Abstract
Currently, 19 species are recognized in the genus Bartonella, 7 of which are involved in an increasing variety of human diseases. Development of molecular tools for detection, identification, and subtyping of strains and isolates has promoted research on Bartonella spp. We amplified and sequenced the portion of the ftsZ gene encoding the N-terminal region of the cell division protein for 13 Bartonella species: Bartonella alsatica, B. birtlesii, B. doshiae, B. elizabethae, B. grahami, B. koehlerae, B. schoenbuchensis, B. taylorii, B. tribocorum, Bartonella vinsonii subsp. arupensis, Bartonella vinsonii subsp. berkhoffii, Bartonella vinsonii subsp. vinsonii, and B. bovis Bermond et al.(“B. weissii”). Phylogenetically derived trees revealed four statistically supported groups, indicating that sequencing of the ftsZ gene is a useful tool for identifying evolutionary relationships among Bartonella species. Furthermore, we amplified and sequenced the portion of the ftsZ gene encoding the C-terminal region of the protein for 4 B. bacilliformis isolates, 14 B. clarridgeiae isolates, 14 B. quintana isolates, and 30 B. henselae isolates that were obtained from different geographic regions, hosts, and clinical specimens. B. clarridgeiae and B. quintana sequences were highly conserved, while those of the four B. bacilliformis isolates differed from the type strain at 5 positions. Among B. henselae strains isolated from cats and patients, only two genotypes were detected: Houston and Marseille. Among 80 clinical samples we detected Bartonella spp. in 35 (43.75%) and found the assay to be comparable to that of a combined intergenic-spacer-region- and pap31-based PCR assay. Our results show the usefulness of the portion of the ftsZ gene encoding the C-terminal region for diagnosis of Bartonella infections. More samples should be tested to study its usefulness for epidemiological investigations.
The genus Bartonella contains aerobic, fastidious, gram-negative bacilli belonging to the alpha-2 subgroup of the class Proteobacteria. Recently the number of Bartonella species isolated has increased markedly (5, 6, 15, 27), and the bacteria are considered emerging pathogens involved in an increasing number of recognized diseases (1, 28, 38). Currently, 19 Bartonella species are recognized, and all are associated with mammalian hosts. Bartonella taylorii, B. elizabethae, B. tribocorum, and B. birtlesii have been isolated from rats (6, 7, 11, 24); B. grahamii, Bartonella vinsonii subsp. vinsonii, and B. doshiae have been recovered from voles (7, 11); Bartonella vinsonii subsp. arupensis has been isolated from mice (54); B. alsatica has been isolated from rabbits (23); B. koehlerae, B. clarridgeiae, B. henselae, and “B. weissii,” recently described as B. bovis Bermond et al., have been found in cats (5, 17, 29, 31, 35); and B. bovis Bermond et al. has also been detected in cattle (5, 10). Bartonella vinsonii subsp. berkhoffii has been isolated from dogs (10) and coyotes (13); “B. washoensis” has been demonstrated in rodents (R. L. Regnery, personal communication); B. quintana and B. bacilliformis have been isolated from humans (22, 40), and B. schoenbuchensis and B. capreoli have been isolated from wild roe deer (5, 15). To date, 7 of the 19 species have been implicated in human disease (28). B. bacilliformis is the agent of bartonellosis (Carrion's disease), which is endemic in Andean valleys in South America. B. quintana and B. henselae, etiologic agents of trench fever and cat scratch disease (CSD), respectively, have also been associated with endocarditis and bacillary angiomatosis in immunocompromised patients (1). B. elizabethae and B. vinsonii subsp. berkhoffii cause endocarditis (14, 46), and B. vinsonii subsp. arupensis was first isolated from a febrile patient with heart valve disease in the United States (54). B. grahamii has been implicated in cases of neuroretinitis (30), and B. clarridgeiae is also suspected to be an agent of CSD (32, 51). Because no distinguishing phenotypic characteristics have been described for Bartonella species, their identification and phylogenetic classification has been based mainly on genetic studies. DNA hybridization and pulsed-field gel electrophoresis can be used for molecular characterization of Bartonella species (39, 47), but these techniques are not suitable for routine use in a clinical laboratory. PCR-derived assays allow detection and identification of the bacteria directly from clinical samples even in conditions such as CSD, where organisms are infrequently isolated in culture. Many DNA regions and encoding gene sequences have been used in genetic studies: the 16S rRNA gene, the 16S-23S rRNA intergenic spacer region (ITS) (26, 37), the citrate synthase gene (gltA) (8, 9, 25), the riboflavin synthase alpha chain gene (ribC) (2), the heat shock protein gene (groEL) (36, 55), the genes encoding the PAP31 and 35-kDa proteins (33, 56), and the cell division protein gene (ftsZ) (19, 29).
The FtsZ protein plays an important role in bacterial cell division, and its gene sequence has been used to differentiate three Bartonella species (29). Compared to other bacteria, the FtsZ proteins of Bartonella species are nearly twice as large and have an additional region at the C-terminal end (29, 42). The C-terminal region has a higher degree of sequence divergence than the N-terminal region and has recently been used for B. henselae subtyping (19).
In our study we determined a partial 900-base nucleotide sequence of ftsZ encoding the N-terminal region for the main Bartonella species and assessed its usefulness in species differentiation and for inferring interspecies phylogenetic relationships. Furthermore, we investigated PCR of the portion of the ftsZ gene encoding the C-terminal region as a means of detecting and identifying Bartonella spp. in 80 clinical samples. We also studied the usefulness of sequencing the portion of the ftsZ gene encoding the C-terminal end in subtyping B. henselae, B. quintana, B. clarridgeiae, and B. bacilliformis isolates from patients and cats and for epidemiological investigations of infections.
MATERIALS AND METHODS
Bartonella strains, isolates, and DNA extraction.
Strains and isolates used in this study are detailed in Tables 1 and 2. Bartonella isolates were grown on 5% sheep blood agar (Biomerieux, Marcy l'Étoile, France) at 37°C under a 5% CO2-enriched atmosphere. Bacteria were harvested after 7 days of culture, and DNA was extracted by the Chelex method (52). Genomic DNA was stored at 4°C until use as a template in PCR assays.
TABLE 1.
