coat the ELISA wells. None of the MAbs reacted in Western blots, precluding the use of this technique to test the specificities of the MAbs (data not shown). MAbs were therefore tested for binding to whole cells of strain 35000 and to its isogenic hgbA mutant, strain FX504, but again, no binding was observed (data not shown). We surmised that the MAbs were binding to conformational epitopes that were not surface exposed on intact H. ducreyi.
Because the MAbs recognized conformation-dependent epitopes that were stable when HgbA was solubilized in Zw 3,14 in the ELISA format, we performed immunoprecipitation experiments using Zw 3,14-solubilized OMP (Fig. 1). As seen in Fig. 1A, by using iodinated OMP, each anti-HgbA MAb was able to immunoprecipitate a 100-kDa protein. This 100-kDa band was much enriched compared to its abundance in the starting OMP material. Neither an irrelevant MAb (MAb A4.70) (55) nor the no-MAb control (None in Fig. 1A) produced signals at the 100-kDa position. Minor nonspecific binding of other more abundant OMP bands was observed in all lanes whether or not a MAb was present.
In order to confirm that the 100-kDa protein was HgbA, we performed additional immunoprecipitation experiments using unlabeled OMP as the antigen. Figure 1B shows a Western blot in which we used an antibody prepared against a synthetic peptide of HgbA that recognizes the denatured form of HgbA (26). Each MAb immunoprecipitated a 100-kDa protein that comigrated with purified HgbA and that was recognized by the antipeptide HgbA antiserum. Immunoprecipitation done in the absence of MAb yielded no bands at this position. Immunoprecipitation by MAb 1.51 in the absence of OMP yielded smaller weakly reactive bands that presumably were of IgG origin. HgbA was recognized only in the OMP antigen lane, the purified HgbA lane, and the appropriate four MAb immunoprecipitate lanes. One limitation of immunoprecipitation is the possibility of coprecipitation of complexes (35). It has been shown that antibodies to lipooligosaccharide (LOS) can immunoprecipitate proteins. LOS, however, does not label under the conditions that we used in this study. To test for reactivity to LOS we performed dot blots in which purified HgbA, proteinase K-digested purified HgbA (39a), or purified LOS (42, 63) was immobilized onto the NC. Each of the four MAbs bound to purified HgbA but not to proteinase K-treated HgbA or to the LOS (data not shown). A control LOS MAb (MAb 3E6) (50, 52) recognized the LOS only. We concluded that the MAbs specifically recognized HgbA, a conclusion that was reconfirmed in subsequent experiments (see below).
Use of capture ELISA.
We used a capture ELISA method to determine how broadly cross-reactive each MAb was to a geographically diverse panel of H. ducreyi isolates (Table 2). Twenty-six laboratory strains (24) were grown under heme-limiting conditions in order to induce expression of HgbA. All four of the MAbs tested recognized laboratory strain 35000, while none recognized FX504, the hgbA mutant. MAbs 1.51, 4.65, and 6.18 recognized all 26 strains. MAb 4.23, however, recognized only 25 of 26 strains tested, resulting in a lower mean value. MAb 4.23 did not recognize isolate 112. In the course of experiments designed to explain this discrepancy, we found that the deduced amino acid sequence of HgbA from isolate 112 contains residues that are different from those of hgbA in strain 35000 (Elkins, unpublished), including seven contiguous residues between amino acids 438 and 444. This variability most likely results in epitope alterations that prevent recognition of HgbA by MAb 4.23. These results, in general, confirm that the MAbs used for preparation of the IC strips are able to detect diverse strains of H. ducreyi by capture ELISA.
TABLE 2.
MAbs recognize all strains of H. ducreyi, as determined by capture ELISA
| MAb | Optical density
|
No. Positive/no. testedc | |||
|---|---|---|---|---|---|
| 35000a | FX504b | Meand | Ranged | ||
| 1.51 | 2.05 | −0.094 | 2.15 | 1.77-2.56 | 26/26 |
| 4.23 | 0.62 | 0.005 | 0.43 | 0.05-1.23 | 25/26e |
| 4.65 | 1.37 | −0.045 | 1.39 | 0.57-2.59 | 26/26 |
| 6.18 | 2.28 | 0.004 | 2.36 | 1.64-2.73 | 26/26 |
Positive control strain 35000.
