Abstract
Pyrazinamide (PZA) is an integral component of the short-course chemotherapy regimen for tuberculosis. The BACTEC 460TB PZA susceptibility test for Mycobacterium tuberculosis with a daily (D) reading schedule has been available for more than 10 years, but weekend laboratory staffing is necessary. A nonweekend (NW) reading schedule has not been validated in a multicenter study. This prospective multicenter study compares the interlaboratory reproducibility of PZA susceptibility results by following both the D and NW schedules. A total of 181 cultures were shared among four laboratories. Isolates were selected based on resistance or borderline resistance to at least one streptomycin-isoniazid-rifampin-ethambutol drug or PZA. One laboratory used a D reading schedule, and three laboratories used a NW schedule. Both reading schedules are based on the standard BACTEC 460TB PZA protocol. With the NW schedule, the growth index (GI) is not available for test interpretation on Saturday, Sunday, and Monday. Of the 181 shared cultures, 154 were found to be susceptible by all laboratories, 19 were found to be resistant, and 8 had discordant results. The overall pairwise interlaboratory agreement was 97.7%. The discrepancies were not associated with the type of reading schedule used. However, the median control GI was significantly higher for the NW schedule (321) than for the D schedule (259) (P < 0.0001) although results were available on average in about 7 days from setup for both schedules. These results show that the NW schedule is a suitable alternative for laboratories that do not read and interpret PZA susceptibility tests on weekends.
Pyrazinamide (PZA) is an integral part of the short-course treatment regimen for tuberculosis (TB) (2). Drug susceptibility testing of Mycobacterium tuberculosis isolates against PZA is recommended by the Centers for Disease Control and Prevention (CDC) for areas where drug resistance is prevalent (2). Several tests have been developed to determine the susceptibility of M. tuberculosis isolates to PZA. In vitro tests for PZA susceptibility have been demonstrated in low-pH 7H10 agar medium (1, 16, 17). A simple pyrazinamidase test initially developed for identifying mycobacteria revealed that PZA resistance was associated with the absence of this enzyme (10, 19).
Only the radiometric method (BACTEC 460TB; Becton Dickinson Diagnostic Systems, Sparks, Md.) is presently recommended by NCCLS for the PZA susceptibility testing of M. tuberculosis isolates (9). Although the reproducibility of BACTEC 460TB PZA susceptibility testing has been questioned, no systematic evaluation of the interlaboratory reproducibility of this test has been reported (3, 5, 6, 7, 8, 11, 20).
The manufacturer's recommended procedure for the BACTEC 460TB test involves reading the growth of mycobacteria on a daily (D) basis. A nonweekend (NW) reading schedule is provided by the manufacturer for the streptomycin-isoniazid-rifampin-ethambutol drugs as an alternative to allow laboratories that do not read the radiometric tests on weekends to perform drug susceptibility testing (4). For laboratories that also test for PZA susceptibility, however, no NW reading schedule is recommended. A small study in one laboratory (W. Gross, F. S. Wadney, W. Forenbach, D. A. Bonato, and S. Campbell, Abstr. 94th Gen. Meet. Am. Soc. Microbiol. 1994, abstr. U-140, 1994) revealed 100% agreement in the use of NW and D reading schedules with 24 PZA-susceptible M. tuberculosis strains and 96% agreement with 23 PZA-resistant strains.
A prospective multicenter study involving four laboratories was performed to evaluate the interlaboratory reproducibility of M. tuberculosis drug susceptibility testing for PZA. This study provides reproducibility results comparing a NW reading schedule with the manufacturer's recommended D reading schedule for PZA susceptibility testing (S. Siddiqi, BACTEC 460TB Systems product and procedure manual, Becton-Dickinson Diagnostic Instrument Systems, Sparks, Md., 1996).
MATERIALS AND METHODS
Study design.
Mycobacteriology laboratories enrolled in the study included the Veterans Administration TB reference laboratory, two state public health laboratories, and one large county public health laboratory. A total of 181 isolates were shared among the four study laboratories. A total of 169 of the isolates were found by the study laboratories to be resistant or borderline resistant by the BACTEC 460TB method to one or more of the streptomycin-isoniazid-rifampin-ethambutol drugs or PZA and were then distributed to the other study laboratories for PZA susceptibility testing. The remaining 12 isolates were originally tested by the CDC Mycobacteriology Laboratory and then distributed to the study laboratories for PZA susceptibility testing. All 12 isolates were tested by the CDC by following the D reading schedule, and 6 of the 12 isolates were characterized for pyrazinamidase and pncA gene analysis.
