Abstract
A pilot study was conducted to determine the genetic diversity of multiple colonies of pneumococci recovered from 37 nasopharyngeal (NP) samples of children. A total of 239 pneumococcal isolates (typically, six to eight colonies per sample) were typed by pulsed-field gel electrophoresis (PFGE). In most NP samples (89%) the multiple colonies shared common PFGE types and serotypes. However, four samples were heterogeneous (samples A through D): each contained two strains with different PFGE types, antibiotypes, and serotypes. Samples A and B each contained one strain of a vaccine capsular type and another expressing a non-vaccine type (according to the currently licensed seven-valent conjugate vaccine). In samples B and C the penicillin MIC for one strain was elevated and the other strain was susceptible. In each of the heterogeneous samples, one of the strains was a representative of an internationally disseminated clone. Samples A, C, and D contained strains which carried prophages that were inducible by mitomycin C and that could be visualized by electron microscopy. The comC gene allele (which encodes the competence-stimulating peptide) was the same in both strains found in each of samples A, B, and D. Carriage of multiple pneumococci with distinct properties should favor genetic exchange and provide a dynamic population structure for pneumococci in their ecological reservoir. Quantitative resolution of majority and minority components of the pneumococcal NP flora will be of importance for evaluation of the impact of intervention strategies such as vaccination or introduction of new antimicrobial agents.
Streptococcus pneumoniae is a major cause of morbidity and mortality among young children worldwide (1). With the emergence of antimicrobial resistance in this bacterium, it has become increasingly important to monitor the prevalence of drug-resistant strains carried by young children since the nasopharynx of individuals in this age group is known to be a major reservoir of this bacterial species (13).
Several projects are going on worldwide to monitor the prevalence of drug-resistant pneumococci among invasive and carried isolates. In Portugal, since 1996 we have been studying S. pneumoniae strains isolated from nasopharyngeal (NP) samples of a large group of children attending day care centers (4, 26). To evaluate the overall genetic diversity of over 2,000 NP samples we have routinely picked a single colony of pneumococci from each positive sample, and these were characterized for antibiotic susceptibility, serotype, and molecular type (pulsed-field gel electrophoresis [PFGE]). An interesting finding made in those studies was the high prevalence of eight clones that accounted for over half of drug-resistant pneumococci colonizing children attending day care centers (26). Subsequently, each one of the eight epidemic clones was also identified in international samples (24, 25).
The purpose of the pilot study described in this report was to use molecular typing techniques to determine the homogeneity of S. pneumoniae strains recovered from NP samples of children. Although some studies have described the serotype diversity among carriage pneumococcal isolates in cultures (8, 11, 12), to our knowledge, only two recent studies have addressed their genetic diversity (16, 28). We further characterized the strains isolated from heterogeneous samples for genetic determinants involved with DNA transfer and for susceptibility to lysis by antimicrobial agents. Studies of this nature are of particular importance for the proper evaluation of the impact of intervention strategies aimed at decreasing the rates of carriage of drug-resistant pneumococci and the incidence of disease.
MATERIALS AND METHODS
Study design.
In 1996 we performed a prevalence study in Lisbon, Portugal, of NP carriage of S. pneumoniae among 586 day care center attendees (4). The study was based on the characterization of one pneumococcal colony from each carrier, which was arbitrarily designated the “primary isolate.” In addition, several individual colonies (typically, six to eight colonies) were cultured from each NP sample positive for pneumococci and frozen.
In this study, we characterized 239 pneumococcal isolates from 37 selected NP samples. The selection criteria were based on the already known properties of primary isolates in order to have a diversity of antimicrobial resistance patterns, capsular types, and genetic profiles among the primary isolates.
Properties of primary isolates.
The 37 NP samples were plated on selective agar, and a single colony from each of these primary plates was characterized. The 37 primary isolates showed a variety of antimicrobial resistance patterns and belonged to eight serogroups and 12 PFGE types (Table 1) which included five internationally disseminated clones: penicillin-resistant clones Spain23F-1 and Spain9V-3 (15) (PFGE types A and B in Table 1), a penicillin-susceptible clone, and two penicillin-intermediate macrolide-resistant clones (PFGE types E, M, and H in Table 1) (24, 25).
TABLE 1.