Bacterial strains and sequences used for N-terminal sequencing and phylogeny
| Species (strain) | Collection no. or sourcea | GenBank accession no. for:
|
|
|---|---|---|---|
| 16S rRNA | ftsZ | ||
| Bartonella alsatica (IBS382T) | CIP 105477 | AJ002139 | AF467763 |
| Bartonella bacilliformis (KC584T) | ATCC 35686 | Z11683 | AF007266 |
| Bartonella birtlesii (IBS 325T) | CIP 106294 | AF204274 | AF467762 |
| Bartonella clarridgeiae (Houston-2T) | ATCC 51734 | U64691 | AF141018 |
| Bartonella doshiae (R18T) | NCTC 12862 | Z31351 | AF467754 |
| Bartonella elizabethae (F9251T) | ATCC 49927 | L01260 | AF467760 |
| Bartonella grahamii (V2T) | NCTC 12860 | Z31349 | AF467753 |
| Bartonella henselae (Houston-1T) | ATCC 49882 | M73229 | AF061746 |
| Bartonella koehlerae (C-29T) | ATCC 700693 | AF076237 | AF467755 |
| Bartonella quintana (OklahomaT) | CDC | M11927 | AF061747 |
| Bartonella schoenbuchensis (R1T) | NCTC 13165T | AJ278187 | AF467765 |
| Bartonella taylorii (M6T) | NCTC 12861 | Z31350 | AF467756 |
| Bartonella tribocorum (IBS 506T) | CIP 104576 | AJ003070 | AF467759 |
| Bartonella vinsonii subsp. arupensis (OK 94-513T) | ATCC 700727 | AF214558 | AF467758 |
| Bartonella vinsonii subsp. berkhoffii (93-CO1T) | ATCC 51672 | U26258 | AF467764 |
| Bartonella vinsonii subsp. vinsonii (BakerT) | ATCC VR-152 | M73230 | AF467757 |
| Bartonella “weissi” (FC7049UT)b | AF199502 | AF467761 | |
Abbreviations: CIP, Collection de l'Institut Pasteur; Paris, France; ATCC, American Type Culture Collection, Manassas, Va.; NCTC, National Collection of Type Cultures, Central Public Health Laboratory, London, United Kingdom; CDC, Centers for Disease Control and Prevention, Atlanta, Ga.
Recently described as B. bovis Bermond et al.
TABLE 2.
Bartonella strains and isolates used for C-terminal amplification and sequencing
| Species (strain) | Clinical sourcea | Geographic origin | B. henselae genotype |
|---|---|---|---|
| B. quintana (FullerT) | Trench fever patient | Yugoslavia | |
| B. quintana (OklahomaT) | Bacteremia, HIV-positive patient | United States | |
| B. quintana (URBQMTF 20) | Blood culture | France | |
| B. quintana (URBQMTF 47) | Bacteremia, homeless patient | France | |
| B. quintana (URBQMIE 48) | Endocarditis patient | France | |
| B. quintana (URBQMTF 88) | Blood culture | France | |
| B. quintana (URBQMTF 95) | Bacteremia, homeless patient | France | |
| B. quintana (URBQMTF 96) | Blood culture | France | |
| B. quintana (URBQMNHP 90) | Body louse | France | |
| B. quintana (URBQMNHP 94) | Body louse | France | |
| B. quintana (URBQMNHP 102) | Body louse | France | |
| B. quintana (URBQMNHP 103) | Body louse | France | |
| B. quintana (URBQMTF 108) | Body louse | France | |
| B. henselae Marseille (U8) | Lymph node, CSD patient | France | Marseille |
| B. henselae Marseille (U9) | Endocarditis patient | France | Marseille |
| B. henselae Houston-1 | HIV-positive patient | United States | Houston |
| B. henselae (SA-2) | Suspected CSD patient | United States | Houston |
| B. henselae (90-615) | Lymph node, CSD patient | United States | Houston |
| B. henselae (CAL-1) | Septicemia | United States | Marseille |
| B. henselae EN1 | Endocarditis patient | France | Marseille |
| B. henselae EN2 | Endocarditis patient | France | Marseille |
| B. henselae EN3 | Endocarditis patient | France | Marseille |
| B. henselae 1129 | Endocarditis patient | France | Houston |
| B. henselae 5327 | Endocarditis patient | France | Marseille |
| B. henselae 5097 | Endocarditis patient | France | Houston |
| B. henselae C20 | Cat | France | Houston |
| B. henselae C45 | Cat | France | Houston |
| B. henselae C51 | Cat | France | Marseille |
| B. henselae C52 | Cat | France | Marseille |
| B. henselae C53 | Cat | France | Marseille |
| B. henselae C77 | Cat | France | Houston |
| B. henselae C78 | Cat | France | Houston |
| B. henselae C85 | Cat | France | Houston |
| B. henselae C87 | Cat | France | Houston |
| B. henselae C96 | Cat | France | Houston |
| B. henselae NZ1 | Cat of patient with CSD | Australia | Marseille |
| B. henselae NZ2 | Cat of patient with CSD | Australia | Marseille |
| B. henselae NZ3 | Cat of patient with CSD | Australia | Marseille |
| B. henselae NZ4 | Cat of patient with CSD | Australia | Marseille |
| B. henselae NZ5 | Cat of patient with CSD | Australia | Marseille |
| B. henselae NZ6 | Cat of patient with CSD | Australia | Marseille |
| B. henselae NZ7 | Cat of patient with CSD | Australia | Houston |
| B. henselae NZ8 | Cat of patient with CSD | Australia | Marseille |
| B. bacilliformisT | Bartonellosis patient | Peru | |
| B. bacilliformis Cuzco 8 | Bartonellosis patient | Peru | |
| B. bacilliformis Cuzco 14 | Bartonellosis patient | Peru | |
| B. bacilliformis Cuzco 269 | Bartonellosis patient | Peru | |
| B. bacilliformis Cuzco 812 | Bartonellosis patient | Peru | |
| B. clarridgeiae | Cat | United States | |
| B. clarridgeiae C23 | Cat | France | |
| B. clarridgeiae C44 | Cat | France | |
| B. clarridgeiae C48 | Cat | France | |
| B. clarridgeiae C49 | Cat | France | |
| B. clarridgeiae C53 | Cat | France | |
| B. clarridgeiae C68 | Cat | France | |
| B. clarridgeiae C69 | Cat | France | |
| B. clarridgeiae C71 | Cat | France | |
| B. clarridgeiae C73 | Cat | France | |
| B. clarridgeiae C74 | Cat | France | |
| B. clarridgeiae C75 | Cat | France | |
| B. clarridgeiae C76 | Cat | France | |
| B. clarridgeiae C157 | Cat | France |
HIV, human immunodeficiency virus.