PZA susceptibility determination.
The drug concentration of PZA tested in the BACTEC 460TB medium was 100 μg/ml. Three laboratories used the NW reading schedule (4), and one laboratory used the D reading schedule (BACTEC manual, Becton-Dickinson). Of the three laboratories that used a NW reading schedule, two set up PZA susceptibility tests only on Friday while the third laboratory set up on Wednesday, Thursday, or Friday based on test volume. The D reading laboratory set up twice per week on any day of the week.
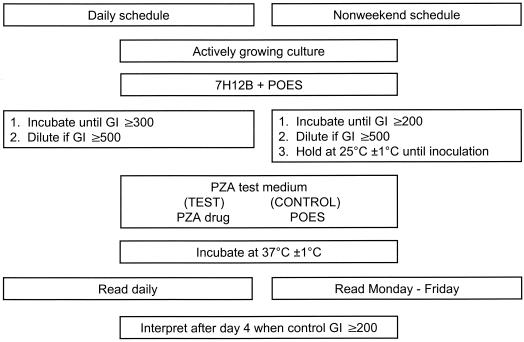
Figure 1 shows the flowchart for the D (BACTEC manual, Becton-Dickinson) and NW testing and reading schedules. Test results were recorded when the growth index (GI) of the control vial reached ≥200. The GI is a measure of the metabolic activity of the isolate in the growth medium. PZA susceptibility test results were interpreted as susceptible when the GI in the drug vial was <9% of the GI in the control vial. The isolate was interpreted as PZA resistant when the GI in the drug vial was >11% of that in the control vial. When the GI in the PZA vial was between 9 and 11% of that in the control vial, the isolate was interpreted as borderline resistant. The D schedule consisted of reading the control and test vials each day, including weekends (BACTEC manual, Becton-Dickinson), whereas in the NW testing protocol, control and drug vials were not read on Saturday and Sunday. Vials were read on Monday, but GIs were disregarded because they represented a 3-day accumulation of 14CO2 in the vials. GIs read on Tuesday through Friday were used for the interpretation. Vials are read within 2 h of the same time each day in both protocols. On the NW reading schedule, an isolate that gave a control GI of at least 999 and a PZA GI above 90 could not be interpreted as sensitive or resistant and was categorized as indeterminate in this study. The susceptibility testing data recorded included the number of days needed to reach an interpretable result based on the initial test, control GI, and susceptibility interpretation for PZA.
FIG. 1.
Flowchart for the D and NW reading schedules for BACTEC 460TB PZA susceptibility testing. POES, polyoxyethylene stearate.
RESULTS
Time for reporting results.
The 181 shared isolates yielded a total of 710 susceptibility results. Of the 710 M. tuberculosis PZA susceptibility results, 134 of 176 (76.1%) were reported within 4 to 7 days of test inoculation by the laboratory following a D reading schedule while 424 of 534 (79.4%) were reported within 4 to 7 days by the three laboratories following the NW reading schedule. Although this difference was not statistically significant (Fisher's exact test, P = 0.45), we found considerable differences among the NW laboratories in the percentage of results reported within 4 to 7 days (68.5, 82.2, and 87.5%) (Fisher's exact test, P < 0.0001).
GI of PZA control.