Properties of primary isolates from multiple colonies of S. pneumoniae picked from NP samples and selected for further characterization
| Participant | Properties of primary isolate
|
PFGE type | No. of colonies characterized | ||
|---|---|---|---|---|---|
| Serogroup or serotype | Penicillin MIC (μg/ml) | Antibiotypea (resistance) | |||
| 149 | 3 | 0.023 | r1 | 8 | |
| 123 | 6 | 0.032 | E, Cc, Te, SXT | L1 | 8 |
| 127 | 6 | 0.032 | E, Cc, Te, SXT | L1 | 7 |
| 122 | 6A | 0.023 | z1 | 8 | |
| 119 | 6B | 0.023 | E, Cc, Te, SXT | M2 | 8 |
| 135 | 6 | 0.032 | E, Cc, Te, SXT | M2 | 8 |
| 449 | 6 | 0.023 | E, Cc, Te, SXT | M7 | 7 |
| 511 | 6 | 0.016 | Te | M6 | 7 |
| 541 | 6 | 0.023 | Te, SXT | M6 | 6 |
| 166 | 6B | 0.19 | E, Cc, Te | E | 7 |
| 500 | 6B | 0.125 | E, Cc, Te, SXT | E | 7 |
| 439 | 9V | 2 | SXT | B1 | 3 |
| 106 | 11 | 0.016 | E, Cc | SSS1 | 7 |
| 88 | 14 | 1.5 | SXT | B1 | 7 |
| 99 | 14 | 1.5 | SXT | B1 | 6 |
| 114 | 14 | 1 | SXT | B1 | 7 |
| 79 | 16 | 0.032 | n1 | 8 | |
| 432 | 19 | 0.094 | SXT | D2 | 8 |
| 437 | 19 | 0.25 | SXT | D2 | 7 |
| 448 | 19 | 0.094 | SXT | D2 | 7 |
| 98 | 19F | 0.047 | E, Cc | H5 | 6 |
| 325 | 19F | 0.047 | H1 | 4 | |
| 238 | 23F | 0.75 | C, Te, SXT | A1 | 4 |
| 241 | 23F | 1 | C, Te, SXT | A1 | 6 |
| 244 | 23F | 1.5 | C, Te, SXT | A4 | 5 |
| 308 | 23F | 1.5 | C, Te, SXT | A3 | 6 |
| 407 | 23F | 1 | C, Te, SXT | A1 | 6 |
| 415 | 23F | 0.75 | C, Te, SXT | A1 | 5 |
| 425 | 23F | 2 | C, Te, SXT | A1 | 5 |
| 450 | 23F | 2 | C, Te, SXT | A2 | 5 |
| 453 | 23F | 2 | C, Te, SXT | A2 | 5 |
| 455 | 23F | 2 | C, Te, SXT | A3 | 6 |
| 456 | 23F | 2 | C, Te, SXT | A1 | 6 |
| 458 | 23F | 1 | C, Te, SXT | A2 | 8 |
| 459 | 23F | 1 | C, Te, SXT | A1 | 8 |
| 561 | 23F | 2 | C, Te, SXT | A2 | 6 |
| 460 | 23F | 0.016 | h1 | 7 | |
C, chloramphenicol; E, erythromycin; Cc, clindamycin; Te, tetracycline; SXT, sulfamethoxazole-trimethoprim.
NP swabs and culture.
NP samples were collected by pediatric nurses by using sterile swabs (Mini-Tip Culturette; Becton Dickinson Microbiology Systems, Cockeysville, Md.) and were inoculated within 4 h in 5% blood Trypticase soy agar plates containing gentamicin (5 mg/liter) to select for S. pneumoniae. The plates were incubated overnight at 37°C under anaerobic conditions with an optochin disk. Growth of pneumococci was quantified from − (no growth) to +++ (growth in four quadrants of the plate). Individual colonies were picked from each of the primary selective plates, streaked onto 5% blood agar plates, and incubated overnight with an optochin disk at 37°C in a 5% CO2-enriched atmosphere. Cultures were frozen in Mueller-Hinton broth containing 15% glycerol (vol/vol) and kept at −70°C. All culture media were purchased from Difco (Detroit, Mich.).
Antimicrobial susceptibility testing.
Agar disk diffusion testing was performed with chloramphenicol, erythromycin, clindamycin, tetracycline, and trimethoprim-sulfamethoxazole disks by the guidelines of the National Committee for Clinical Laboratory Standards (17). Penicillin MICs were determined by the E-test (AB Biodisk, Solna, Sweden) according to the recommendations of the manufacturer.
Serotyping.
Capsular types and groups were determined by the Quellung reaction with commercially available antisera (Statens Serum Institut, Copenhagen, Denmark).
Molecular typing.
Preparation of chromosomal DNA, restriction with SmaI endonuclease, and PFGE were done as described previously (25).
Southern blot hybridization.
DNA fragments were transferred to nylon membranes and hybridized with a lytA-specific probe, as described previously (27).
Induction of lytic cycle in putative lysogenic strains.
Mitomycin C was added to cultures in the exponential phase (optical density at 620 nm, between 0.2 and 0.3) at 0.1 μg/ml (final concentration), and the cultures were monitored by determination of the optical density (23). Strains URU481 (lysogenic clinical isolate), R6 (nonlysogenic laboratory strain), and Lyt-4-4 (nonlysogenic autolysin-deficient laboratory mutant) were used as controls.
Analysis of comC allelic variation.