PCR amplification and DNA sequencing of the portion of the ftsZ gene encoding the N-terminal region.
Primers (Eurobio, Les Ulis, France) used for amplification and sequencing are shown in Table 3. PCRs were carried out in a PTC-200 automated thermocycler (MJ Research, Waltham, Mass.) using an Elongase DNA polymerase kit (Gibco-BRL, Cergy Pontoise, France) and primers Bfp1 and Bfp2 (Table 3). Reaction mixtures (25 μl) contained the following (final concentrations): primers (0.5 pmol μl−1 each), deoxynucleoside triphosphates (dATP, dCTP, dGTP, and dTTP) (0.2 mM μl−1 each), 1 μl of buffer A, 4 μl of buffer B, 0.6 μl of Elongase enzyme mix, 2.5 μl of DNA (150 to 200 ng), and sterile water. PCR amplifications were performed as follows: a 4-min denaturation at 94°C was followed by 44 cycles of denaturation for 30 s at 94°C, annealing for 30 s at 55°C, and extension for 60 s at 68°C. Amplification was completed by holding the reaction mixture at 68°C for 10 min to ensure complete extension of the PCR products. These were separated by electrophoresis on 1% agarose gels, visualized by staining with ethidium bromide, and purified with the QIAquick PCR purification kit (Qiagen, Hilden, Germany) according to the manufacturer's instructions. PCR products were sequenced in both directions using primers Bfp1, Bfp2, Bfs3, and Bfs4 and the d-Rhodamine Terminator Cycle Sequencing Ready Reaction kit (Perkin-Elmer, Coignieres, France) according to the manufacturer's instructions. Sequencing products were resolved using an ABI 3100 automated sequencer (Perkin-Elmer).
TABLE 3.
Primers used for PCR and/or sequencing
| Primera | Bartonella species | Primer sequence | Source or reference |
|---|---|---|---|
| Bfp1 (ap,s) | All | 5′-ATTAATCTGCAYCGGCCAGA-3′ | This study |
| Bfp2 (ap,s) | All | 5′-ACVGADACACGAATAACACC-3′ | This study |
| Bfs3 (s) | All | 5′-TTACAAAAATCYGTTGATAC-3′ | This study |
| Bfs4 (s) | All | 5′-GTATCAACRGATTTTTGTAA-3′ | This study |
| BaftsZF (ap,s) | All | 5′-GCTAATCGTATTCGCGAAGAA-3′ | This study |
| BaftsZR (ap,s) | All | 5′-GCTGGTATTTCCAAYTGATCT-3′ | This study |
| BhftsZ 1393.n (s) | B. henselae, B. clarridgeiae | 5′-GCGAACTACGGCTTACTTGC-3′ | 19 |
| Bh ftsZ 1247.p (s) | B. henselae, B. clarridgeiae | 5′-CGGTTGGAGAGCAGTTTCGTC-3′ | 19 |
| Bq ftsZseqF (s) | B. quintana | 5′-GCACATATTCTTGATGAGAT-3′ | This study |
| Bq ftsZseqR (s) | B. quintana | 5′-CCCCTATCATCTCATCAAG-3′ | This study |
| Bb ftsZseqF (s) | B. bacilliformis | 5′-GCGCATGTTCTTAGTGAAAT-3′ | This study |
| Bb ftsZseqR (s) | B. bacilliformis | 5′-CCTGTATACGTGATGCATTT-3′ | This study |
| FTS1p (ap,s) | All | 5′-GCCTTCTCATCCTCAACTT-3′ | This study |
| FTS2p (ap,s) | All | 5′-CAGCCTCTTCACGATGTG-3′ | This study |
ap, amplification primer; s, sequencing primer.
Analysis of sequences and construction of phylogenetic trees.
Sequence analysis was performed with ABI Prism DNA Sequencing Analysis Software, version 3.0 (Perkin Elmer), and multisequence alignment was performed with CLUSTAL W software, version 1.81 (53). DNA sequence similarities were calculated by use of MEGA 2.1 software (S. Kumar, K. Tamura, I. B. Jakobsen, and M. Nei, Molecular Evolutionary Genetics Analysis software, Tempe, Ariz., 2001). Phylogenetic trees were obtained from DNA sequences by using the maximum-parsimony method (DNAPARS software in PHYLIP) (20), distance methods (DNADIST [distance matrix with Kimura 2 parameters or Jukes-Cantor parameters] and NEIGHBOR [neighbor joining]), and the maximum-likelihood method (DNAMLK software in PHYLIP). Bootstrap replicates were performed to estimate the node reliability of the phylogenetic trees obtained by the three methods (12). Bootstrap values were obtained from 100 trees (18) generated randomly with SEQBOOT and CONSENSE in the PHYLIP software package. Only values above 90 were considered significant. Phylogenetic trees were established by using TreeView, version 1.5 (43). Only neighbor-joining trees are presented in this report. The phylogenetic trees we obtained were compared with those available for Bartonella species in GenBank, which were inferred from analyses of the 16S rRNA, gltA, rpoB, ITS, and groEL gene sequences.
PCR amplification and DNA sequencing of the portion of the ftsZ gene encoding the C-terminal region.
Primers used for amplification and sequencing of Bartonella isolates and clinical samples are described in Table 3. PCR was carried out as described above by using primers BaftsZF and BaftsZR with 56°C as the annealing temperature. Sequencing was performed as described above by using primers BaftsZF, BaftsZR, BhftsZ 1393.n, Bh ftsZ 1247.p, Bq ftsZseqF, Bq ftsZseqR, Bb ftsZseqF, and Bb ftsZseqR. The resulting sequences from the different Bartonella species were compared in order to investigate the usefulness of the C-terminal region in genotyping.
Clinical samples and DNA extraction.
