Abstract
Background
Dengue virus infected patients have high plasminogen activator inhibitor type I (PAI-1) plasma concentrations. Whether the insertion/deletion (4G/5G) polymorphism in the promotor region of the PAI-1 gene is associated with increased PAI-1 plasma concentrations and with death from dengue is unknown. We, therefore, investigated the relationship between the 4G/5G polymorphism and PAI-1 plasma concentrations in dengue patients and risk of death from dengue.
Methods
A total of 194 patients admitted to the Dr. Kariadi Hospital in Semarang, Indonesia, with clinical suspected severe dengue virus infection were enrolled. Blood samples were obtained on day of admission, days 1, 2 and 7 after admission and at a 1-month follow-up visit. Plasma concentrations of PAI-1 were measured using a sandwich ELISA kit. The PAI-1 4G/5G polymorphism was typed by allele-specific PCR analysis.
Results
Concentrations of PAI-1 on admission and peak values of PAI-1 during admission were higher than the values measured in healthy controls. Survival was significantly worse in patients with PAI-1 concentrations in the highest tertile (at admission: OR 4.7 [95% CI 0.9–23.8], peak value during admission: OR 6.3 [95%CI 1.3–30.8]). No association was found between the PAI-1 4G/5G polymorphism, and PAI-1 plasma concentrations, dengue disease severity and mortality from dengue.
Conclusion
These data suggest that the 4G/5G polymorphism has no significant influence on PAI-1 concentrations in dengue virus infected patients and is not associated with the risk of death from dengue. Other factors contributing to the variability of PAI-1 plasma concentrations in patients with dengue need to be explored.
Background
Dengue is the most prevalent viral disease transmitted by arthropod vectors worldwide [1]. An estimated 50–100 million cases of dengue fever and 500.000 cases of dengue haemorrhagic fever resulting in around 24.000 deaths occur annually depending on epidemic activity [1,2]. At present, almost 30% of the world population is at risk for dengue virus infection and it is expected that this number will increase substantially as transmission spreads to other yet unaffected geographic regions [3]. The viruses are transmitted to humans through infected mosquitoes, and may induce clinical manifestations ranging from a mild, uncomplicated febrile illness to the more severe dengue haemorrhagic fever and dengue shock syndrome. Increased vascular permeability is thought to be central in the pathogenesis of dengue haemorrhagic fever and dengue shock syndrome, since it results in the loss of plasma from the vascular compartment, which may give rise to shock in severe cases. Bleeding manifestations are believed to result from thrombocytopenia and thrombocytopathia, but increasing evidence suggests an important role for other abnormalities in the coagulation and fibrinolytic systems [4].
Nearly all patients suffering from dengue haemorrhagic fever show some evidence of a deranged coagulation system, but coagulation abnormalities are most marked in dengue shock syndrome patients [5]. The activity of the fibrinolytic system is amongst others regulated by plasminogen activator inhibitor type I (PAI-1), of which levels may greatly increase during acute phase reactions. Indeed, levels of PAI-1 are high in particular in dengue shock syndrome patients with an adverse clinical outcome [6,7]. A single base pair insertion/deletion (4G/5G) polymorphism in the promotor region of the PAI-1 gene has been associated with increased plasma concentrations of PAI-1 and with the development of shock and death after infection with Neisseria meningitides [8,9]. We therefore investigated whether in dengue virus infected patients increased PAI-1 levels are associated with a greater risk of death from dengue and whether the 4G/4G genotype contributes to these higher levels.
Methods
Patients were selected from two observational studies conducted in the Dr. Kariadi Hospital in Semarang, Indonesia. In the first study, performed from 1996 to 1997 at the paediatric intensive care unit, a total of 50 patients with a clinical diagnosis of suspected dengue shock syndrome were studied. Blood samples were obtained from these patients on day of admission and on days 1, 2 and 7 after admission. The study protocol and the results of the measurement of PAI-1 plasma levels have been described previously [6]. The second study was performed from 2001 to 2003 at the paediatric intensive care unit and the paediatric ward. Patients, aged 3 to 14 years, admitted to the hospital with a clinical diagnosis of suspected dengue shock syndrome or with a clinical diagnosis of suspected dengue haemorrhagic fever were included. Demographic data, medical history, physical examination findings and subsequent progress for each patient were recorded on a standard data form. Blood samples were obtained on day of admission, days 1, 2 and 7 after admission and at a 1-month follow-up visit. A formal classification, according to WHO criteria [2], using all available clinical and laboratory data, was done after completion of both studies. If dengue virus infected patients did not meet the criteria for dengue haemorrhagic fever or dengue shock syndrome, they were considered to suffer from dengue fever. The controls were healthy school-aged children who had no history of dengue haemorrhagic fever or dengue shock syndrome and originated from the same geographical area as the cases.