Negative control strain FX504.
Threshold for a positive result, 0.005.
Mean and range for 26 experimental H. ducreyi strains.
Isolate 112 was the only strain not recognized by MAb 4.23.
MAb competition.
Most detection methods require a capture and detection MAb to bind to separate epitopes on the target antigen. We tested the four MAbs for binding to separate epitopes by a competitive binding assay (Fig. 2). Each MAb bound to the immobilized HgbA protein in the absence of competitor. Each MAb also competed for binding to itself. We found that MAb 4.65 could prevent the binding of MAb 4.23, but not vice versa. This suggested that MAbs 4.23 and 4.65 bound to the same epitope or to closely spaced epitopes and/or that the affinity of MAb 4.65 was greater than that of MAb 4.23. However, other MAb combinations were noncompetitive. On the basis of the broadly cross-reactive nature of the four MAbs and on our observation that three combinations were noncompetitive, we proceeded to test these MAb pairs in a first-generation format, IC.
FIG. 2.
Competition between anti-HgbA MAbs. Purified HgbA was immobilized onto an NC membrane (in duplicate) and probed with each of the iodinated MAbs in the absence (none) or presence of a 50-fold excess of each unlabeled MAb. After washing of the unbound MAbs, the dot blots were exposed to film to detect binding to HgbA by the labeled MAb.
Generation of IC test strips.
IC relies on the migration of soluble samples across the surface of an NC membrane. IC tests typically use a labeled antibody (often conjugated to colloidal gold) that binds to the target antigen in solution. The antigen-antibody complex migrates through the NC toward a second antibody, immobilized in a line across the strip, that captures this complex via a separate epitope on the antigen. When the immobilized antibody captures the antigen-detection antibody complex, a visible colored line is generated. Preliminary tests were done with various pairs of MAbs in the IC test strips to determine the best combination. These results revealed that use of MAb 1.51 as the detection MAb and MAb 4.65 conjugated to colloidal gold as the capture MAb gave the strongest signal. The use of more than one MAb for either detection or capture did not increase the sensitivity of the test (data not shown).
Once the IC test strips were generated, H. ducreyi strain 35000 and its isogenic hgbA mutant, strain FX504, were tested, and the results confirmed that the MAbs were directed against HgbA. A positive reaction was obtained with strain 35000 but not with strain FX504. These strains were used as controls in all subsequent experiments.
Testing of IC.
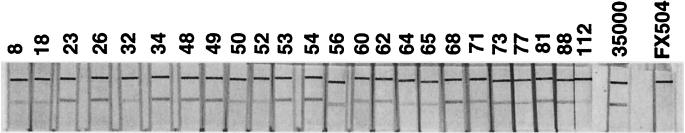
To examine the clinical utility of the IC test, we tested a broad range of bacterial isolates (Table 1). As a first step, we tested a diverse panel of laboratory-maintained H. ducreyi clinical isolates in order to determine how broadly reactive the MAbs were in the IC format. A total of 24 strains were tested, in addition to laboratory strain 35000 and hgbA mutant FX504. All strains gave a positive reaction (Fig. 3). Having established the broad reactivity of these IC strips against a diverse panel of H. ducreyi isolates, we next determined the specificity of the IC strips by testing their reactivity against other pathogenic bacteria species. We tested S. aureus and enteric gram-negative and anaerobic bacteria, including E. coli, E. faecalis, K. pneumoniae, E. aerogenes, and B. fragilis, in order to determine whether false-positive reactions might be obtained from other bacteria commonly associated with skin and bowel floras. In addition, hemoglobin receptor-expressing N. gonorrhoeae and H. influenzae strains were tested to assess the possibility that the MAbs might recognize other bacteria containing related TonB-dependent hemoglobin receptors. Finally, we tested the reactivity of the IC test against the related species H. influenzae. Because the species H. influenzae is a heterogeneous group, we tested both typeable and nontypeable clinical strains as well as isolates characterized as biotype IV genital strains (Table 1). In contrast to the reactivity of this test with all H. ducreyi strains, which were uniformly positive, the IC test results for all non-H. ducreyi bacteria were indistinguishable from the result for the negative control (strain FX504) (specificity, 100%).
