Abstract
Rapid detection of metallo-β-lactamase (MBL)-producing gram-negative bacilli is necessary to prevent their dissemination. The method using a disk with imipenem plus 750 μg of EDTA differentiated all MBL-producing pseudomonads, and the sensitivity and specificity for acinetobacters were 95.7 and 91.0%, respectively. The imipenem-EDTA disks were stable for 12 and 16 weeks at 4 and −20°C, respectively.
An increasing prevalence of carbapenem resistance mediated by acquired metallo-β-lactamases (MBLs) is being reported, particularly for Pseudomonas aeruginosa clinical isolates in several countries (4, 6, 8, 9, 11-14, 17, 18). In Korea, approximately 10 and 50% of imipenem resistance in P. aeruginosa (8) and Acinetobacter spp. (19), respectively, are due to MBL production. The resistance may spread rapidly to various species of gram-negative bacilli, as the MBL genes reside in mobile gene cassettes inserted in integrons (3). The rapid detection of MBL-positive gram-negative bacilli is necessary to aid infection control and to prevent their dissemination (5). A PCR method was simple to use in detecting MBL-producing isolates initially (16), but it became more difficult with the increased number of types of MBLs.
MBL activity is inhibited by chelating agents. Double-disk synergy tests using a ceftazidime disk and a 2-mercaptopropionic acid disk (1), or an imipenem disk and an EDTA disk (7), have been reported as a simple method to detect MBL-producing clinical isolates. However, occasional adjustment of the distance between the two disks is required to obtain optimal results (1, 7), as is the case with the double-disk test for the detection of extended-spectrum β-lactamase-producing isolates (2). For the phenotypic confirmation of extended-spectrum-β-lactamase-producing isolates, inhibition zones are compared by using both ceftazidime and cefotaxime disks with and without clavulanic acid (10). The aim of this study was to determine the feasibility of using an imipenem disk with added EDTA to confirm MBL-producing clinical isolates of Pseudomonas spp. and Acinetobacter spp.
The MBL-producing gram-negative bacilli used in this study were 102 isolates of P. aeruginosa, 14 of Pseudomonas putida, 20 of Acinetobacter baumannii, and 3 of Acinetobacter genomospecies 3. All of the isolates were VIM-2 MBL producers except for one isolate of P. aeruginosa and five isolates of acinetobacters, which were IMP-1 MBL producers. MBL genes were detected by PCR, and MBL production was detected by the imipenem-EDTA double-disk synergy test as described previously (8). Imipenem-resistant or -intermediate but non-carbapenemase-producing isolates were included for comparison.
Test organisms were inoculated onto plates of Mueller-Hinton agar (Becton Dickinson, Cockeysville, Md.) as recommended by the National Committee for Clinical Laboratory Standards (10). A 0.5 M EDTA solution was prepared by dissolving 186.1 g of disodium EDTA · 2H2O (Junsei Chemical, Tokyo, Japan) in 1,000 ml of distilled water and adjusting it to pH 8.0 by using NaOH. The mixture was sterilized by autoclaving (15). Two 10-μg-imipenem disks (Becton Dickinson) were placed on the plate, and appropriate amounts of an EDTA solution were added to one of them to obtain the desired concentration. The inhibition zones of the imipenem and imipenem-EDTA disks were compared after 16 to 18 h of incubation in air at 35°C.
To test the stability of the EDTA-added imipenem disks, an EDTA solution was added to 10-μg-imipenem disks to obtain a concentration of 1,000 μg. The disks were dried immediately in an incubator and stored at 4 or at −20°C in airtight vials without desiccant. The inhibition zones produced for MBL-positive and -negative isolates were compared after storage of the disks.
A preliminary study showed that a disk with imipenem plus 150 μg of EDTA could increase the mean inhibition zone diameter by 12 mm for four MBL-positive P. aeruginosa isolates, but the increase was only 6 mm for three MBL-positive Acinetobacter isolates. A disk with imipenem plus 1,500 μg of EDTA increased the mean inhibition zones for three MBL-negative isolates by 7 mm. Therefore, 750- and 1,000-μg EDTA concentrations were chosen for further study.