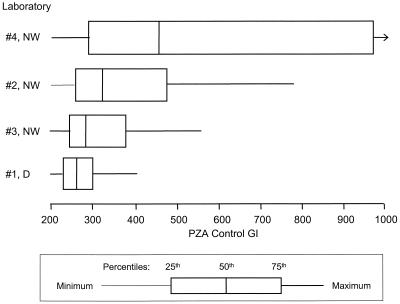
Of the numbers of samples on the D reading schedule, 171 of 176 (97.2%) isolates tested had a PZA control GI between 200 and 400 and all of them had control GIs less than 600 at the time of interpretation of the test results. For the two laboratories on the NW schedule that set up on Friday (labs 2 and 3), 97 of 354 (27.4%) isolates had a control GI greater than 400, including 33 (9.3%) which had a control GI of at least 999. For the laboratory on the NW schedule which did not necessarily set up on Friday (laboratory 4), 98 of 180 (54.4%) isolates had a control GI greater than 400, including 44 (24.4%) which had a control GI of at least 999 (Fig. 2). The median control GI at interpretation was significantly higher for the NW laboratory without Friday setup (436) than that for the NW laboratories with Friday setup (298) (Wilcoxon signed-rank test, P < 0.0001), which in turn was significantly higher than that for the D laboratory (259) (Wilcoxon signed-rank test, P < 0.0001). Test results could still be interpreted as susceptible for an isolate with a control GI of at least 999 and a PZA GI below 90. However, an isolate with a control GI of at least 999 and a PZA GI above 90 could not be interpreted as sensitive, resistant, or borderline. Of the 77 isolates with a control GI of at least 999, 9 (11.7%) had a PZA GI above 90 and hence were characterized as indeterminate.
FIG. 2.
Boxplot of BACTEC PZA control GIs for laboratories using the D and NW reading schedules.
Isolates shared between laboratories.
Results were compared for 181 isolates tested in the three laboratories using the NW reading schedule and the one laboratory using the D reading schedule. We found good agreement between the NW and D reading schedule results among the laboratories for susceptible, borderline, and resistant results (Table 1). There were nine indeterminate results. Two of these indeterminate results were from an isolate with no test result provided by the D laboratory. Of the remaining seven isolates with indeterminate results from a NW laboratory, six were found to be resistant by the laboratory using the D reading schedule.
TABLE 1.
Agreement between results from D and NW schedule laboratories
| NW schedule laboratory and result | No. of isolates from D schedule that were:
|
||
|---|---|---|---|
| Susceptible | Borderline | Resistant | |
| Laboratory 2 | |||
| Susceptible | 147 | 2 | 2 |
| Borderline | 1 | 0 | 0 |
| Resistant | 2 | 0 | 14 |
| Indeterminatea | 0 | 0 | 2 |
| Laboratory 3 | |||
| Susceptible | 149 | 1 | 3 |
| Borderline | 0 | 0 | 0 |
| Resistant | 2 | 1 | 15 |
| Indeterminatea | 0 | 0 | 1 |
| Laboratory 4 | |||
| Susceptible | 148 | 0 | 2 |
| Borderline | 0 | 0 | 0 |
| Resistant | 2 | 2 | 14 |
| Indeterminatea | 1 | 0 | 3 |
Control GI, ≥999; PZA GI, ≥90.
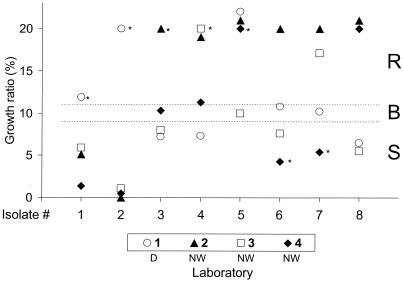
Of the 181 shared cultures, 154 were found to be susceptible by all laboratories that tested them, 19 were found to be resistant by all laboratories that tested them, and 8 produced discordant results. The eight cultures with discordant results included four with borderline results. The overall pairwise interlaboratory agreement was 97.7%. The cultures with discordant results and their GI ratios from each laboratory are shown in Fig. 3. These discrepancies do not appear to be associated with the reading schedule used or with a particular laboratory.
FIG. 3.
BACTEC growth ratios of discordant isolates. An asterisk indicates a source laboratory. Isolate 8 was from the CDC. R, resistant; B, borderline; S, susceptible.
Reproducibility of results on characterized isolates.