Eighty lymph node biopsy, lymph node aspirate, or valve samples from 79 patients with suspected CSD or endocarditis were sent to the Unité des Rickettsies to be tested for the presence of Bartonella spp. during December 2001. Thirty-nine samples had been found positive for Bartonella spp. by use of ITS- and pap31-based PCR assays (47, 56). Ten to 25 mg of tissue or 200 μl of aspirate was used for extraction of total genomic DNA with the QIAamp tissue kit (Qiagen) according to the manufacturer's instructions. Samples were handled under sterile conditions to avoid the risk of cross-contamination. Extracted DNA was suspended in 125 μl of elution buffer and stored at 4°C. DNAs from 10 bacterial strains and isolates were used as a negative control: Rickettsia helvetica, Escherichia coli, Mycobacterium tuberculosis, Pseudomonas aeruginosa, Tropheryma whipplei, Afipia felis, Coxiella burnetii, Bosea massiliensis, Staphylococcus aureus, and Enterococcus faecalis.
For the PCR assays, samples were thawed at room temperature and amplified with primers FTS1p and FTS2p by using 56°C as the annealing temperature. Sterile distilled water was used in negative controls. A seminested groEL-derived assay was carried out (56) on samples for which discrepant results had been obtained in the ftsZ assay and the combined ITS/pap31 assay, which was performed as previously described (47, 56).
Statistical analysis.
Fisher's exact test was used to compare the results of the combined ITS/pap31 assay and the ftsZ assay. Observed differences were considered significant when the P value was <0.05 for two-tailed tests.
RESULTS
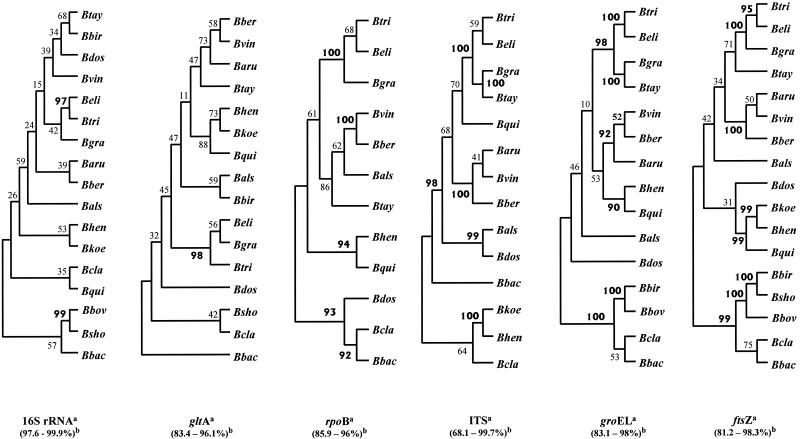
Amplification of the portion of the ftsZ gene encoding the N-terminal region for all the Bartonella species used in the experiments yielded a single product of nearly 900 bp. Pairwise comparison of these and the reported (Table 1) ftsZ sequences revealed a sequence similarity ranging from 81.2 to 98.3% (Table 4). When compared to the sequence similarities of other genes of Bartonella species available in GenBank, ftsZ sequence similarity was found to be similar to those of gltA (83.4 to 96.1%), rpoB (85.9 to 96%), and groEL (83.1 to 98%), higher than that of the ITS (69.1 to 99.7%), and lower than that of the 16S ribosomal DNA (rDNA) (97.7 to 99.8%) (Fig. 1).
TABLE 4.
Level of ftsZ DNA sequence similarity for Bartonella species
| Taxon | Similarity (%) with taxon:
|
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | |
| 1) B. alsatica | ||||||||||||||||
| 2) B. vinsonii subsp. arupensis | 92.9 | |||||||||||||||
| 3) B. bacilliformis | 86.8 | 87.9 | ||||||||||||||
| 4) B. vinsonii subsp. Berkhoffii | 92.6 | 98.0 | 88.4 | |||||||||||||
| 5) B. birtlesii | 98.3 | 88.6 | 88.6 | 88.7 | ||||||||||||
| 6) B. clarridgeiae | 87.5 | 88.3 | 88.9 | 88.2 | 88.2 | |||||||||||
| 7) B. doshiae | 90.5 | 91.4 | 86.7 | 91.2 | 88.4 | 86.9 | ||||||||||
| 8) B. elizabethae | 89.5 | 91.6 | 86.9 | 91.2 | 87.2 | 86.8 | 89.6 | |||||||||
| 9) B. grahamii | 90.9 | 91.7 | 86.7 | 91.5 | 87.5 | 87.8 | 88.9 | 95.4 | ||||||||
| 10) B. henselae | 92.0 | 93.1 | 87.7 | 92.2 | 88.9 | 87.9 | 91.5 | 90.5 | 90.1 | |||||||
| 11) B. koehlerae | 91.2 | 92.2 | 87.7 | 91.6 | 88.8 | 88.2 | 90.3 | 90.5 | 90.0 | 95.9 | ||||||
| 12) B. quintana | 91.5 | 92.9 | 81.2 | 92.1 | 88.9 | 88.3 | 91.6 | 91.6 | 91.3 | 94.4 | 93.8 | |||||
| 13) B. schoenbuchensis | 89.3 | 88.7 | 88.6 | 88.6 | 98.0 | 87.7 | 88.3 | 87.2 | 87.4 | 88.7 | 88.8 | 88.8 | ||||
| 14) B. taylorii | 91.6 | 92.9 | 87.3 | 93.0 | 87.5 | 87.7 | 90.3 | 91.0 | 92.4 | 91.0 | 90.1 | 91.0 | 87.5 | |||
| 15) B. tribocorum | 89.7 | 91.2 | 87.8 | 91.1 | 87.2 | 87.3 | 89.8 | 96.3 | 95.2 | 90.2 | 90.0 | 90.3 | 87.2 | 91.0 | ||
| 16) B. vinsonii subsp. vinsonii | 93.8 | 98.3 | 87.8 | 97.7 | 89.1 | 87.8 | 91.6 | 91.1 | 92.0 | 92.8 | 91.6 | 92.5 | 89.2 | 93.3 | 91.1 | |
| 17) “B. weissii” (B. bovis Bermond et al.) | 89.2 | 88.1 | 88.4 | 87.9 | 94.4 | 86.9 | 86.8 | 86.7 | 87.3 | 88.9 | 88.2 | 88.6 | 94.3 | 87.5 | 87.7 | 88.6 |
FIG. 1.
