Abstract
By using a TaqMan assay we monitored longitudinal changes in Mycoplasma genitalium loads in five men with recurrent M. genitalium-positive nongonococcal urethritis. We observed regrowth of M. genitalium persisting in hosts after treatment and a possible association of the increase in the M. genitalium load with emergence of symptoms and signs of nongonococcal urethritis in four of these patients.
In 1981, when Mycoplasma genitalium was initially identified, two strains were isolated from two urethral specimens in culture (16). Despite repeated attempts to isolate M. genitalium from the urogenital tract, however, culture of M. genitalium is still immensely difficult. In the 1990s, the advent of PCR-based assays facilitated studies on the association of M. genitalium with acute nongonococcal urethritis (NGU) (9, 11). This mycoplasma has been detected significantly more often in patients with acute NGU, particularly in patients with nonchlamydial NGU, than in subjects without urethritis (6, 8). In 2002 we developed a TaqMan assay for quantification of M. genitalium DNA and quantified M. genitalium in first-pass urine of men with urethritis and of asymptomatic men (17). We reported that the M. genitalium load was significantly higher in men with acute nonchlamydial NGU than in asymptomatic men (17). The various results reported to date suggest that M. genitalium may be associated with the development of acute NGU independent of Chlamydia trachomatis (2, 13).
For chronic NGU, it has been suggested that persistence of M. genitalium in the urethra after antimicrobial chemotherapy might be associated with this condition (5, 7, 10, 15). However, quantitative analysis of M. genitalium has been not performed in cases of chronic NGU. In the present study, therefore, we used the TaqMan assay to monitor longitudinal changes in M. genitalium loads in patients with recurrent NGU and to examine those cases for association of the M. genitalium load with clinical findings and inflammatory responses.
Five men with recurrent NGU, who had been included in a previous study (10), were enrolled in the present study. They attended the Department of Urology, Toyota Memorial Hospital, Toyota, Japan, between July 1999 and December 2001. Each of these patients had symptoms and signs consistent with acute urethritis at the first visit. For each patient, Gram-stained urethral smears showed five or more polymorphonuclear leucocytes (PMNLs) per high-power (×1,000) microscopic field in at least three fields. Urine specimens were subjected to AMPLICOR STD-1 assay (Roche Diagnostics, Indianapolis, Ind.) for detecting Neisseria gonorrhoeae and C. trachomatis and to a PCR- and phylogeny-based assay for detecting mycoplasmas and ureaplasmas. The AMPLICOR STD-1 assay was done according to the manufacturer's instructions. The PCR- and phylogeny-based assay, which could detect at least 10 copies of the 16S rRNA gene of M. genitalium, was done as described in our previous report (18). Two patients were positive for both C. trachomatis and M. genitalium, and three were positive only for M. genitalium. We treated all five patients with levofloxacin at 100 mg three times daily for 14 days. We asked them to practice sexual abstinence during treatment and to return for reexamination 7 days after the start of treatment and at the end of treatment, irrespective of the presence or absence of symptoms. With successive visits, we reexamined urethral smears and retested urine for pathogens.
DNA samples, which had been prepared from the first-pass urine for the phylogeny-based assay and stored at −70°C, were subjected to the TaqMan assay for quantification of M. genitalium DNA (17, 18). Briefly, precipitate from 1 ml of the first-pass urine was obtained by centrifugation and treated with digestion solution containing proteinase K (18). The DNA was purified by a classic phenol-chloroform procedure followed by ethanol precipitation and was then dissolved in 50 μl of TE buffer (10 mM Tris-HCl [pH 8.0], 1 mM EDTA). A portion of the DNA solution was used for the PCR- and phylogeny-based assay, and the remaining solution was frozen and stored.
Sequences of primers and a probe for the TaqMan assay were derived from the 16S rRNA gene of M. genitalium (17). In our previous study this assay was confirmed to be highly specific for M. genitalium (17). For first-pass urine specimens the working range of this assay was from 5 × 107 to 5 × 101 copies of the M. genitalium 16S rRNA gene per 1 ml of urine (17). In this study, after thawing the DNA solutions we used 10 μl of each sample as template DNA for the TaqMan assay. All standard dilutions of M. genitalium DNA, negative controls including no DNAs, and clinical samples were run simultaneously (17). Amplification, data acquisition, and analyses were performed with the ABI Prism 7700 Sequence Detection System (Applied Biosystems, Foster City, Calif.). The number of targets initially present was determined according to a standard curve (4).