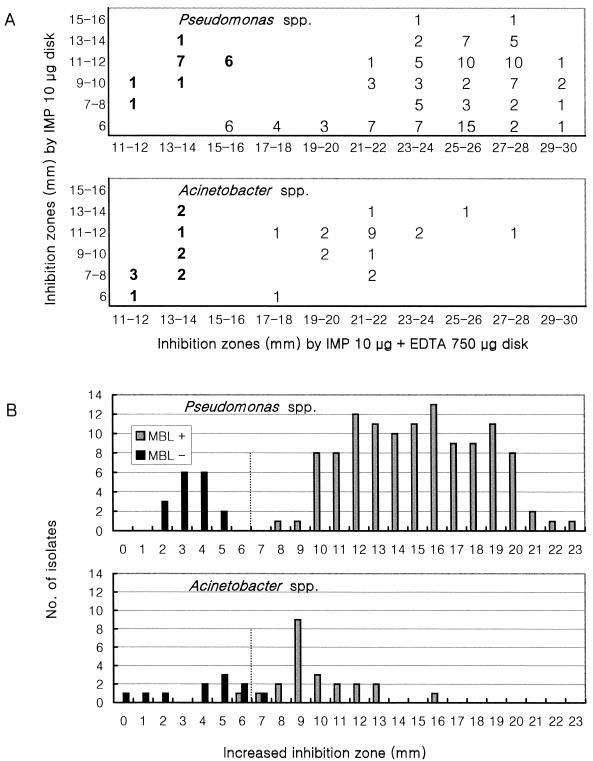
Figure 1 shows inhibition zones for MBL-positive and -negative imipenem-resistant pseudomonads and acinetobacters produced by disks with imipenem alone and disks with imipenem plus 750 μg of EDTA (data for the disk with imipenem plus 1,000 μg of EDTA are not shown). For MBL-positive isolates of Pseudomonas spp., disks with imipenem plus 750 and 1,000 μg of EDTA increased inhibition zones by 8 to 15 mm (mean, 10.5 mm) and 9 to 16 mm (mean, 11.5 mm), respectively, while the increases for MBL-negative isolates were 1 to 5 mm (mean, 3.8 mm) and 2 to 6 mm (mean, 5.0 mm), respectively (data not shown). The inhibition zone diameter for MBL-positive Acinetobacter spp. increased by 6 to 13 mm (mean, 9.4 mm) and 7 to 14 mm (mean, 10.4 mm), respectively, while that for MBL-negative isolates increased by 1 to 7 mm (mean, 4.0 mm) and 3 to 9 mm (mean, 6.0 mm), respectively. The disks with imipenem plus 750 μg of EDTA and those with imipenem plus 1,000 μg of EDTA produced comparable results, and therefore, a disk containing 750 μg of EDTA was chosen.
FIG. 1.
Comparison of inhibition zone diameters produced by disks with imipenem and imipenem plus 750 μg of EDTA. (A) Scattergram comparing inhibition zone diameters. Boldface indicates imipenem-resistant or -intermediate, but MBL-negative isolates, and lightface indicates MBL-positive isolates. (B) Increased inhibition zones with imipenem-EDTA disks. IMP, imipenem.
With Pseudomonas spp., all of the MBL-positive isolates were well separated from MBL-negative isolates by the criterion of a ≥7-mm increase of inhibition zone with the disks to which 750 μg of EDTA was added (Fig. 1B). However, by the same criterion, 1 of 23 (4.3%) MBL-positive and 1 of 11 (9.1%) MBL-negative acinetobacters showed false-negative and false-positive results, respectively (Fig. 1B). The size of inhibition zones was helpful for resolving this problem with equivocal isolates. The inhibition zones with imipenem-EDTA disks were ≤14 mm for the MBL-negative isolates, while they were ≥17 mm for the MBL-positive isolates (Fig. 1A).
A ceftazidime disk method was reported elsewhere to be more sensitive than an imipenem disk method for the detection of MBL by the double-disk synergy test (1). However, the use of a 2-mercaptopropionic acid-containing ceftazidime disk was not considered in this study, because the chemical is volatile and the MICs of imipenem for all of our MBL-producing isolates were ≥8 μg/ml.
An EDTA stock solution is stable, but addition of the solution at each performance of the test is time-consuming. To determine the stability of imipenem disks containing 1,000 μg of EDTA, the dried disks were stored at 4 or at −20°C without desiccant to simulate the most unfavorable laboratory condition. The inhibition zones with the imipenem-EDTA disks did not decrease much for one each of MBL-positive or -negative, imipenem-resistant P. aeruginosa and A. baumannii isolates for 12 and 16 weeks at 4 or at −20°C, respectively (Table 1). The imipenem-susceptible P. aeruginosa ATCC 27853 showed only slightly smaller inhibition zones with the imipenem-EDTA disk than with imipenem disks for routine use. The inhibition zone for the National Committee for Clinical Laboratory Standards control strain was within the acceptable range (10) for at least 24 weeks.