Comparison of neighbor-joining trees based on 16S rDNA, gltA, rpoB, ITS, groEL, and ftsZ partial or complete sequences. Bootstrap values at tree nodes are based on 100 replicates; values of >90 are boldfaced. Trees were unrooted, and only topology was shown for these trees. a, gltA, citrate synthase; rpoB, beta subunit of RNA polymerase; ITS, 16S-23S rRNA ITS; groEL, heat shock protein; ftsZ, cell division protein. b, range of the level of DNA sequence similarity for each gene used. Designations for species and subspecies consist of the letter B (for Bartonella) and the following abbreviations: ber, vinsonii subsp. berkhoffii; vin, vinsonii subsp. vinsonii; aru, vinsonii subsp. arupensis; tri, tribocorum; eli, elizabethae; gra, grahamii; tay, taylorii; als, alsatica; dos, doshiae; hen, henselae; qui, quintana; koe, koehlerae; cla, clarridgeiae; bir, birtlesii; sho, schoenbuchensis; bac, bacilliformis; bov, bovis Bermond et al.
Bartonella phylogeny derived from ftsZ sequences.
For each of the 17 Bartonella species, a sequence of 788 bp could be used for alignment and comparison. Phylogenetic trees derived by using parsimony and distance methods showed consistent topologies and statistical support (Fig. 1). The Bartonella species were divided into two clades with significant bootstrap values (99%); the first contained B. birtlesii, B. schoenbuchensis, and B. bovis Bermond et al. in one arm and B. clarridgeiae and B. bacilliformis in the second arm. The second clade contained three clusters. All three B. vinsonii subspecies (B. vinsonii subsp. vinsonii, B. vinsonii subsp. arupensis, and B. vinsonii subsp. berkhoffii) grouped together in the first cluster (100%); the second contained B. henselae, B. koehlerae, and B. quintana (99%); the third contained B. elizabethae, B. tribocorum, and B. grahamii (99%). The branching of B. taylorii, B. alsatica, and B. doshiae in the different groups was not reliable (70, 46, and 32%, respectively).
Comparison of the sequences of the ftsZ gene encoding the C-terminal region for subtyping Bartonella species isolates.
A fragment of nearly 885 bp of the portion of the ftsZ gene encoding the C-terminal region was amplified from four isolates of B. bacilliformis, 14 of B. clarridgeiae, 14 of B. quintana, and 30 of B. henselae. The sequences of all the fragments were compared with one another and with those available in GenBank (Table 2). The sequences of each of the 14 B. quintana isolates were identical to one another and to that previously described for B. quintana (Oklahoma) (29). The sequences of all B. clarridgeiae isolates used were identical to that reported for B. clarridgeiae (GenBank accession no. AF141018). While the sequences of all four of our B. bacilliformis isolates were identical (accession no. AF467752), they differed from that of B. bacilliformisT at 5 positions: 1071, 1279, 1490, 1587, and 1676 (numbered relative to the ftsZ gene of B. bacilliformis, accession no. AF007266). Variation at the first and fourth of these positions yielded silent mutations. Two previously described genotypes (4, 16, 19, 49, 50, 55, 56) were detected among the B. henselae isolates we tested (Table 2). The Houston sequence (accession no. AF161249) was found in 43.3% of our isolates; the remainder were of the Marseille genotype (accession no. AF161251).
Use of ftsZ C-terminal-derived primers for detection and identification of Bartonella spp. directly from clinical samples and comparison of their efficiency with that of the combined ITS/pap31 PCR assay.
All negative controls gave no PCR products. When the 80 clinical samples which had previously been tested with the combined ITS/pap31 PCR assay (41, 48) were assayed, C-terminal ftsZ amplicons were detected in 35 samples (43.75%) from 34 patients. The overall correlation between the C-terminal ftsZ assay results and those of the ITS- and pap31-derived assay was 89.7%, but this was not significant (P = 0.052). Four samples were negative by the ftsZ assay but positive with the ITS-pap31 assay; three of these samples were also positive in the seminested groEL-derived assay (56).
DISCUSSION
In the past decade, a number of new Bartonella species have been described (6, 15, 27) and comparisons of 16S rDNA sequences have led to many taxonomic changes in the genus Bartonella (7, 11). Although comparison of 16S rDNA gene sequences has been useful in phylogenetic studies at the genus level (41), its use has been questioned in studies at the species level (21; M. Hasegawa and T. Hashimoto, Letter, Nature 361:23, 1993). Other genes have been used empirically in attempts to classify the Bartonella species: the gltA gene (9), the rpoB gene (45), the 16S-23S rRNA ITS (26), and the groEL (55) gene. The FtsZ protein plays an important role in bacterial cell division, and recently its sequence was established for four Bartonella species (29). In our study we sequenced the 900-base sequence encoding the N-terminal region (partial) of the ftsZ gene for all recognized Bartonella species. The sequences were generally well conserved (81.2 to 98.3% [Table 4]) between species, but the sequence divergence present allowed us to develop a phylogenetic tree (Fig. 1) which was well supported for most of the strains studied. We compared this tree with those inferred from sequences of the 16S rDNA, gltA, rpoB, ITS, and groEL genes of Bartonella species available in GenBank (Fig. 1). With the ftsZ sequences, the Bartonella species were divided into two well supported clades which were also obtained with the groEL and rpoB sequences. Within these clades, various supported clusters could be found with the different DNA sequences. The statistically supported cluster formed by the subspecies of B. vinsonii in the ftsZ tree was also found in the rpoB-, ITS-, and groEL-derived trees. A cluster containing B. henselae and B. koehlerae was found in the ITS-derived tree, while a cluster containing B. henselae and B. quintana was obtained in the rpoB- and groEL-derived trees. A cluster including B. tribocorum, B. elizabethae, and B. grahamii was present in the phylogenetic trees established by using the gltA, rpoB, ITS, and groEL sequences. B. taylorii was included in this cluster in the ITS- and groEL-derived trees. A cluster formed by B. bovis Bermond et al. and B. birtlesii was found in the groEL-derived tree, and a cluster of B. bovis Bermond et al. and B. schoenbuchensis was found in the 16S rDNA-inferred tree. The similarities we found between the phylogenetic trees derived with the ftsZ gene sequences and those derived with other genes shows that fstZ gene sequencing should be considered a useful tool to be included in phylogeny studies.
We believe that it is important to consider the sequences of several genes in phylogeny studies. Although each gene-derived tree will differ from the others and will have different levels of statistical support, it has been found that groupings obtained with two different sequences at bootstrapping values over 90% are stable and reliable (48). In previous phylogenetic studies B. bacilliformis was chosen to be the outgroup, but because new Bartonella species have been described recently (6, 15) we chose to draw an unrooted tree.