The strains were grown in liquid medium until the early stationary phase. An appropriate amount of culture was diluted (1:50) in water, boiled for 5 min, and cooled on ice. Templates were stored at −20°C. A total of 70 μl of template was used in each 100-μl PCR mixture. Primer 3 (5′-TGA CAG TTG AGA GAA TCT T-3′) and primer 4 (5′-CTT TTC TAT TTA TTT GAC CT-3′) (30) were used to amplify a PCR product containing comC, the genetic determinant of the competence-stimulating peptide (10). PCR products were purified by using Wizard PCR Preps (Promega, Madison, Wis.). Sequencing reactions were done with the same primers used for PCR amplification at the Rockefeller University Protein/DNA Technology Center by the TaqFS fluorescent dye terminator sequencing method with an ABI Prism 3700 DNA analyzer (Perkin-Elmer Applied Biosystems, Foster City, Calif.). Nucleotide and derived amino acid sequence data were analyzed with DNASTAR software (Lasergene, Madison, Wis.). Homology searches were performed with the BLAST utility (2), available through the National Center for Biotechnology Information website.
Lysis curves and viability counts.
Six-milliliter cultures of S. pneumoniae in the early exponential phase were exposed to 10 times the MIC of benzylpenicillin. Absorbances were monitored at 620 nm for 6 h after addition of the antibiotic. The number of viable cells in cultures exposed to penicillin was determined at various times by diluting cultures in liquid medium supplemented with 100 U of penicillinase/ml (Sigma) and plating them in 5% blood Trypticase soy agar (14). Strains R6 and Lyt-4-4 were used as controls.
Electron microscopy.
Preparation of samples for electron microscopy, staining, and observation were done as described previously (23, 29).
RESULTS
Serotyping and molecular typing of multiple individual colonies.
Multiple colonies (six to eight per plate) were picked from each primary plate, generating 239 pneumococcal isolates which were serotyped and whose PFGE profiles were determined. Each colony in 33 of 37 (89%) NP samples had the same serotype, PFGE profile, and lytA hybridization pattern as the corresponding primary isolates, suggesting that only one pneumococcal strain was present in the sample (Fig. 1). However, four of the NP samples (samples A through D) were heterogeneous: each sample contained two strains differing in serotype and PFGE type. There was no correlation between the amount of growth on the original selective agar plate and the presence of multiple strains. None of the children who concomitantly carried two pneumococcal strains had taken antibiotics in the month preceding collection of the sample.
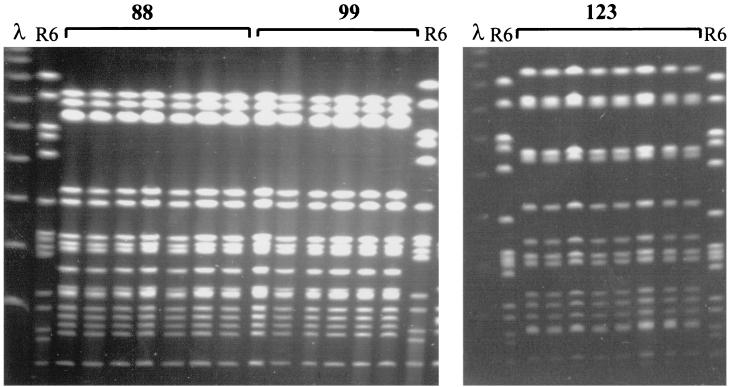
FIG. 1.
Example of PFGE profiles obtained from the analysis of multiple colonies isolated from the same NP sample. The profiles of the samples from participants 88, 99, and 123 are shown. All individual colonies analyzed have the same PFGE pattern. λ, bacteriophage λ ladder pulsed-field gel marker; R6, control strain.
Properties of heterogeneous samples.
Samples A through D each contained two strains which could be distinguished by serotype, antibiotype, PFGE type, and lytA hybridization pattern after Southern blot hybridization of PFGE gels (Table 2 and Fig. 2). These samples provided examples of concomitant carriage of pneumococci of vaccine types and non-vaccine types (according to the currently licensed seven-valent conjugate vaccine) as well as antimicrobial-susceptible and antimicrobial-resistant strains. For the strains in NP samples A and B, one of the strains was of a vaccine serotype (serotypes 6B and 19F, respectively), while the other strain was of a non-vaccine type (serotype 11 and nontypeable, respectively). For the strains in both samples B and C, the penicillin MIC for one of the two strains was elevated (0.75 and 2 μg/ml, respectively), while the penicillin MICs were less than 0.1 μg/ml for the other strains. There were differences in the levels of resistance to tetracycline in the pairs of strains from samples A, C, and D; and, additionally, there were differences in the levels of resistance to chloramphenicol in the pair of strains from sample C. These antibiotic resistance determinants can be transferred between pneumococcal isolates by transformation (of altered pbp genes) (5) or by conjugation of transposons (which carry the cat, tet, and erm genes) (31). Interestingly, in each heterogeneous sample, one of the strains was a representative of previously described internationally disseminated epidemic clones (24, 25) (PFGE types M, H, A, and M, in samples A, B, C, and D, respectively).
TABLE 2.