FIG. 3.
Detection of HgbA in clinical isolates by an IC test. H. ducreyi cells (108 CFU) were solubilized in detergent buffer, and a test strip was placed into the test tube. After 15 min, the strips were observed for the presence of single or duplicate lines. The upper line is the positive control line that contains a capture antibody (goat anti-mouse IgG) for detection of colloidal gold-conjugated MAb 1.51. The lower line contains anti-HgbA capture MAb 4.65. The numbers above the lanes indicate the isolates described in Table 1; 35000, parent strain 35000 (positive control); FX504, hemoglobin receptor mutant (hgbA) in a strain 35000 background (negative control).
Having established the broad reactivity and specificity of the IC test strips, we determined the sensitivity of the IC test against purified HgbA protein and solubilized whole cells of strain 35000. With serial dilutions, the limit of detection of purified HgbA by this first iteration of the IC test was found to be 8.5 ng/ml. Similarly, we found that the test could consistently detect 2 × 106 CFU of H. ducreyi strain 35000 by limiting dilution. Taken together, these results indicate that the IC test has broad reactivity against a diverse panel of H. ducreyi strains, is highly specific for H. ducreyi when it is tested against other clinically relevant bacteria, and has moderate sensitivity in its present configuration.
DISCUSSION
In this study we demonstrate the use of novel MAbs in an IC format for the rapid, direct detection of H. ducreyi. Such a test could be useful in resource-poor countries where chancroid is endemic. These countries also have the highest prevalence of HIV infections (20, 62). Therefore, the transmission of HIV could potentially be decreased if chancroid were eliminated (1). IC tests have been developed for other bacterial antigens (11, 31) and have a number of advantages over conventional microbiological techniques, including ease of performance, stability in temperate climates, and comparatively low cost, among many others. In the case of H. ducreyi, the greatest advantage would be the rapidity of diagnosis of the etiology of the genital ulcer. In making a rapid diagnosis, prompt, appropriate treatment could be instituted, thereby hastening the time of curing the chancroid ulcer. In doing so, the spread of HIV could be diminished.
IC relies upon the capture of an antigen by a labeled MAb prepared against that specific antigen. A second immobilized MAb, directed against a separate antigenic epitope, allows the antigen-antibody complex to be concentrated and visually detected. In developing our IC test for H. ducreyi, the HgbA receptor was chosen as the antigen because it is abundantly expressed, it is readily purified for the development of antigens, and it is antigenically and functionally conserved. It is likely that naturally occurring mutants do not occur in vivo because an isogenic mutant that does not synthesize HgbA was unable to initiate an infection in the human model of chancroid (5). Furthermore, two studies have shown that HgbA is expressed during natural infection (30, 56). Therefore, the generation of MAbs to the HgbA protein seemed to be a logical target.
To be clinically useful, an IC test strip must be able to recognize a diverse panel of clinical isolates and have a high specificity in order to distinguish between other related pathogenic bacteria while it must maintain a sensitivity that is high enough. We found that the MAbs used in our IC test were able to accurately identify all laboratory strains of H. ducreyi tested. These strains are broadly representative of the known pathogenic strains of H. ducreyi (24). It is likely, therefore, that the present configuration of the IC test will be useful in detecting H. ducreyi under a variety of clinical circumstances and in a variety of locations.
Using a limited panel of pathogenic bacteria, we also found the test to be highly specific. As they are specific for the H. ducreyi HgbA protein, the designated MAbs do not cross-react with related TonB-dependent hemoglobin receptors found in other bacteria, nor do the MAbs generate false-positive results when they are tested against skin and genital commensal bacteria, but they are able to differentiate H. ducreyi from cryptic genospecies of Haemophilus (biotype IV), which are also isolated from genital sites.
This first-generation test maintains only a moderate level of sensitivity. Similar IC tests, such as those for diphtheria toxin (31) and Plasmodium falciparum HRP-2 antigen (11), have greater sensitivities. Our IC test may have a lower sensitivity due to its dependence upon the extraction of an OMP. Compared to other antigens, OMPs may be more difficult to extract and therefore may result in less antigen-antibody binding. In testing laboratory strains, we used a 50/50 mixture of bacteria and suspension buffer. The current detergent in the suspension buffer may need to be altered to increase the level of extraction. During the course of developing the IC test, the original suspension buffer that contained Zwittergent produced false-positive results. The buffer was modified to contain Triton X-100, another nondenaturing detergent. Other detergents may result in the extraction of larger amounts of the HgbA protein from the outer membrane.