Because Bartonella species are implicated in an increasing variety of human diseases, the development of species-specific tools for their detection and identification in clinical samples is becoming more crucial, especially in light of the difficulties in culturing these bacteria (34). In 1996, Drancourt et al. (16) reported two serotypes of B. henselae (Houston and Marseille), and later Bergmans et al. (4) confirmed by 16S rDNA gene sequence analysis that there were two genotypes of B. henselae, genotypes I and II, corresponding to the Houston and Marseille serotypes, respectively. More recently, further studies have confirmed the presence of these two subspecies (3, 4, 19, 33, 49, 50, 56). Many genes have been used to characterize Bartonella isolates (2, 8, 9, 27, 36, 37, 55), and the cell division protein (FtsZ) has also been used for detection (29) and subtyping of Bartonella species (19). In our study we amplified and sequenced the ftsZ sequence corresponding to the C-terminal region for 4 B. bacilliformis isolates, 14 B. clarridgeiae isolates, 14 B. quintana isolates, and 30 B. henselae isolates from different geographic regions, hosts, and clinical samples. The sequences of the B. clarridgeiae and B. quintana isolates were identical to those of the type strains. Similarly, sequencing of the groEL gene could not be used to differentiate B. quintana isolates (55). When the ITS sequence was used for subtyping, however, B. quintana isolates were found to belong to three genotypes and different sequences were found for all the B. clarridgeiae isolates studied (26). This difference may be explained by the high degree of variability of ITS sequences. The ftsZ sequence data may show the homogeneity of the B. clarridgeiae and B. quintana isolates. The sequences of the four B. bacilliformis isolates we studied were identical to one another but different from that of B. bacilliformisT at 5 positions, only 3 of which yielded significant amino acid substitutions. Among the 30 B. henselae isolates we studied, only Houston and Marseille genotypes were found and there was no evidence of genotype III, detected by Ehrenborg et al. (19).
We also tested whether the C-terminal ftsZ assay could detect the DNAs of Bartonella species in clinical samples and compared its sensitivity with that of a combined ITS-pap31 assay. We believe that false-positive PCR results due to contamination problems may be prevented by using a number of primer pairs which target different genes. Addition of the ftsZ gene to the panel of genes available for diagnosis of infections by PCR may be useful, and it may be a good tool for the “suicide” PCR application (44).
Conclusion.
We confirmed that using one pair of primers enables the comparison of partial ftsZ sequences for all Bartonella species and that this is a useful tool for detection and identification, which should facilitate routine work on clinical samples. The sequences obtained were also useful in phylogenetic analyses at the species level, and the results obtained correlated closely with those obtained in previous studies using other markers. Furthermore, we showed that Bartonella species occur in two clades and that B. bacilliformis belongs to a robust and well-defined clade. Using multiple DNA sequences seems to be the most suitable way to reliably infer phylogeny. We also showed the usefulness of ftsZ C-terminal region sequencing in the direct detection and identification of Bartonella species in clinical samples and for subtyping B. henselae and B. bacilliformis isolates. Its usefulness for epidemiological studies should be further investigated by using a diverse range of clinical samples.
Acknowledgments
We thank Yves Piemont for providing the B. schoenbuchensis strain, Jennifer Robson for providing B. henselae isolates from Australian cats, and Pat Kelly for reviewing the manuscript.
REFERENCES
- 1.Anderson, B. E., and M. A. Neuman. 1997. Bartonella spp. as emerging human pathogens. Clin. Microbiol. Rev. 10:203-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bereswill, S., S. Hinkelmann, M. Kist, and A. Sander. 1999. Molecular analysis of riboflavin synthesis genes in Bartonella henselae and use of the ribC gene for differentiation of Bartonella species by PCR. J. Clin. Microbiol. 37:3159-3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bergmans, A. M. C., C. M. A. de Jong, G. Van Amerongen, C. S. Schot, and L. M. Schouls. 1997. Prevalence of Bartonella species in domestic cats in The Netherlands. J. Clin. Microbiol. 35:2256-2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bergmans, A. M. C., J. F. P. Schellekens, J. D. A. Van Embden, and L. M. Schouls. 1996. Predominance of two Bartonella henselae variants among cat scratch disease patients in The Netherlands. J. Clin. Microbiol. 34:254-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bermond, D., H. J. Boulouis, R. Heller, G. Van Laere, H. Monteil, B. B. Chomel, A. Sander, C. Dehio, and Y. Piemont. 2002. Bartonella bovis Bermond et al. sp.nov. and Bartonella capreoli sp. nov., isolated from European ruminants. Int. J. Syst. Evol. Microbiol. 52:383-390. [DOI] [PubMed] [Google Scholar]
- 6.Bermond, D., R. Heller, F. Barrat, G. Delacour, C. Dehio, A. Alliot, H. Monteil, B. Chomel, H. Boulouis, and Y. Piémont. 2000. Bartonella birtlesii sp. nov., isolated from small mammals (Apodemus spp.). Int. J. Syst. Evol. Microbiol. 50:1973-1979. [DOI] [PubMed] [Google Scholar]
- 7.Birtles, R. J., T. G. Harrison, N. A. Saunders, and D. H. Molyneux. 1995. Proposals to unify the genera Grahamella and Bartonella, with descriptions of Bartonella talpae comb. nov., Bartonella peromysci comb. nov., and three new species, Bartonella grahamii sp. nov., Bartonella taylorii sp. nov., and Bartonella doshiae sp. nov. Int. J. Syst. Bacteriol. 45:1-8. [DOI] [PubMed] [Google Scholar]
- 8.Birtles, R. J., S. Hazel, K. Brown, D. Raoult, M. Begon, and M. Bennett. 2000. Subtyping of uncultured bartonellae using sequence comparison of 16S/23S rRNA intergenic spacer regions amplified directly from infected blood. Mol. Cell. Probes 14:79-87. [DOI] [PubMed] [Google Scholar]
- 9.Birtles, R. J., and D. Raoult. 1996. Comparison of partial citrate synthase gene (gltA) sequences for phylogenetic analysis of Bartonella species. Int. J. Syst. Bacteriol. 46:891-897. [DOI] [PubMed] [Google Scholar]
- 10.Breitschwerdt, E. B., and D. L. Kordick. 2000. Bartonella infection in animals: carriership, reservoir potential, pathogenicity, and zoonotic potential for human infection. Clin. Microbiol. Rev. 13:428-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brenner, D. J., S. O'Connor, H. H. Winkler, and A. G. Steigerwalt. 1993. Proposals to unify the genera Bartonella and Rochalimaea, with descriptions of Bartonella quintana comb. nov., Bartonella vinsonii comb. nov., Bartonella henselae comb. nov., and Bartonella elizabethae comb. nov., and to remove the family Bartonellaceae from the order Rickettsiales. Int. J. Syst. Bacteriol. 43:777-786. [DOI] [PubMed] [Google Scholar]