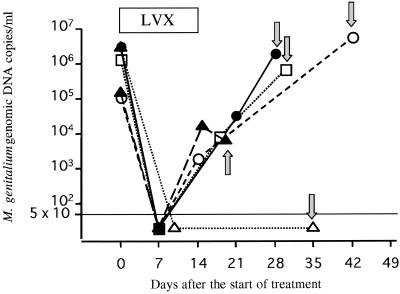
Bacterial load in the five patients ranged from 1.1 × 105 to 3.3 × 106 copies of the M. genitalium 16S rRNA gene per 1 ml of urine (mean, 1.54 × 106 copies/ml) before treatment (Fig. 1). Four patients visited the clinic 7 days after the start of treatment. There were no urethritis symptoms, and the urethral smears were negative for PMNLs. The PCR- and phylogeny-based assay results were positive for the mycoplasma, but M. genitalium loads were suppressed to below the detection level of 50 copies/ml. The four patients returned again for follow-up examination within 7 days after completing the 14-day levofloxacin treatment. They complained of no urethritis symptoms, the smears were still negative for PMNLs, and two of them, who had been positive for C. trachomatis at the first visit, were negative for chlamydia; thus, their NGU was judged clinically cured. In these four patients, however, the M. genitalium load was higher than it was 7 days after the start of the treatment and ranged from 2.0 × 103 to 2.8 × 104 copies/ml (mean, 1.35 × 104 copies/ml) (Fig. 1). From 4 to 28 days after completion of the 14-day levofloxacin treatment, each of these four patients noticed urethral symptoms and returned to the clinic. Urethral smears were positive for PMNLs. We considered the recurrent NGU to have occurred by M. genitalium persisting in the urethra, since all four patients reported abstinence or protected sexual intercourse with the use of condoms after completing the treatment. The first-pass urine samples were positive for M. genitalium by the PCR- and phylogeny-based assay, and the M. genitalium load ranged from 6.0 × 103 to 5.5 × 106 copies/ml (mean, 2.01 × 106 copies/ml) according to the TaqMan assay (Fig. 1). The load at the time of recurrence of NGU was as high as it was at the first visit when definite NGU was observed.
FIG. 1.
Longitudinal changes in M. genitalium loads in five patients with recurrent M. genitalium-positive NGU. The M. genitalium 16S rRNA gene in the first-pass urine samples of the patients was quantified by the TaqMan assay. The M. genitalium load was suppressed to below the detection level of 50 copies/ml in some urine samples during and after the levofloxacin (LVX) treatment, but all samples were positive for the mycoplasma according to the PCR- and phylogeny-based assay. Arrows indicate the M. genitalium load at the time when the patient returned to the clinic with recurrent urethritis symptoms. For four (○, •, □, and ▴) of the five patients, the load at the time of recurrence of NGU was as high as it was at the time of the first visit when definite NGU was observed. For the remaining patient (▵), no increase in the number of M. genitalium organisms was observed at the time of NGU recurrence.
The remaining patient, for whom the M. genitalium load was 3.0 × 106 copies/ml before treatment, visited the clinic 10 days after the start of treatment (Fig. 1). He had no symptoms of urethritis, and the urethral smear was negative. We judged the NGU to be clinically cured. The PCR- and phylogeny-based assay showed the first-pass urine to be positive for M. genitalium, but the bacterial load was <50 copies/ml according to the TaqMan assay. When he returned with symptoms 21 days after completing the 14-day levofloxacin treatment, his urethral smear was positive for PMNLs. Although the PCR- and phylogeny-based assay was positive for the mycoplasma, the M. genitalium load still remained <50 copies/ml. No increase in the number of M. genitalium organisms was observed at the time of NGU recurrence; thus, other pathogens might have been responsible for the presence of NGU in this case.
Presently, guidelines or recommendations are not available for the treatment of M. genitalium-positive urethritis. The in vitro antimicrobial susceptibility profile of M. genitalium is similar to that of Mycoplasma pneumoniae (14). Tetracyclines, macrolides, and some of the newer quinolones are active against M. genitalium (1, 14). However, clinical data on treating M. genitalium-positive NGU with such anitmicrobial agents are extremely limited. In managing M. pneumoniae-induced disease, the mycoplasma is not easily eliminated from the respiratory tract, even when individuals are given adequate doses of antibiotics to which the mycoplasma is known to be sensitive in vitro (3, 12). Antibiotic treatments decrease numbers of the mycoplasma to undetectable levels, but in some cases the mycoplasma reappears and persists in the respiratory tract without clinical signs (3, 12). In this study, M. genitalium loads from men with M. genitalium-positive NGU were suppressed to below the detection level of 50 copies/ml during levofloxacin therapy, though the PCR- and phylogeny-based assay results were positive for the mycoplasma and urethritis symptoms and signs disappeared. At the end of the treatment the mycoplasma loads became detectable by the TaqMan assay, but there were still no symptoms or signs. Such persistent M. genitalium in the urethra for NGU might be analogous to persistent M. pneumoniae in the respiratory tract. Although the number of cases was quite limited in our present study, we observed regrowth of M. genitalium persisting in hosts and a possible association of an increase in the M. genitalium load with the emergence of symptoms and signs of NGU. In our previous study we observed no recurrence of NGU in seven patients with M. genitalium-positive NGU who became negative for the mycoplasma by the PCR and phylogeny assay after antimicrobial chemotherapy. Further studies are required to establish a new treatment algorithm for NGU, including M. genitalium-positive NGU. However, our results suggest that the antimicrobial chemotherapy that is capable of eliminating the mycoplasma from the urethra may be recommended in the management of patients with M. genitalium-positive NGU to prevent the recurrence of NGU.