The absolute number of H. ducreyi cells present in ulcers is not known. One study on pustules (the lesion just prior to the formation of ulcers) in an experimental human infection reported that there were approximately 105 CFU per lesion (56). Therefore, it is unclear what the minimum limit of detection is that is needed to accurately detect a reasonable amount of the HgbA released from H. ducreyi or what the number of organisms is that are heme stressed in clinical specimens. It should be noted, however, that the IC test is not dependent upon viable organisms. The test is able to detect HgbA that has been extracted from both viable and nonviable organisms. Therefore, even if only nonviable organisms were sampled, the test potentially could produce a positive result.
The test described is designed as a “proof of principle,” an initial test to determine whether an immunologic assay with the particular MAbs might be feasible for the diagnosis of H. ducreyi infection. To increase the sensitivity, and therefore, to provide greater clinical utility, the test may require modifications, perhaps by use of a different format. Nevertheless, the MAbs used in this test demonstrated the required specificity, noncompetitiveness, and cross-reactivity required for diagnostic detection.
To date, all testing of this prototype IC test has used well-characterized laboratory strain 35000 as well as clinical isolates that have been frozen for up to 40 years rather than recently obtained patient specimens. We envision testing of swabs of future patient samples and then solubilization of the material in suspension buffer. The swab would be briefly but vigorously swirled to dislodge the specimen, pressed to the wall of the tube to extract as much buffer as possible, and then discarded prior to addition of the test strip. Such a study would require confirmation and comparison with culture and PCR. As an alternative to patient samples, swabs from experimental lesions of rabbits or pigs infected with H. ducreyi could be used.
This IC test is a means to the rapid diagnosis of a genital ulcer-causing disease that has been shown to increase the rate of transmission of HIV (45). The test, which provides results in as little as 15 min, would allow the treatment of H. ducreyi-infected patients at their first visit. For HIV-positive patients, reducing their infectivity for chancroid could, in turn, reduce their infectivity for HIV. It has been shown that effective control of chancroid in core groups reduces or eliminates it in an untreated noncore group population (48, 49; R. Steen et al., Int. Congr. Sex. Transm. Dis., 1999). In a study by Steen (48), commercial sex workers were treated with the long-acting antibiotic azithromycin. This treatment not only eliminated the incidence of chancroid ulcerations in the core group (the commercial sex workers), but chancroid was also eliminated in their partners. Although the study was not designed to demonstrate a decline in HIV transmission, computer modeling suggested that 40 of 400 new cases of HIV were prevented in the core group and 135 cases were prevented in their partners. Thus, a targeted intervention may have broad-reaching effects on the general population. Through rapid diagnosis and treatment, our results suggest that this IC test method may prove to be an important tool in the slowing of the spread of the sexually transmitted infection chancroid and, thus, HIV.
Acknowledgments
This work was supported by WHO/UNAIDS grant STDDIA06 (to C.E.) and University of North Carolina at Chapel Hill Center for AIDS Research grant P 30AI 50410 (to C.E.).
We thank Cam Patterson for editorial comments, Pat Totten for the generous gift of H. ducreyi strains, Peter Gilligan for American Type Culture Collection bacterial strains and the H. influenzae strains, Tim Murphy for H. influenzae type IV genital strains, Steve Simkins for production of hybridomas, Eric Hansen for the gift of LOS MAb, Isabelle Leduc for the gift of purified LOS, and Fred Sparling for MAb A4.70 and N. gonorrhoeae strains FA1090 and FA19.