Properties of multiple isolates obtained from NP samples containing two strains of S. pneumoniae
| Sample | Isolate code | Serotype | Penicillin MIC (μg/ml) | Antibiotypea (resistance) | PFGE type | lytA hybridization pattern by PFGE (kb) | Result of addition of mitomycin C | Phage DNAb | comC allelec |
|---|---|---|---|---|---|---|---|---|---|
| A | 106 | 11 | 0.016 | E, Cc | SSS | 85 | No lysis | comC1 | |
| A | 106-1 | 6B | 0.023 | E, Cc, Te | M | 280, 110, 90 | lysis | Yes | comC1 |
| A | 106-2 | 11 | 0.006 | E, Cc | SSS | 85 | |||
| A | 106-3 | 11 | 0.008 | E, Cc | SSS | 85 | |||
| A | 106-4 | 11 | 0.008 | E, Cc | SSS | 85 | |||
| A | 106-5 | 11 | 0.008 | E, Cc | SSS | 85 | |||
| A | 106-6 | 6B | 0.016 | E, Cc, Te | M | 280, 110, 90 | |||
| B | 325 | 19F | 0.047 | H | 90, 35 | No lysis | comC2.1 | ||
| B | 325-1 | NT | 0.5 | TTT | 170, 90, 40, 30 | NDd | comC2.1 | ||
| B | 325-2 | NT | 0.75 | TTT | 170, 90, 40, 30 | ||||
| B | 325-3 | NT | 1 | TTT | 170, 90, 40, 30 | ||||
| C | 448 | 19A | 0.094 | SXT | D | 235, 90, 80 | Lysis | Yes | comC1 |
| C | 448-1 | 19A | 0.064 | SXT | D | 235, 90, 80 | |||
| C | 448-2 | 23F | 2 | C, Te, SXT | A | 230, 100, 40 | Lysis | Yes | comC2.2 |
| C | 448-3 | 19A | 0.094 | SXT | D | 235, 90, 80 | |||
| C | 448-4 | 19A | 0.094 | SXT | D | 235, 90, 80 | |||
| C | 448-5 | 19A | 0.094 | SXT | D | 235, 90, 80 | |||
| C | 448-6 | 19A | 0.064 | SXT | D | 235, 90, 80 | |||
| D | 541 | 6B | 0.023 | Te, SXT | M | 280, 90, 60 | Lysis | Yes | comC1 |
| D | 541-1 | 19A | 0.023 | SXT | UUU | 90 | No lysis | comC1 | |
| D | 541-2 | 19A | 0.023 | SXT | UUU | 90 | |||
| D | 541-3 | 6B | 0.016 | Te, SXT | M | 280, 90, 60 | |||
| D | 541-4 | 6B | 0.012 | Te, SXT | M | 280, 90, 60 | |||
| D | 541-5 | 6B | 0.008 | Te, SXT | M | 280, 90, 60 |
C, chloramphenicol; E, erythromycin; Cc, clindamycin; Te, tetracycline; SXT, sulfamethoxazole-trimethoprim.
Detected by PFGE separation of total DNA after induction by mitomycin C followed by hybridization with lytA and by electron microscopy (see Materials and Methods).
Allele nomenclature according to reference 30.
ND, not determined.
FIG. 2.
(A) PFGE profiles of SmaI-restricted S. pneumoniae DNA from NP samples A to D. Lanes 1 to 7 (unnumbered), sample A (isolates 106, 106-1, 106-2, 106-3, 106-4, 106-5, and 106-6, respectively); lanes 8 to 11, sample B (isolates 325, 325-1, 325-2, and 325-3, respectively); lanes 12 to 18, sample C (isolates 448, 448-1, 448-2, 448-3, 448-4, 448-5, and 448-6, respectively); lanes 19 to 24, sample D (isolates 541, 541-1, 541-2, 541-3, 541-4, and 541-5, respectively); lane 25, bacteriophage λ ladder. (B) Hybridization of gel with a lytA probe.
For all isolates from samples A through D, Southern hybridization of SmaI-restricted DNA on PFGE gels with a lytA probe identified a band of approximately 90 kb that is known to contain the host autolytic gene (23). Additional bands were observed for six of the eight strains which produced unique lytA hybridization patterns that could distinguish the two strains present in the heterogeneous samples (Fig. 2B). The multiple lytA hybridization bands suggested that the six strains contained prophages (23).
In the next set of experiments one isolate representing each of the pairs of strains from heterogeneous samples was selected for further characterization. These strains were as follows: isolates 106 and 106-1 from sample A, isolates 325 and 325-1 from sample B, isolates 448 and 448-2 from sample C, and isolates 541 and 541-1 from sample D.
Detection of prophages.
To investigate whether the strains carried prophages, mitomycin C was added to cultures in the exponential phase of growth. In four of the six strains (strains 106-1, 448, 448-2, and 541), which contained multiple lytA hybridization bands, rapid lysis was observed following addition of mitomycin C, suggesting prophage induction (Table 2; Fig. 3). One of the two remaining strains (strain 325-1, sample B) was not tested since it did not grow well in liquid medium, and the other one (strain 325, sample B) did not lyse in the presence of mitomycin C. Strain 106 from sample A and strain 541-1 from sample D, which had only one lytA band, did not respond to mitomycin C.