REFERENCES
- 1.Bebear, C. M., H. Renaudin, A. Bryskier, and C. Bebear. 2000. Comparative activities of telithromycin (HMR 3647), levofloxacin, and other antimicrobial agents against human mycoplasmas. Antimicrob. Agents Chemother. 44:1980-1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deguchi, T., and S.-I. Maeda. 2002. Mycoplasma genitalium: another important pathogen of nongonococcal urethritis. J. Urol. 167:1210-1217. [DOI] [PubMed] [Google Scholar]
- 3.Foy, H. M., J. T. Grayston, G. E. Kenny, E. R. Alexander, and R. McMahan. 1966. Epidemiology of Mycoplasma pneumoniae infection in families. JAMA 197:859-866. [PubMed] [Google Scholar]
- 4.Heid, C. A., J. Stevens, K. J. Livak, and P. M. Williams. 1996. Real time quantitative PCR. Genome Res. 6:986-994. [DOI] [PubMed] [Google Scholar]
- 5.Hooton, T. M., M. C. Roberts, P. L. Roberts, K. K. Holmes, W. E. Stamm, and G. E. Kenny. 1988. Prevalence of Mycoplasma genitalium determined by DNA probe in men with urethritis. Lancet i:266-268. [DOI] [PubMed]
- 6.Horner, P. J., C. B. Gilroy, B. J. Thomas, R. O. Naidoo, and D. Taylor-Robinson. 1993. Association of Mycoplasma genitalium with acute non-gonococcal urethritis. Lancet 342:582-585. [DOI] [PubMed] [Google Scholar]
- 7.Horner, P., B. Thomas, C. B. Gilroy, M. Egger, and D. Taylor-Robinson. 2001. Role of Mycoplasma genitalium and Ureaplasma urealyticum in acute and chronic nongonococcal urethritis. Clin. Infect. Dis. 32:995-1003. [DOI] [PubMed] [Google Scholar]
- 8.Jensen, J. S., R. Orsum, B. Dohn, S. Uldum, A. M. Worm, and K. Lind. 1993. Mycoplasma genitalium: a cause of male urethritis? Genitourin. Med. 69:265-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jensen, J. S., S. A. Uldum, J. Sondergard-Andersen, J. Vuust, and K. Lind. 1991. Polymerase chain reaction for detection of Mycoplasma genitalium in clinical samples. J. Clin. Microbiol. 29:46-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maeda, S. I., M. Tamaki, K. Kojima, T. Yoshida, H. Ishiko, M. Yasuda, and T. Deguchi. 2001. Association of Mycoplasma genitalium persistence in the urethra with recurrence of nongonococcal urethritis. Sex. Transm. Dis. 28:472-476. [DOI] [PubMed] [Google Scholar]
- 11.Palmer, H. M., C. B. Gilroy, P. M. Furr, and D. Taylor-Robinson. 1991. Development and evaluation of the polymerase chain reaction to detect Mycoplasma genitalium. FEMS Microbiol. Lett. 61:199-203. [DOI] [PubMed] [Google Scholar]
- 12.Smith, C. B., W. T. Friedewald, and R. M. Chanock. 1967. Shedding of Mycoplasma pneumoniae after tetracycline and erythromycin therapy. N. Engl. J. Med. 276:1172-1175. [DOI] [PubMed] [Google Scholar]
- 13.Taylor-Robinson, D. 2002. Mycoplasma genitalium—an up-date. Int. J. STD AIDS 13:145-151. [DOI] [PubMed] [Google Scholar]
- 14.Taylor-Robinson, D., and C. Bebear. 1997. Antibiotic susceptibilities of mycoplasmas and treatment of mycoplasmal infections. J. Antimicrob. Chemother. 40:622-630. [DOI] [PubMed] [Google Scholar]
- 15.Taylor-Robinson, D., C. B. Gilroy, and P. E. Hay. 1993. Occurrence of Mycoplasma genitalium in different populations and its clinical significance. Clin. Infect. Dis. 17(Suppl. 1):66-68. [DOI] [PubMed] [Google Scholar]
- 16.Tully, J. G., D. Taylor-Robinson, R. M. Cole, and D. L. Rose. 1981. A newly discovered mycoplasma in the human urogenital tract. Lancet i:1288-1291. [DOI] [PubMed]
- 17.Yoshida, T., T. Deguchi, M. Ito, S.-I. Maeda, M. Tamaki, and H. Ishiko. 2002. Quantitative detection of Mycoplasma genitalium from first-pass urine of men with nongonococcal urethritis and asymptomatic men by real-time PCR. J. Clin. Microbiol. 40:1451-1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yoshida, T., S.-I. Maeda, T. Deguchi, and H. Ishiko. 2001. Phylogeny-based rapid identification of mycoplasmas and ureaplasmas from urethritis patients. J. Clin. Microbiol. 40:105-110. [DOI] [PMC free article] [PubMed] [Google Scholar]