- 12.Brown, J. K. M. 1994. Bootstrap hypothesis tests for evolutionary trees and other dendrograms. Proc. Natl. Acad. Sci. USA 91:12293-12297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chang, C. C., R. W. Kasten, B. B. Chomel, D. C. Simpson, C. M. Hew, D. L. Kordick, R. Heller, Y. Piemont, and E. B. Breitschwerdt. 2000. Coyotes (Canis latrans) as the reservoir for a human-pathogenic Bartonella sp.: molecular epidemiology of Bartonella vinsonii subsp. berkhoffii infection in coyotes from central coastal California. J. Clin. Microbiol. 38:4193-4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Daly, J. S., M. G. Worthington, D. J. Brenner, W. C. Moss, D. G. Hollis, R. S. Weyant, A. G. Steigerwalt, R. E. Weaver, M. I. Daneshvar, and S. P. O'Connor. 1993. Rochalimaea elizabethae sp. nov. isolated from a patient with endocarditis. J. Clin. Microbiol. 31:872-881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dehio, C., C. Lanz, R. Pohl, P. Behrens, D. Bermond, Y. Piémont, K. Pelz, and A. Sander. 2001. Bartonella schoenbuchii sp. nov., isolated from the blood of wild roe deer. Int. J. Syst. Evol. Microbiol. 51:1557-1565. [DOI] [PubMed] [Google Scholar]
- 16.Drancourt, M., R. Birtles, G. Chaumentin, F. Vandenesch, and D. Raoult. 1996. New serotype of Bartonella henselae in endocarditis and cat-scratch disease. Lancet 347:441-443. [DOI] [PubMed] [Google Scholar]
- 17.Droz, S., B. Chi, E. Horn, A. G. Steigerwalt, A. M. Whitney, and D. J. Brenner. 1999. Bartonella koehlerae sp. nov., isolated from cats. J. Clin. Microbiol. 37:1117-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Efron, B., E. Halloran, and S. Holmes. 1996. Bootstrap confidence levels for phylogenetic trees. Proc Natl. Acad. Sci USA 93:13429-13434. (Original printing, 93:7085-7090.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ehrenborg, C., L. Wesslen, A. Jakobson, G. Friman, and M. Holmberg. 2000. Sequence variation in the ftsZ gene of Bartonella henselae isolates and clinical samples. J. Clin. Microbiol. 38:682-687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Felsenstein, J. 1989. PHYLIP—phylogeny inference package (version 3.2). Cladistics 5:164-166. [Google Scholar]
- 21.Fox, G. E., J. D. Wisotzkey, and P. J. Jurtshuk. 1992. How close is close: 16S rRNA sequence identity may not be sufficient to guarantee species identity. Int. J. Syst. Bacteriol. 42:166-170. [DOI] [PubMed] [Google Scholar]
- 22.Gray, G. C., A. A. Johnson, S. A. Thornton, W. A. Smith, J. Knobloch, P. W. Kelley, E. L. Obregon, H. M. Arones, and F. S. Wignall. 1990. An epidemic of Oroya fever in the Peruvian Andes. Am. J. Trop. Med. Hyg. 42:215-221. [DOI] [PubMed] [Google Scholar]
- 23.Heller, R., M. Kubina, P. Mariet, P. Riegel, G. Delacour, C. Dehio, F. Lamarque, R. Kasten, H. J. Boulouis, H. Monteil, B. Chomel, and Y. Piemont. 1999. Bartonella alsatica sp. nov., a new Bartonella species isolated from the blood of wild rabbits. Int. J. Syst. Bacteriol. 49:283-288. [DOI] [PubMed] [Google Scholar]
- 24.Heller, R., P. Riegel, Y. Hansmann, G. Delacour, D. Bermond, C. Dehio, F. Lamarque, H. Monteil, B. Chomel, and Y. Piémont. 1998. Bartonella tribocorum sp. nov., a new Bartonella species isolated from the blood of wild rats. Int. J. Syst. Bacteriol. 48:1333-1339. [DOI] [PubMed] [Google Scholar]
- 25.Houpikian, P., P. E. Fournier, and D. Raoult. 2001. Phylogenetic position of Bartonella vinsonii subsp. arupensis based on 16S rDNA and gltA gene sequences. Int. J. Syst. Evol. Microbiol. 51:179-182. [DOI] [PubMed] [Google Scholar]
- 26.Houpikian, P., and D. Raoult. 2001. 16S/23S rRNA intergenic spacer regions for phylogenetic analysis, identification, and subtyping of Bartonella species. J. Clin. Microbiol. 39:2768-2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Houpikian, P., and D. Raoult. 2001. Molecular phylogeny of the genus Bartonella: what is the current knowledge? FEMS Microbiol. Lett. 200:1-7. [DOI] [PubMed] [Google Scholar]
- 28.Jacomo, V., P. J. Kelly, and D. Raoult. 2002. Natural history of Bartonella infections (an exception to Koch's postulate). Clin. Diagn. Lab. Immunol. 9:8-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kelly, T. M., I. Padmalayam, and B. R. Baumstark. 1998. Use of the cell division protein FtsZ as a means of differentiating among Bartonella species. Clin. Diagn. Lab. Immunol. 5:766-772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kerkhoff, F. T., A. M. Bergmans, Z. van Der, and A. Rothova. 1999. Demonstration of Bartonella grahamii DNA in ocular fluids of a patient with neuroretinitis. J. Clin. Microbiol. 37:4034-4038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koehler, J. E., C. A. Glaser, and J. W. Tappero. 1994. Rochalimaea henselae infection: a new zoonosis with the domestic cat as a reservoir. JAMA 271:531-535. [DOI] [PubMed] [Google Scholar]
- 32.Kordick, D. L., E. J. Hilyard, T. L. Hadfield, K. H. Wilson, A. G. Steigerwalt, D. J. Brenner, and E. B. Breitschwerdt. 1997. Bartonella clarridgeiae, a newly recognized zoonotic pathogen causing inoculation papules, fever, and lymphadenopathy (cat scratch disease). J. Clin. Microbiol. 35:1813-1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.La Scola, B., Z. Liang, Z. Zeaiter, P. Houpikian, P. Grimont, and D. Raoult. 2002. Genotypic characteristics of two serotypes of Bartonella henselae. J. Clin. Microbiol. 40:2002-2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.La Scola, B., and D. Raoult. 1999. Culture of Bartonella quintana and Bartonella henselae from human samples: a 5-year experience (1993 to 1998). J. Clin. Microbiol. 37:1899-1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lawson, P. A., and M. D. Collins. 1996. Description of Bartonella clarridgeiae sp. nov. isolated from the cat of a patient with Bartonella henselae septicemia. Med. Microbiol. Lett. 5:64-73. [Google Scholar]
- 36.Marston, E. L., J. W. Sumner, and R. L. Regnery. 1999. Evaluation of intraspecies genetic variation within the 60-kDa heat-shock protein gene (groEL) of Bartonella species. Int. J. Syst. Bacteriol. 49:1015-1023. [DOI] [PubMed] [Google Scholar]
- 37.Matar, G. M., B. Swaminathan, S. B. Hunter, L. N. Slater, and D. F. Welch. 1993. Polymerase chain reaction-based restriction fragment length polymorphism analysis of a fragment of the ribosomal operon from Rochalimaea species for subtyping. J. Clin. Microbiol. 31:1730-1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maurin, M., R. J. Birtles, and D. Raoult. 1996. A review of bartonellae and their infections, p. 587-610. In J. Kazar and R. Toman. (ed.), Rickettsiae and rickettsial diseases. Veda, Bratislava, Slovakia.