REFERENCES
- 1.Abinbola, J., A. Ajayi, L. Diamondstone, T. Quinn, W. Blattner, and R. Biggar. 1998. A serosurvey of Haemophilus ducreyi, syphilis and herpes simplex virus type 2 and their association with human immunodeficiency virus among female sex workers in Lagos, Nigeria. Sex. Transm. Dis. 25:237-242. [DOI] [PubMed] [Google Scholar]
- 2.Albritton, W. L. 1989. Biology of Haemophilus ducreyi. Microbiol Rev. 53:377-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alfa, M. J., P. DeGagne, and P. A. Totten. 1996. Haemophilus ducreyi hemolysin acts as a contact cytotoxin and damages human foreskin fibroblasts. Infect. Immun. 64:2349-2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al-Tawfiq, J. A., J. Harezlak, B. P. Katz, and S. M. Spinola. 2000. Cumulative experience with Haemophilus ducreyi 35000 in the human model of experimental infection. Sex. Transm. Dis. 27:111-114. [DOI] [PubMed] [Google Scholar]
- 5.Al-Tawfiq, J. A., K. R. Fortney, B. P. Katz, A. F. Hood, C. Elkins, and S. M. Spinola. 2000. An isogenic hemoglobin receptor-deficient mutant of Haemophilus ducreyi is attenuated in the human model of experimental infection. J. Infect. Dis. 181:1049-1054. [DOI] [PubMed] [Google Scholar]
- 6.Bauer, B. A., S. R. Lumbley, and E. J. Hansen. 1999. Characterization of a WaaF (RfaF) homolog expressed by Haemophilus ducreyi. Infect. Immun. 67:899-907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bauer, B. A., M. K. Stevens, and E. J. Hansen. 1998. Involvement of the Haemophilus ducreyi gmhA gene product in lipooligosaccharide expression and virulence. Infect. Immun. 66:4290-4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bauer, M. E., and S. M. Spinola. 1999. Binding of. Haemophilus ducreyi to extracellular matrix proteins. Infect. Immun. 67:2649-2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bauer, M. E., and S. M. Spinola. 2000. Localization of Haemophilus ducreyi at the pustular stage of disease in the human model of infection. Infect. Immun. 68:2309-2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bauer, M. E., M. P. Goheen, C. A. Townsend, and S. M. Spinola. 2001. Haemophilus ducreyi associates with phagocytes, collagen, and fibrin and remains extracellular throughout infection of human volunteers. Infect. Immun. 69:2549-2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beadle, C. L., W. Gary, W. R. Weiss, P. D. McElroy, S. M. Maret, A. J. Oloo, and S. L. Hoffman.1994. Diagnosis of malaria by detection of Plasmodium falciparum HRP-2 antigen with a rapid dipstick antigen-capture assay. Lancet 343:564-568. [DOI] [PubMed] [Google Scholar]
- 12.Behets, F. M. T., G. Liomba, G. Lule, G. Dalabetta, I. F. Hoffman, H. A. Hamilton, S. Moeng, and M. S. Cohen. 1995. Sexually transmitted diseases and human immunodeficiency virus control in Malawi: a field study of genital ulcer disease. J. Infect. Dis. 171:451-454. [DOI] [PubMed] [Google Scholar]
- 13.Bong, C. T., R. E. Throm, K. R. Fortney, B. P. Katz, A. F. Hood, C. Elkins, and S. M. Spinola. 2001. DsrA-deficient mutant of Haemophilus ducreyi is impaired in its ability to infect human volunteers. Infect. Immun. 69:1488-1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bozue, J. A., L. Tarantino, and R. S. Munson, Jr. 1998. Facile construction of mutations in Haemophilus ducreyi using lacZ as a counter-selectable marker. FEMS Microbiol. Lett. 164:269-273. [DOI] [PubMed] [Google Scholar]
- 15.Bozue, J. A., M. V. Tullius, J. Wang, B. W. Gibson, and R. S. Munson, Jr. 1999. Haemophilus ducreyi produces a novel sialyltransferase. Identification of the sialyltransferase gene and construction of mutants deficient in the production of the sialic acid-containing glycoform of the lipooligosaccharide. J. Biol. Chem. 274:4106-4114. [DOI] [PubMed] [Google Scholar]