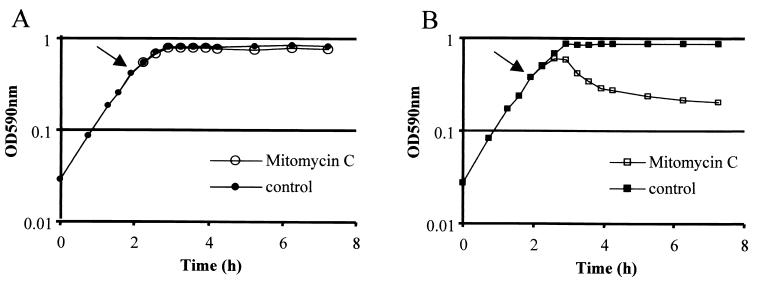
FIG. 3.
Effect of mytomicin C in the growth of S. pneumoniae strains 106 (A) and 106-1 (B). Arrows indicate the time of addition of mitomycin C. OD590nm, optical density at 590 nm.
The four strains which lysed in the presence of mytomycin C were grown again and treated with mitomycin C. After addition of the antibiotic, just before lysis, the cultures were harvested and total DNA was isolated. Separation by PFGE of unrestricted total DNA yielded one band of large molecular size (the bacterial chromosome) for cultures not exposed to mitomycin C and an additional band of small molecular size (<40 kb) for cultures exposed to mitomycin C (Fig. 4A, lanes 2 to 9). Hybridization with lytA showed that all DNA bands gave a positive signal, suggesting that the DNA bands with small molecular sizes corresponded to phage genomes induced by the mitomycin treatment (Fig. 4B, lanes 2 to 9). When the same total DNAs were restricted with SmaI, differences of up to three bands were observed in the PFGE profiles obtained for each strain grown in the absence or in the presence of mitomycin C (Fig. 4A, lanes 11 to 18). Hybridization with lytA showed that these differences in the PFGE profiles could be explained by the induction of prophages and their excision from the chromosome (Fig. 4B, lanes 11 to 18).
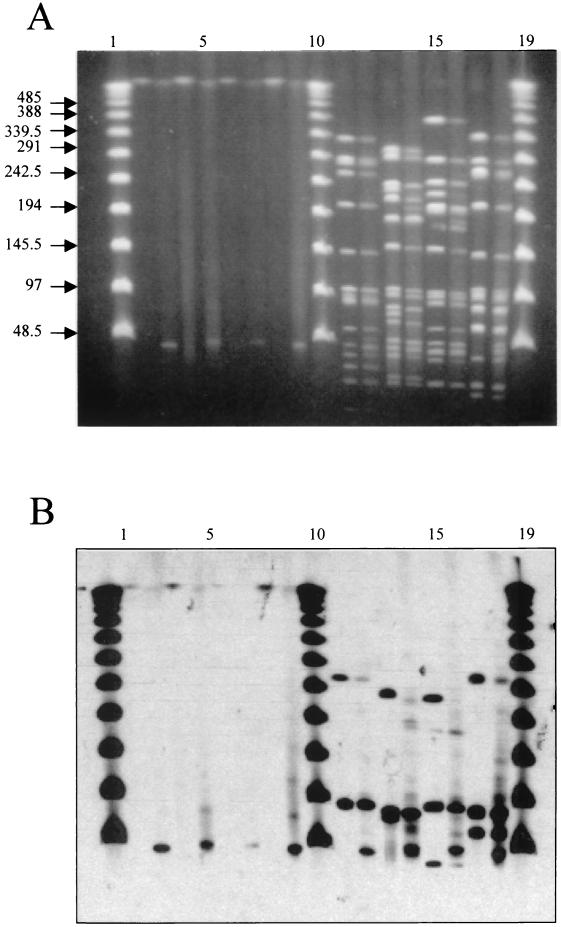
FIG. 4.
(A) Lanes 2 to 9, total unrestricted DNA of strains grown in the presence or absence of mitomycin C. Lanes 2, 4, 6, and 8, strains 106-1, 448, 448-2, and 541, respectively, grown in the absence of mitomycin C (controls); lanes 3, 5, 7, and 9, strains 106-1, 448, 448-2, and 541, respectively, grown in the presence of mitomycin C; lanes 11 to 18, SmaI-restricted DNA of strains grown in the presence or absence of mitomycin C; lanes 11, 13, 15, and 17, strains 106-1, 448, 448-2, and 541, respectively, grown in the absence of mitomycin C (controls); lanes 12, 14, 16, and 18, strains 106-1, 448, 448-2, and 541, respectively, grown in the presence of mitomycin C; lanes 1, 10, and 19, bacteriophage λ ladder. (B) Hybridization of the gel with a lytA probe.
Electron microscopy of the four cultures inducible by mitomycin C showed that the bacteria underwent morphological changes and the presence of intracellular phage particles (Fig. 5).
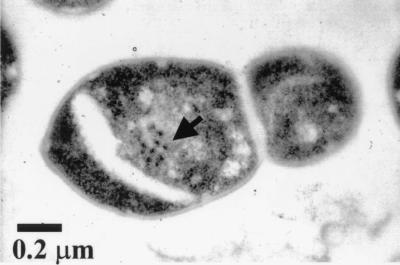
FIG. 5.
Phage particles being assembled (arrow) inside a pneumococcal cell of strain 448 induced with mitomycin C.