- 39.Maurin, M., V. Roux, A. Stein, F. Ferrier, R. Viraben, and D. Raoult. 1994. Isolation and characterization by immunofluorescence, sodium dodecyl sulfate-polyacrylamide gel electrophoresis, Western blot, restriction fragment length polymorphism-PCR, 16S rRNA gene sequencing, and pulsed-field gel electrophoresis of Rochalimaea quintana from a French patient with bacillary angiomatosis. J. Clin. Microbiol. 32:1166-1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McNee, J. W., A. Renshaw, and E. H. Brunt. 1916. “Trench fever”: a relapsing fever occurring with the British forces in France. Br. Med. J. 12:225-234. [Google Scholar]
- 41.Olsen, G. J., and C. R. Woese. 1993. Ribosomal RNA: a key to phylogeny. FASEB J. 7:113-123. [DOI] [PubMed] [Google Scholar]
- 42.Padmalayam, I., B. Anderson, M. Kron, T. Kelly, and B. Baumstark. 1997. The 75-kilodalton antigen of Bartonella bacilliformis is a structural homolog of the cell division protein ftsZ. J. Bacteriol. 179:4545-4552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Page, R. D. 1996. TreeView: an application to display phylogenetic trees on personal computers. Comput. Appl. Biosci. 12:357-358. [DOI] [PubMed] [Google Scholar]
- 44.Raoult, D., G. Aboudharam, E. Crubezy, G. Larrouy, B. Ludes, and M. Drancourt. 2000. Molecular identification by “suicide PCR” of Yersinia pestis as the agent of medieval Black Death. Proc. Natl. Acad. Sci. USA 97:12800-12803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Renesto, P., J. Gouvernet, M. Drancourt, V. Roux, and D. Raoult. 2001. Use of rpoB gene analysis for detection and identification of Bartonella species. J. Clin. Microbiol. 39:430-437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roux, V., S. J. Eykyn, S. Wyllie, and D. Raoult. 2000. Bartonella vinsonii subsp. berkhoffii as an agent of afebrile blood culture-negative endocarditis in a human. J. Clin. Microbiol. 38:1698-1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roux, V., and D. Raoult. 1995. Inter-and intraspecies identification of Bartonella (Rochalimea) species. J. Clin. Microbiol. 33:1573-1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roux, V., and D. Raoult. 2000. Phylogenetic analysis of members of the genus Rickettsia using the gene encoding the outer-membrane protein rOmpB (ompB). Int. J. Syst. Evol. Microbiol. 50:1449-1455. [DOI] [PubMed] [Google Scholar]
- 49.Sander, A., C. Bühler, K. Pelz, E. Von Cramm, and W. Bredt. 1997. Detection and identification of two Bartonella henselae variants in domestic cats in Germany. J. Clin. Microbiol. 35:584-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sander, A., M. Ruess, K. Deichmann, N. Böhm, and W. Bredt. 1998. Two different genotypes of Bartonella henselae in children with cat-scratch disease and their pet cats. Scand. J. Infect. Dis. 30:387-391. [DOI] [PubMed] [Google Scholar]
- 51.Sander, A., A. Zagrosek, W. Bredt, E. Schiltz, Y. Piemont, C. Lanz, and C. Dehio. 2000. Characterization of Bartonella clarridgeiae flagellin (FlaA) and detection of antiflagellin antibodies in patients with lymphadenopathy. J. Clin. Microbiol. 38:2943-2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stein, A., and D. Raoult. 1992. A simple method for amplification of DNA from paraffin-embedded tissues. Nucleic Acids Res. 20:5237-5238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Welch, D. F., K. C. Carroll, E. K. Hofmeister, D. H. Persing, D. A. Robison, A. G. Steigerwalt, and D. J. Brenner. 1999. Isolation of a new subspecies, Bartonella vinsonii subsp. arupensis, from a cattle rancher: identity with isolates found in conjunction with Borrelia burgdorferi and Babesia microti among naturally infected mice. J. Clin. Microbiol. 37:2598-2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zeaiter, Z., P. E. Fournier, H. Ogata, and D. Raoult. 2002. Phylogenetic classification of Bartonella species by comparing groEL sequences. Int. J. Syst. Evol. Microbiol. 52:165-171. [DOI] [PubMed] [Google Scholar]
- 56.Zeaiter, Z., P. E. Fournier, and D. Raoult. 2002. Genomic variation of Bartonella henselae detected in lymph nodes from patients with cat scratch disease. J. Clin. Microbiol. 40:1023-1030. [DOI] [PMC free article] [PubMed] [Google Scholar]