- 16.Brentjens, R., M. Ketterer, M. Apicella, and S. Spinola. 1996. Fine tangled pili expressed by Haemophilus ducreyi are a novel class of pili. J. Bacteriol. 178:808-816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen, C. J., C. Elkins, and P. F. Sparling. 1998. Phase variation of hemoglobin utilization in Neisseria gonorrhoeae. Infect. Immun. 66:987-993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen, C. J., P. F. Sparling, L. A. Lewis, D. W. Dyer, and C. Elkins. 1996. Identification and purification of a hemoglobin-binding outer membrane protein from Neisseria gonorrhoeae. Infect. Immun. 64:5008-5014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chui, L., W. Albritton, B. Paster, I. Maclean, and R. Marusyk. 1993. Development of the polymerase chain reaction for diagnosis of chancroid. J. Clin. Microbiol. 31:659-664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cohen, M. 1998. Sexually transmitted diseases enhance HIV transmission: no longer a hypothesis. Lancet 351:5-7. [DOI] [PubMed] [Google Scholar]
- 21.Cope, L. D., Z. Hrkal, and E. J. Hansen. 2000. Detection of phase variation in expression of proteins involved in hemoglobin and hemoglobin-haptoglobin binding by nontypeable Haemophilus influenzae. Infect. Immun. 68:4092-4101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cope, L. D., S. Lumbley, J. L. Latimer, J. Klesney-Tait, M. K. Stevens, L. S. Johnson, M. Purven, R. S. Munson, Jr., T. Lagergard, J. D. Radolf, and E. J. Hansen. 1997. A diffusible cytotoxin of Haemophilus ducreyi. Proc. Natl. Acad. Sci. USA 94:4056-4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dangor, Y., G. Fehler, F. D. Exposto, and H. J. Koornhof. 1989. Causes and treatment of sexually acquired genital ulceration in southern Africa. S. Afr. Med. J. 76:339-341. [PubMed] [Google Scholar]
- 24.Dutro, S. M., G. Wood, and P. Totten. 1999. Prevalence of, antibody response to, and immunity induced by Haemophilus ducreyi hemolysin. Infect. Immun. 67:3317-3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dylewski, J., H. Nsanze, G. Maitha, and A. Ronald. 1986. Laboratory diagnosis of Haemophilus ducreyi: sensitivity of culture media. Diagn. Microbiol. Infect. Dis. 4:241-245. [DOI] [PubMed] [Google Scholar]
- 26.Elkins, C. 1995. Identification and purification of a conserved heme-regulated hemoglobin-binding outer membrane protein from Haemophilus ducreyi. Infect. Immun. 63:1241-1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Elkins, C., C. J. Chen, and C. E. Thomas. 1995. Characterization of the hgbA locus of Haemophilus ducreyi. Infect. Immun. 63:2194-2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elkins, C., P. A. Totten, B. Olsen, and C. E. Thomas. 1998. Role of the Haemophilus ducreyi Ton system in internalization of heme from hemoglobin. Infect. Immun. 66:151-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Elkins, C., K. B. Barkley, N. H. Carbonetti, A. J. Coimbre, and P. F. Sparling. 1994. Immunobiology of purified recombinant outer membrane porin protein I of Neisseria gonorrhoeae. Mol. Microbiol. 14:1059-1075. [DOI] [PubMed] [Google Scholar]
- 30.Elkins, C., K. Yi, B. Olsen, C. Thomas, K. Thomas, and S. Morse. 2000. Development of a serological test for Haemophilus ducreyi for seroprevalence studies. J. Clin. Microbiol. 38:1520-1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Engler, K. H., A. Efstratiou, D. Norn, R. S. Kozlov, I. Selga, T. G. Glushkevich, M. Tam, V. G. Melnikov, I. K. Mazurova, V. E. Kim, G. Y. Tseneva, L. P. Titov, and R. C. George. 2002. Immunochromatographic strip test for rapid detection of diphtheria toxin: description and multicenter evaluation in areas of low and high prevalence of diphtheria. J. Clin. Microbiol. 40:80-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Filiatrault, M. J., B. W. Gibson, B. Schilling, S. Sun, R. S. Munson, Jr., and A. A. Campagnari. 2000. Construction and characterization of Haemophilus ducreyi lipooligosaccharide (LOS) mutants defective in expression of heptosyltransferase III and β-1,4-glucosyltransferase: identification of LOS glycoforms containing lactosamine repeats. Infect. Immun. 