Analysis of comC allelic variation.
The sequence of comC, the genetic determinant of the competence-stimulating peptide responsible for inducing competence in a pneumococcal population when it reaches a threshold extracellular concentration, was determined for each of the eight strains and compared to those described previously (21, 22, 30). For samples A, B, and D, the two strains present in each of the heterogeneous samples had the same comC allele (Table 2).
Susceptibility to penicillin-induced lysis.
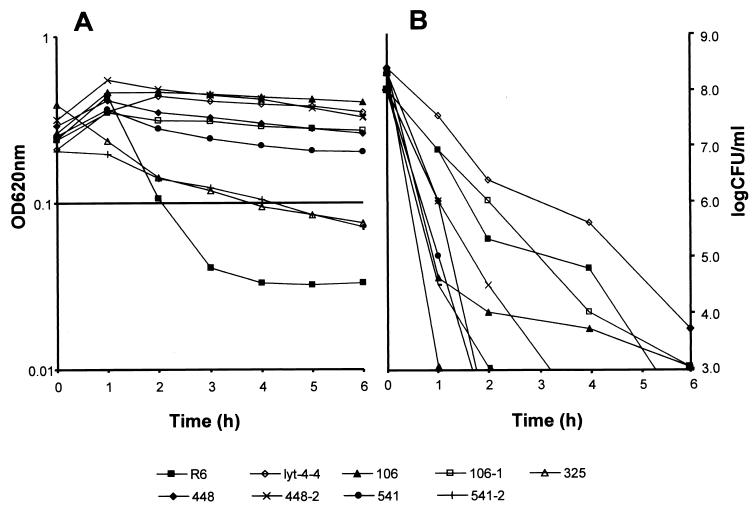
Seven of the eight strains from the heterogeneous samples were tested for lysis in the presence of 10 times the MIC of penicillin. Addition of penicillin to the cultures in exponential growth led to quick lysis of strain R6 and strains 325 and 541-2. The remaining cultures had only a very slow decrease in optical density, suggesting that they were resistant to lysis induction by penicillin (Fig. 6A). Viable titers were determined at hourly intervals for all cultures. After 6 h of exposure to the antibiotic, all cultures had lost viability by 6 or more orders of magnitude (Fig. 6B). All cultures lysed in the presence of 4% deoxycholate. Electron microscopy of strain 106 (nonlysing) after 4 h of exposure to 10 times the MIC of penicillin showed intact cells with normal morphology (data not shown).
FIG. 6.
Penicillin-induced lysis (A) and loss of viability (B) when cultures in the early exponential phase (108 CFU/ml) were treated with 10 times the MIC of penicillin. OD620nm, optical density at 620 nm.
DISCUSSION
We have used PFGE to determine the genetic diversity of pneumococci among multiple isolates from single colonies recovered from 37 NP samples collected from children in day care centers. By picking six to eight colonies from each primary plate we found that in 33 of the NP samples each of the several colonies picked had a common serotype and PFGE type, indicating that the majority of pneumococci recovered in these particular NP samples belonged to a single strain. However, four of the NP samples were heterogeneous: each contained two pneumococcal strains distinguishable by their PFGE patterns as well as their serotypes.
Carriage of multiple pneumococci has been demonstrated before. In 1946, Hodges et al. (11) studied an epidemic of pneumococcal pneumonia in an Army Air Force technical school and found that 15% of the subjects simultaneously carried more than one serotype of pneumococcus. In 1980, Gray et al. (8) described that more than one serotype of pneumococcus was found in 8% of the cultures of NP samples obtained from 82 children studied from birth to 24 months of life. In a recent study, which used PFGE to study the genetic diversity of S. pneumoniae and Haemophilus influenzae strains isolated in cultures of throat swab specimens from children in day care centers, the investigators found that two genetically diverse strains could be identified in 10% of the positive samples (28).
The strains in our heterogeneous samples expressed different antimicrobial resistance types and capsular types and carried prophages that could be induced to produce phage particles and lyse the host strains, and several of the pairs of strains had the same comC allele, suggesting that both strains could be activated simultaneously to a competent state since they would respond to the same competence-stimulating peptide pherotype. Such strains could participate in DNA transfer events through genetic transformation or pseudotransduction (20, 30). Pneumococci are naturally transformable; and evidence of in vivo capsular transformation (3, 18), transfer of murM mosaic genes (6), and antimicrobial resistance markers such as penicillin-binding protein mosaic genes (5) and other antimicrobial resistance determinants (26) has been presented. Concomitant carriage of multiple lineages of pneumococci with such diverse traits represents an opportunity for these DNA transfer events. In fact, such shifts have been observed to occur in the nasopharynx (3, 7). In the four heterogeneous samples, at least one of the strains appeared to be lysis defective in the presence of high concentrations of penicillin (10 times the MIC), and although the cultures were loosing viability, intact cells were observed by electron microscopy after 4 h of exposure to the antibiotic. It has been suggested that selection of the lysis-defective and/or antibiotic-tolerant phenotype may favor and/or precede acquisition of antibiotic resistance (9).