TABLE 1.
Stability of imipenem disks with 1,000 μg of EDTA added during storage as determined by change of inhibition zone diameter
| Storage (wk) | Diam (mm) of:
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| IMPb-EDTA disk zone
|
IMP disk zone, P. aeruginosa ATCC 27853a | ||||||||||
| IMP resistant, MBL positive
|
IMP resistant, MBL negative
|
P. aeruginosa ATCC 27853
|
|||||||||
|
P. aeruginosa
|
A. baumannii
|
P. aeruginosa
|
A. baumannii
|
||||||||
| 4°C | −20°C | 4°C | −20°C | 4°C | −20°C | 4°C | −20°C | 4°C | −20°C | ||
| Immediate | 24 | 24 | 23 | 23 | 15 | 15 | 17 | 17 | 24 | 24 | 27 |
| 1 | 23 | 23 | 22 | 23 | 12 | 13 | 15 | 15 | 22 | 23 | 24 |
| 4 | 21 | 22 | 22 | 22 | 13 | 13 | 14 | 16 | 21 | 22 | 25 |
| 8 | 20 | 21 | 22 | 23 | 13 | 12 | 15 | 15 | 20 | 21 | 25 |
| 12 | 21 | 24 | 23 | 23 | 11 | 13 | 14 | 16 | 20 | 22 | 22 |
| 16 | 20 | 23 | 23 | 25 | 11 | 13 | 9 | 15 | 19 | 22 | 25 |
| 24 | 18 | 23 | 22 | 23 | 11 | 12 | 12 | 13 | 19 | 22 | 25 |
| Mean | 21.0 | 22.9 | 22.4 | 23.1 | 12.3 | 13.0 | 13.7 | 15.3 | 20.7 | 22.3 | 24.7 |
Routine quality control results with disks stored as recommended by the National Committee for Clinical Laboratory Standards. The acceptable imipenem disk zone diameter is 20 to 28 mm.
IMP, imipenem.
In conclusion, the method using a disk with imipenem plus 750 μg of EDTA is simple to perform and highly sensitive in differentiating MBL-producing isolates. The specificity was excellent for pseudomonads and good for acinetobacters. The imipenem-EDTA disks can be stored at −20°C without significant loss of activity for at least 16 weeks.
Acknowledgments
We are grateful to Yong Hee Suh, research assistant, for collecting and storing the clinical isolates and for technical assistance.
REFERENCES
- 1.Arakawa, Y., N. Shibata, K. Shibayama, H. Kurokawa, T. Yagi, H. Fujiwara, and M. Goto. 2000. Convenient test for screening metallo-β-lactamase-producing gram-negative bacteria by using thiol compounds. J. Clin. Microbiol. 38:40-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bradford, P. A. 2001. Extended-spectrum β-lactamases in the 21st century: characterization, epidemiology, and detection of this important resistance threat. Clin. Microbiol. Rev. 14:933-951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fluit, A. C., and F. T. Schmitz. 1999. Class 1 integrons, gene cassettes, mobility, and epidemiology. Eur. J. Clin. Microbiol. Infect. Dis. 18:761-770. [DOI] [PubMed] [Google Scholar]
- 4.Gibb, A. P., C. Tribuddharat, R. A. Moore, T. J. Louie, W. Krulicki, D. M. Livermore, M.-F. I. Palepou, and N. Woodford. 2002. Nosocomial outbreak of carbapenem-resistant Pseudomonas aeruginosa with a new blaIMP allele, blaIMP-7. Antimicrob. Agents Chemother. 46:255-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hirakata, Y., K. Izumikawa, T. Yamaguchi, H. Takemura, H. Tanaka, R. Yoshida, J. Matsuda, M. Nakano, K. Tomono, S. Maesaki, M. Kaku, Y. Yamada, S. Kamihira, and S. Kohno. 1998. Rapid detection and evaluation of clinical characteristics of emerging multiple-drug resistant gram-negative rods carrying the metallo-β-lactamase gene blaIMP. Antimicrob. Agents Chemother. 42:2006-2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lauretti, L., M. L. Riccio, A. Mazzariol, G. Cornaglia, G. Amicosante, R. Fontana, and G. M. Rossolini. 1999. Cloning and characterization of blaVIM, a new integron-borne metallo-β-lactamase gene from a Pseudomonas aeruginosa clinical isolate. Antimicrob. Agents Chemother. 43:1584-1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee, K., Y. Chong, H. B. Shin, Y. A. Kim, D. Yong, and J. H. Yum. 2001. Modified Hodge and EDTA-disk synergy tests to screen metallo-β-lactamase-producing strains of Pseudomonas and Acinetobacter species. Clin. Microbiol. Infect. 7:88-91. [DOI] [PubMed] [Google Scholar]