68:3352-3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Filiatrault, M. J., R. S. Munson, Jr., and A. A. Campagnari. 2001. Genetic analysis of a pyocin-resistant lipooligosaccharide (LOS) mutant of Haemophilus ducreyi: restoration of full-length LOS restores pyocin sensitivity. J. Bacteriol. 183:5756-5761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Greenblatt, R. M., S. A. Lukehart, F. A. Plummer, T. C. Quinn, C. W. Critchlow, R. L. Ashley, L. J. DCosta, A. J. O. Ndinya, L. Corey, A. R. Ronald, et al. 1988. Genital ulceration as a risk factor for human immunodeficiency virus infection. AIDS 2:47-50. [DOI] [PubMed] [Google Scholar]
- 35.Gulig, P. A., and E. J. Hansen. 1985. Coprecipitation of lipopolysaccharide and the 39,000-molecular-weight major outer membrane protein of Haemophilus influenzae type B by lipopolysaccharide-directed monoclonal antibody. Infect. Immun. 49:819-827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hansen, E. J., J. L. Latimer, S. E. Thomas, M. Helminen, W. L. Albritton, and J. D. Radolf. 1992. Use of electroporation to construct isogenic mutants of Haemophilus ducreyi. J. Bacteriol. 174:5442-5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harlow, E., and D. Lane. 1988. Antibodies: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 38.Hiltke, T., A. Campagnari, and S. Spinola. 1996. Characterization of a novel lipoprotein expressed by Haemophilus ducreyi. Infect. Immun. 64:5047-5052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hiltke, T. J., M. E. Bauer, J. Klesney-Tait, E. J. Hansen, R. S. Munson, Jr., and S. M. Spinola. 1999. Effect of normal and immune sera on Haemophilus ducreyi 35000HP and its isogenic MOMP and LOS mutants. Microb. Pathog. 26:93-102. [DOI] [PubMed] [Google Scholar]
- 39a.Hitchcock, P. J., and T. M. Brown. 1983. Morphological heterogeneity among Salmonella lipopolysaccharide cheomotypes in silver-stained polyacrylanide gels. J. Bacteriol. 154:269-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Johnson, S. R., D. H. Martin, C. Cammarata, and S. A. Morse. 1995. Alterations in sample preparation increase sensitivity of PCR assay for diagnosis of chancroid. J. Clin. Microbiol. 33:1036-1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jones, C. C., and T. Rosen. 1991. Cultural diagnosis of chancroid. Arch. Dermatol. 127:1823-1827. [PubMed] [Google Scholar]
- 42.Leduc, I. 2001. Ph.D. thesis. University of Ottawa, Ottawa, Ontario, Canada.
- 43.Macdonald, K., D. W. Cameron, G. Irungu, L. J. DCosta, F. A. Plummer, L. A. Slaney, A. J. O. Ndinya, and A. R. Ronald. 1989. Comparison of Sheffield media with standard media for the isolation of Haemophilus ducreyi. Sex. Transm. Dis. 16:88-90. [DOI] [PubMed] [Google Scholar]
- 44.Morse, S. A., D. L. Trees, Y. Htun, F. Radebe, K. A. Orle, Y. Dangor, C. M. Beck-Sague, S. Schmid, G. Fehler, J. B. Weiss, and R. C. Ballard. 1997. Comparison of clinical diagnosis and standard laboratory and molecular methods for the diagnosis of genital ulcer disease in Lesotho: association with human immunodeficiency virus infection. J. Infect. Dis. 175:583-589. [DOI] [PubMed] [Google Scholar]
- 45.Plummer, F. A., J. N. Simonsen, D. W. Cameron, J. O. Ndinya-Achola, J. K. Kreiss, M. N. Gakinya, P. Waiyaki, M. Cheang, P. Piot, A. R. Ronald, and E. N. Ngugi. 1991. Cofactors in male-female sexual transmission of human immunodeficiency virus type 1. J. Infect. Dis. 163:233-239. [DOI] [PubMed] [Google Scholar]
- 46.Purcell, B. K, J. A. Richardson, J. D. Radolf, and E. J. Hansen. 1991. A temperature-dependent rabbit model for production of dermal lesions by Haemophilus ducreyi J. Infect. Dis. 164:359-367. [DOI] [PubMed] [Google Scholar]