In our heterogeneous samples the strains that made up the minority represented a substantial proportion (14 to 30%) of the total pneumococcal population of that particular sample. Carriage of multiple strains of pneumococci at substantially lower levels may very well occur in the nasopharynx, and such minority populations may play an important role in the dynamics of population changes that occur when the NP flora is perturbed by interventions such as the introduction of conjugate vaccines, treatment with antimicrobial agents, or improvements in infection control measures (12, 19). Detection of carriage of multiple strains that make up less than 1% of the pneumococcal population will require molecular detection techniques such as multiplex PCR or DNA arrays.
Acknowledgments
Partial support for this work was provided by Projects Projecto PRAXIS/P/SAU/14051/1998 from the Fundação para a Ciência e Tecnologia of Portugal, contract QLK2-CT-2000-01020 from the European Union (to H. de Lencastre), and funds from the Lounsbery Foundation and the Irene Diamond Foundation (to A. Tomasz). R.S.-L. received doctoral grants from Fundação para a Ciência e Tecnologia of Portugal (grant BD/4259/96) and Fundação Calouste Gulbenkian of Portugal.
We thank Rosario Mato, Idalina Bonfim, and Marta Aires de Sousa for participation in the collection and isolation of samples and Duarte Oliveira for helpful discussions.
REFERENCES
- 1.Advisory Committee on Immunization Practices. 1997. Prevention of pneumococcal disease: recommendations of the Advisory Committee on Immunization Practices. Morb. Mortal. Wkly. Rep. 46:1-24. [PubMed] [Google Scholar]
- 2.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barnes, D. M., S. Whittier, P. H. Gilligan, S. Soares, A. Tomasz, and F. W. Henderson. 1995. Transmission of multidrug-resistant serotype 23F Streptococcus pneumoniae in group day care: evidence suggesting capsular transformation of the resistant strain in vivo. J. Infect. Dis. 171:890-896. [DOI] [PubMed] [Google Scholar]
- 4.de Lencastre, H., K. G. Kristinsson, A. Brito-Avô, I. S. Sanches, R. Sá-Leão, J. Saldanha, E. Sigvaldadottir, S. Karlsson, D. Oliveira, R. Mato, M. A. de Sousa, and A. Tomasz. 1999. Carriage of respiratory tract pathogens and molecular epidemiology of Streptococcus pneumoniae colonization in healthy children attending day care centers in Lisbon, Portugal. Microb. Drug Resist. 5:19-29. [DOI] [PubMed] [Google Scholar]
- 5.Dowson, C. G., A. Hutchison, J. A. Brannigan, R. C. George, D. Hansman, J. Linares, A. Tomasz, J. M. Smith, and B. G. Spratt. 1989. Horizontal transfer of penicillin-binding protein genes in penicillin-resistant clinical isolates of Streptococcus pneumoniae. Proc. Natl. Acad. Sci. USA 86:8842-8846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Filipe, S. R., E. Severina, and A. Tomasz. 2000. Distribution of the mosaic structured murM genes among natural populations of Streptococcus pneumoniae. J. Bacteriol. 182:6798-6805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gherardi, G., J. S. Inostrozo, M. O'Ryan, V. Prado, S. Prieto, C. Arellano, R. R. Facklam, and B. Beall. 1999. Genotypic survey of recent β-lactam-resistant pneumococcal nasopharyngeal isolates from asymptomatic children in Chile. J. Clin. Microbiol. 37:3725-3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gray, B. M., G. M. D. Converse, and H. C. Dillon, Jr. 1980. Epidemiologic studies of Streptococcus pneumoniae in infants: acquisition, carriage, and infection during the first 24 months of life. J. Infect. Dis. 142:923-933. [DOI] [PubMed] [Google Scholar]
- 9.Handwerger, S., and A. Tomasz. 1985. Antibiotic tolerance among clinical isolates of bacteria. Annu. Rev. Pharmacol. Toxicol. 25:349-380. [DOI] [PubMed] [Google Scholar]
- 10.Havarstein, L. S., G. Coomaraswamy, and D. A. Morrison. 1995. An unmodified heptadecapeptide pheromone induces competence for genetic transformation in Streptococcus pneumoniae. Proc. Natl. Acad. Sci. USA 92:11140-11144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hodges, R. G., C. M. MacLeod, and W. G. Bernhard. 1946. Epidemic pneumococcal pneumonia. III. Carrier studies. Am. J. Hyg. 44:207-230. [DOI] [PubMed] [Google Scholar]
- 12.Huebner, R. E., R. Dagan, N. Porath, A. D. Wasas, and K. P. Klugman. 2000. Lack of utility of serotyping multiple colonies for detection of simultaneous nasopharyngeal carriage of different pneumococcal serotypes. Pediatr. Infect. Dis. J. 19:1017-1020. [DOI] [PubMed] [Google Scholar]
- 13.Kristinsson, K. G. 1997. Effect of antimicrobial use and other risk factors on antimicrobial resistance in pneumococci. Microb. Drug Resist. 3:117-123. [DOI] [PubMed] [Google Scholar]