As a further check on the reproducibility between laboratories performing testing according to the D and NW reading schedules, 12 M. tuberculosis isolates from the CDC Mycobacteriology Laboratory were sent to each of the four study laboratories for BACTEC 460TB PZA susceptibility testing. Six of the isolates were characterized for mutations in the pncA gene and for pyrazinamidase activity. Three of the six isolates had pncA gene mutations and lacked pyrazinamidase activity. We found agreement among all four laboratories, and in the CDC laboratory, there was agreement on five of the six isolates. For the one isolate with discordant results, two NW laboratories reported the isolate as resistant and one reported it as susceptible, while both the D laboratory and CDC reported the isolate as susceptible. CDC reported that the isolate lacked a mutation in the pncA gene and was positive for pyrazinamidase activity. Of the other six uncharacterized cultures, there were no discordant results among the laboratories that tested the isolates, including the CDC laboratory.
DISCUSSION
For achieving optimal results when using the NW protocol for PZA drug susceptibility testing, the standard manufacturer's protocol should be followed with close attention when preparing and storing the inoculum. Deviations may result in PZA control and test results with GIs of at least 999 that may be difficult to interpret.
Although the CDC recommends that drug susceptibility tests be set up promptly and that samples not be held for batching (18), results from NW testing laboratories show that the best day for setting up test vials is Friday because the PZA control and test vials will be at the required fourth day of incubation after the first weekend and these test vials have a high probability of reaching a GI of 200 prior to being held over the second weekend. This conclusion is based on the percentage of cultures with GIs of at least 999 and the number of cultures that are read between 4 and 7 days. In addition, there were no isolates with GIs of at least 999 on the D schedule.
Several studies point to a lack of reproducibility of PZA susceptibility testing results among different laboratories (6, 10). In our study, however, the overall pairwise interlaboratory agreement was high (97.7%).
Isolates of M. tuberculosis that produced PZA susceptibility results that were in the borderline range of 9 to 11% designated in the BACTEC test may be easily influenced by the number of resistant mycobacteria in the population and other factors in the test procedure (6, 21). Of the eight isolates with discordant results, five might have been resolved if the borderline range of the PZA ratio were widened from 9 to 11% to 7 to 13% and all isolates with borderline results were retested. Doing so, however, would have increased the number of borderline results from 4 (0.6%) to 17 (2.4%).
Our findings do not suggest a significant difference in results between the D and NW reading schedules for PZA susceptibility tests by the BACTEC 460TB method. Reproducibility of PZA susceptibility test results between laboratories may improve in the future with new methods and as the mechanisms of PZA resistance in M. tuberculosis are better understood (3, 10, 12, 13, 14, 15, 16).
Acknowledgments
We thank Angie Schooley, Steve Church, Dale Berry, Don Bonato, Lynn Ladutko, Raymond Jansen, Paul Temprendola, and David Sikes for technical assistance. We also thank James Thompson for editorial review.
This work was supported by CDC cooperative agreements with the Michigan Department of Community Health, Lansing, Mich. (U52/CCU500499); the San Diego County Department of Public Health, San Diego, Calif. (U52/CCU900452); and the State Laboratory Institute, Boston, Mass. (U52/CCU100516) and an interagency agreement (98-FED-14558) with the Veterans Administration Hospital TB Reference Laboratory, West Haven, Conn.
Use of trade names and commercial sources is for identification only and does not imply endorsement by the Public Health Service or by the U.S. Department of Health and Human Services.