- 47.Spinola, S. M., M. E. Bauer, and R. S. Munson. 2002. Immunopathogenesis of Haemophilus ducreyi infection (chancroid). Infect. Immun. 70:1667-1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Steen, R. 2001. Eradicating chancroid. Bull W. H. O. 79:218-228. [PMC free article] [PubMed] [Google Scholar]
- 49.Steen, R., B. Vuylsteke, T. De Coito, S. Ralepeli, et al. 2000. Evidence of declining STD prevalence in a South African mining community following a core group intervention. Sex. Transm. Dis. 21:1-8. [DOI] [PubMed] [Google Scholar]
- 50.Stevens, M. K., L. D. Cope, J. D. Radolf, and E. J. Hansen. 1995. A system for generalized mutagenesis of Haemophilus ducreyi. Infect. Immun. 63:2976-2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sturm, A. W., G. J. Stolting, R. H. Cormane, and H. C. Zanen. 1987. Clinical and microbiological evaluation of 46 episodes of genital ulceration. Genitourin. Med. 63:98-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sun, S., B. Schilling, L. Tarantino, M. V. Tullius, B. W. Gibson, and R. S. Munson, Jr. 2000. Cloning and characterization of the lipooligosaccharide galactosyltransferase II gene of Haemophilus ducreyi. J. Bacteriol. 182:2292-2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thomas, C. E., B. Olsen, and C. Elkins. 1998. Cloning and characterization of tdhA, a locus encoding a TonB-dependent heme receptor from Haemophilus ducreyi. Infect. Immun. 66:4254-4262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thomas, K., I. LeDuc, B. Olsen, C. Thomas, W. Cameron, and C. Elkins. 2001. Cloning, overexpression, purification and immunobiology of an 85-kilodalton outer membrane protein from Haemophilus ducreyi. Infect. Immun. 69:4438-4446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thompson, S., L. Wand, A. West, and P. Sparling. 1993. Neisseria meningitidis produces iron-regulated proteins related to the RTX family of exoproteins. J. Bacteriol. 175:811-818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Throm, R. E., and S. M. Spinola. 2001. Transcription of candidate virulence genes of Haemophilus ducreyi during infection of human volunteers. Infect. Immun. 69:1483-1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Totten, P., J. Kuypers, and S. Morse. 1998. Haemophilus ducreyi detection, p. 47-65. In R. Peeling and P. Sparling (ed.), Sexually transmitted diseases: methods and protocols in molecular medicine. Humana Press, Inc., Totowa, N.J.
- 58.Totten, P. A., J. M. Kuypers, C. Y. Chen, M. J. Alfa, L. M. Parsons, S. M. Dutro, S. A. Morse, and N. B. Kiviat. 2000. Etiology of genital ulcer disease in Dakar, Senegal, and comparison of PCR and serologic assays for detection of Haemophilus ducreyi infection. J. Clin. Microbiol. 38:268-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Totten, P. A., W. R. Morton, G. H. Knitter, A. M. Clark, N. B. Kiviat, and W. E. Stamm. 1993. A primate model for chancroid. J. Infect. Dis. 169:1284-1290. [DOI] [PubMed] [Google Scholar]
- 60.Trees, D. L., and S. A. Morse. 1995. Chancroid and Haemophilus ducreyi: an update. Clin. Microbiol. Rev. 8:357-375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ward, C. K., S. R. Lumbley, J. L. Latimer, L. D. Cope, and E. J. Hansen. 1998. Haemophilus ducreyi secretes a filamentous hemagglutinin-like protein. J. Bacteriol. 180:6013-6022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wasserheit, J. 1992. Epidemiological survey. Interrelationships between human immunodeficiency virus infection and other sexually transmitted diseases. Sex. Transm. Dis. 19:61-77. [PubMed] [Google Scholar]
- 63.Westphal, O., and K. Jann. 1965. Bacterial lipopolysaccharides: extraction with phenol-water and further applications of the procedure, p. 83-91. In R. Whistler (ed.), Carbohydrate chemistry. Academic Press, Inc., New York, N.Y.
- 64.Wood, G. E., S. M. Dutro, and P. A. Totten. 1999. Target cell range of the Haemophilus ducreyi hemolysin and its involvement in invasion of human epithelial cells. Infect. Immun. 67:3740-3749. [DOI] [PMC free article] [PubMed] [Google Scholar]