- 14.Liu, H. H., and A. Tomasz. 1985. Penicillin tolerance in multiply drug-resistant natural isolates of Streptococcus pneumoniae. J. Infect. Dis. 152:365-372. [DOI] [PubMed] [Google Scholar]
- 15.McGee, L., L. McDougal, J. Zhou, B. G. Spratt, F. C. Tenover, R. George, R. Hakenbeck, W. Hryniewicz, J. C. Lefevre, A. Tomasz, and K. P. Klugman. 2001. Nomenclature of major antimicrobial-resistant clones of Streptococcus pneumoniae defined by the pneumococcal molecular epidemiology network. J. Clin. Microbiol. 39:2565-2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muller-Graf, C. D., A. M. Whatmore, S. J. King, K. Trzcinski, A. P. Pickerill, N. Doherty, J. Paul, D. Griffiths, D. Crook, and C. G. Dowson. 1999. Population biology of Streptococcus pneumoniae isolated from oropharyngeal carriage and invasive disease. Microbiology 145:3283-3293. [DOI] [PubMed] [Google Scholar]
- 17. National Committee for Clinical Laboratory Standards. 1997. Performance standards for antimicrobial disk susceptibility tests. Approved standard M2-A6. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 18.Nesin, M., M. Ramirez, and A. Tomasz. 1998. Capsular transformation of a multidrug-resistant Streptococcus pneumoniae in vivo. J. Infect. Dis. 177:707-713. [DOI] [PubMed] [Google Scholar]
- 19.Obaro, S. K., R. A. Adegbola, W. A. Banya, and B. M. Greenwood. 1996. Carriage of pneumococci after pneumococcal vaccination. Lancet 348:271-272. [DOI] [PubMed] [Google Scholar]
- 20.Porter, R. D., N. B. Shoemaker, G. Rampe, and W. R. Guild. 1979. Bacteriophage-associated gene transfer in pneumococcus: transduction or pseudotransduction? J. Bacteriol. 137:556-567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pozzi, G., L. Masala, F. Iannelli, R. Manganelli, L. S. Havarstein, L. Piccoli, D. Simon, and D. A. Morrison. 1996. Competence for genetic transformation in encapsulated strains of Streptococcus pneumoniae: two allelic variants of the peptide pheromone. J. Bacteriol. 178:6087-6090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramirez, M., D. A. Morrison, and A. Tomasz. 1997. Ubiquitous distribution of the competence related genes comA and comC among isolates of Streptococcus pneumoniae. Microb. Drug Resist. 3:39-52. [DOI] [PubMed] [Google Scholar]
- 23.Ramirez, M., E. Severina, and A. Tomasz. 1999. A high incidence of prophage carriage among natural isolates of Streptococcus pneumoniae. J. Bacteriol. 181:3618-3625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sá-Leão, R., A. Tomasz, and H. de Lencastre. 2001. Multilocus sequence typing of Streptococcus pneumoniae clones with unusual drug resistance patterns: genetic backgrounds and relatedness to other epidemic clones. J. Infect. Dis. 184:1206-1210. [DOI] [PubMed] [Google Scholar]
- 25.Sá-Leão, R., A. Tomasz, I. S. Sanches, A. Brito-Avô, S. E. Vilhelmsson, K. G. Kristinsson, and H. de Lencastre. 2000. Carriage of internationally spread clones of Streptococcus pneumoniae with unusual drug resistance patterns in children attending day care centers in Lisbon, Portugal. J. Infect. Dis. 182:1153-1160. [DOI] [PubMed] [Google Scholar]
- 26.Sá-Leão, R., A. Tomasz, I. S. Sanches, S. Nunes, C. R. Alves, A. B. Avô, J. Saldanha, K. G. Kristinsson, and H. de Lencastre. 2000. Genetic diversity and clonal patterns among antibiotic-susceptible and -resistant Streptococcus pneumoniae colonizing children: day care centers as autonomous epidemiological units. J. Clin. Microbiol. 38:4137-4144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Severina, E., M. Ramirez, and A. Tomasz. 1999. Prophage carriage as a molecular epidemiological marker in Streptococcus pneumoniae. J. Clin. Microbiol. 37:3308-3315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.St. Sauver, J., C. F. Marrs, B. Foxman, P. Somsel, R. Madera, and J. R. Gilsdorf. 2000. Risk factors for otitis media and carriage of multiple strains of Haemophilus influenzae and Streptococcus pneumoniae. Emerg. Infect. Dis. 6:622-630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tomasz, A., J. D. Jamieson, and E. Ottolenghi. 1964. The fine structure of Streptococcus pneumoniae. J. Cell Biol. 22:453-467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Whatmore, A. M., V. A. Barcus, and C. G. Dowson. 1999. Genetic diversity of the streptococcal competence (com) gene locus. J. Bacteriol. 181:3144-3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Widdowson, C. A., and K. P. Klugman. 1999. Molecular mechanisms of resistance to commonly used non-beta-lactam drugs in Streptococcus pneumoniae. Semin. Respir. Infect. 14:255-268. [PubMed] [Google Scholar]