REFERENCES
- 1.Butler, W. R., and J. O. Kilburn. 1982. Improved method for testing susceptibility of Mycobacterium tuberculosis to pyrazinamide. J. Clin. Microbiol. 6:1106-1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. 1993. Initial therapy for tuberculosis in the era of multidrug resistance. Recommendations of the Advisory Council for the Elimination of Tuberculosis. Morb. Mortal. Wkly. Rep. 42(No. RR-7):1-8. [PubMed] [Google Scholar]
- 3.Davies, A. P., O. J. Billington, T. D. McHugh, D. A. Mitchison, and T. P. Gillis. 2000. Comparison of phenotypic and genotypic methods for pyrazinamide susceptibility testing with Mycobacterium tuberculosis. J. Clin. Microbiol. 38:3686-3688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hawkins, J. E. 1986. Nonweekend schedule for BACTEC drug susceptibility testing of Mycobacterium tuberculosis. J. Clin. Microbiol. 23:934-937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heifets, L., and T. Sanchez. 2000. New agar medium for testing susceptibility of Mycobacterium tuberculosis to pyrazinamide. J. Clin. Microbiol. 38:1498-1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hewlett, D., D. L. Horn, and C. Alfalla. 1995. Drug resistant tuberculosis: inconsistent results of pyrazinamide susceptibility testing. JAMA 273:916-917. [PubMed] [Google Scholar]
- 7.Libonati, J. P., C. E. Stager, J. R. Davis, and S. H. Siddiqi. 1988. Direct antimicrobial drug susceptibility testing of Mycobacterium tuberculosis by the radiometric method. Diagn. Microbiol. Infect. Dis. 10:41-48. [DOI] [PubMed] [Google Scholar]
- 8.Marttila, H., M. Marjamaki, E. Vyshvskaya, et al. 1999. pncA mutations in pyrazinamide-resistant Mycobacterium tuberculosis isolates from northwestern Russia. Antimicrob. Agents Chemother. 43:1764-1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.NCCLS. 2000. Tentative standard M24-T2. Susceptibility testing of mycobacteria, nocardia, and other aerobic actinomycetes, 2nd ed. NCCLS, Wayne, Pa. [PubMed]
- 10.Raynaud, C., M. A. Laneelle, R. H. Senaratne, P. Draper, G. Laneelle, and M. Daffe. 1999. Mechanisms of pyrazinamide resistance in mycobacteria: importance of lack of uptake in addition to lack of pyrazinamidase activity. Microbiology 145:1359-1367. [DOI] [PubMed] [Google Scholar]
- 11.Salfinger, M., L. B. Reller, B. Demchuk, and Z. T. Johnson. 1989. Rapid radiometric method for pyrazinamide susceptibility testing of Mycobacterium tuberculosis. Res. Microbiol. 140:301-309. [DOI] [PubMed] [Google Scholar]
- 12.Scorpio, A., and Y. Zhang. 1996. Mutations in pncA, a gene encoding pyrazinamide/nicotinamidase, cause resistance to the antituberculous drug pyrazinamide in tubercle bacillus. Nat. Med. 2:662-667. [DOI] [PubMed] [Google Scholar]
- 13.Scorpio, A., P. Lindholm-Levy, L. Heifets, R. Gilman, S. Siddiqi, M. Cynamon, and Y. Zhang. 1997. Characterization of pncA mutations in pyrazinamide-resistant Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 41:540-543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Speirs, R. J., J. T. Welch, and M. H. Cynamon. 1995. Activity of n-propyl pyrazinoate against pyrazinamide-resistant Mycobacterium tuberculosis: investigations into mechanism of action of and mechanism of resistance to pyrazinamide. Antimicrob. Agents Chemother. 39:1269-1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sreevatsan, S., X. Pan, Y. Zhang, B. N. Kreiswirth, and J. M. Musser. 1997. Mutations associated with pyrazinamide resistance in pncA of Mycobacterium tuberculosis complex organisms. Antimicrob. Agents Chemother. 41:636-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stottmeier, K. D., R. E. Beam, and G. P. Kubica. 1967. Determination of drug susceptibility of mycobacteria to pyrazinamide in 7H10 agar. Am. Rev. Respir. Dis. 96:1072-1075. [DOI] [PubMed] [Google Scholar]
- 17.Tarrand, J. J. 1986. Evaluation of a radiometric method for pyrazinamide susceptibility testing of Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 30:852-855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tenover, F., J. T. Crawford, R. E. Hubner, L. J. Geiter, C. R. Horsburgh, Jr., and R. C. Good. 1993. The resurgence of tuberculosis: is your laboratory ready? J. Clin. Microbiol. 31:767-770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wayne, L. G. 1974. Simple pyrazinamidase and urease tests for routine identification of mycobacteria. Am. Rev. Respir. Dis. 109:147-151. [DOI] [PubMed] [Google Scholar]
- 20.Woodley, C., and R. W. Smithwick. 1988. Radiometric method for pyrazinamide susceptibility testing of Mycobacterium tuberculosis in egg yolk-enriched BACTEC 12A medium. Antimicrob. Agents Chemother. 32:125-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang. Y., S. Permar, and Z. Sun. 2002. Conditions that may affect the results of susceptibility testing of Mycobacterium tuberculosis to pyrazinamide. J. Med. Microbiol. 51:42-49. [DOI] [PubMed] [Google Scholar]