- 8.Lee, K., J. B. Lim, J. H. Yum, D. Yong, Y. Chong, J. M. Kim, and D. M. Livermore. 2002. blaVIM-2 cassette-containing novel integrons in metallo-β-lactamase-producing Pseudomonas aeruginosa and Pseudomonas putida isolates disseminated in a Korean hospital. Antimicrob. Agents Chemother. 46:1053-1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mavroidi, A., A. Tsakris, E. Tzelepi, S. Pournaras, V. Loukova, and L. S. Tzouvelekis. 2000. Carbapenem-hydrolyzing VIM-2 metallo-β-lactamase in Pseudomonas aeruginosa from Greece. J. Antimicrob. Chemother. 46:1041-1042. [DOI] [PubMed] [Google Scholar]
- 10.National Committee for Clinical Laboratory Standards. 2001. Performance standards for antimicrobial susceptibility testing. Eleventh informational supplement. M100-S11. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 11.Osano, E., Y. Arakawa, R. Wacharotayankun, M. Ohta, T. Horii, H. Ito, F. Yoshimura, and N. Kato. 1994. Molecular characterization of an enterobacterial metallo-β-lactamase found in a clinical isolate of Serratia marcescens that shows imipenem resistance. Antimicrob. Agents Chemother. 38:71-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Poirel, L., T. Naas, D. Nicolas, L. Collet, S. Bellais, J.-D. Cavallo, and P. Nordmann. 2000. Characterization of VIM-2, a carbapenem-hydrolyzing metallo-β-lactamase and its plasmid- and integron-borne gene from a Pseudomonas aeruginosa clinical isolate in France. Antimicrob. Agents Chemother. 44:891-897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rasmussen, B. A., and K. Bush. 1997. Carbapenem-hydrolyzing β-lactamases. Antimicrob. Agents Chemother. 41:223-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Riccio, M. L., N. Franceschini, L. Boschi, B. Carvelli, G. Cornaglia, R. Fontana, G. Amicosante, and G. M. Rossolini. 2000. Characterization of the metallo-β-lactamase determinant of Acinetobacter baumannii AC-54/97 reveals the existence of blaIMP allelic variants carried by gene cassettes of different phylogeny. Antimicrob. Agents Chemother. 44:1229-1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed., p. A1.26. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 16.Senda, K., Y. Arakawa, S. Ichiyama, K. Nakashima, H. Ito, S. Ohsuka, K. Shimokata, N. Kato, and M. Ohta. 1996. PCR detection of metallo-β-lactamase gene (blaIMP) in gram-negative rods resistant to broad-spectrum β-lactams. J. Clin. Microbiol. 34:2909-2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yan, J.-J., P.-R. Hsueh, W.-C. Ko, K.-T. Luh, S.-H. Tsai, H.-M. Wu, and J.-J. Wu. 2001. Metallo-β-lactamase in clinical isolates of Pseudomonas isolates in Taiwan and identification of VIM-3, a novel variant of the VIM-2 enzyme. Antimicrob. Agents Chemother. 45:2224-2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yan, J.-J., W.-C. Ko, S.-H. Tsai, H.-M. Wu, and J.-J. Wu. 2001. Outbreak of infection with multidrug-resistant Klebsiella pneumoniae carrying blaIMP-8 in a university medical center in Taiwan. J. Clin. Microbiol. 39:4433-4439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yum, J. H., K. Yi, H. Lee, D. Yong, K. Lee, J. M. Kim, G. M. Rossolini, and Y. Chong. 2002. Molecular characterization of metallo-β-lactamase-producing Acinetobacter baumannii and Acinetobacter genomospecies 3 from Korea: identification of two novel integrons carrying blaVIM-2 gene cassettes. J. Antimicrob. Chemother. 49:837-840. [DOI] [PubMed] [Google Scholar]