All blood samples were centrifuged within 1–2 hours after retrieval at 15°C for 20 minutes at 1600*g. Plasma was separated, stored at -80°C and assayed batch-wise in the Netherlands after transportation on dry ice. Since coagulation test results are affected by poor sample quality, only test results of samples with no visible haemolysis or clot formation were included in this analysis. Plasma concentrations of PAI-1 were measured using two separate assays. A sandwich ELISA kit that has been described by de Boer et al [10], was used in the 1996–1997 study. In the second study a commercially available sandwich ELISA kit (Imulyse, Biopool, Sweden) was used. Genomic DNA from patients was prepared either from EDTA whole blood by means of a salting out method as described elsewhere or in case only plasma samples were available with use of the Invisorb® Spin Cell Mini Kit (Invitek GmbH, Berlin, Germany) [11]. The insertion/deletion (4G/5G) polymorphism in the promotor region of the PAI-1 gene was typed by allele-specific PCR and RFLP analysis [11]. The ethics committee of the Dr. Kariadi Hospital approved all clinical and laboratory aspects of both studies. Blood samples were taken from patients and controls provided that a parent or legal guardian gave informed consent.
Paired blood samples from both studies were tested for serologic evidence of acute dengue infection. A commercially available capture and indirect ELISA (Focus Technologies, Cypress, Calif., USA) was used for the detection of dengue virus specific IgM and IgG antibodies respectively. This was performed according to the procedures described by the manufacturer [12]. For some patients, a definitive serodiagnosis was not possible because no convalescent sample was obtained. Detection of dengue antigen and RNA was attempted in these cases using a dot blot immunoassay and a dengue serotype specific revere transcriptase PCR respectively [13]. Patients with serologic evidence of acute dengue infection, a positive dot blot and/or positive PCR were considered to have confirmed dengue virus infection. Those with definite negative serology and/or a well-substantiated alternative clinical diagnosis were classified as not dengue. In the absence of a well-substantiated alternative clinical diagnosis and with inconclusive serology patients were classified as indeterminate.
Categorical data are expressed as numbers and frequencies, and were compared by means of χ2 analysis. Continuous data are expressed as medians with corresponding interquartile ranges and were compared by means of the Kruskal Wallis test. Since PAI-1 plasma levels were measured using two different assays, the nonparametric regression procedure of Passing and Bablok was used to compare these two methods and to validate recalculation of one of the datasets to pool the data [14]. For this purpose, 39 samples were analysed with both assays. The Passing and Bablok regression equation resulting from this comparison is given in the Results section, together with the 95% confidence intervals for the estimates of slope and intercept. PAI-1 plasma levels obtained from the 1996–1997 cohort were converted using the regression equation. We subsequently divided PAI-1 plasma levels on admission and peak PAI-1 plasma levels during admission into tertiles of similar size. Odds ratios and the corresponding 95% confidence intervals (95% CI) were estimated by cross-tabulation using the lowest tertile as reference category. To determine the association between PAI-1 concentration and mortality independent of age, sex, year project and plasminogen activator inhhibitor-1 polymorphism, we used logistic regression analyses. A P-value ≤ 0.05 was considered to indicate statistical significance. Analyses were performed using SPSS 11.0.1. Method comparison was performed using Analyse-it Clinical Laboratory statistics module version 1.62 for Microsoft Excel.
Results
Of 233 enrolled patients with suspected dengue haemorrhagic fever or dengue shock syndrome admitted to the paediatric intensive care unit and the paediatric ward of the Dr. Kariadi Hospital, 202 (87%) were confirmed to have acute dengue, 3 (1%) were categorised as definitely not dengue and 28 (12%) as indeterminate. When a formal classification using the criteria set by the WHO was performed, 106 (52%) were classified as having dengue shock syndrome, 76 (38%) as having dengue haemorrhagic fever and 20 (10%) as having dengue fever. Nineteen of 202 patients with confirmed dengue (9%) died during follow up.
Genomic DNA was obtained from 194 patients. The remaining patients could not be typed because of insufficient volume of blood left or low yield of DNA. Clinical features and basic laboratory investigations on admission of the 194 patients are summarised in Table 1. The numbers of 4G/4G, 4G/5G and 5G/5G PAI-1 genotypes among patients in relation to mortality are summarised in Table 2. Of 192 control samples tested, 45 patients (23%) were 4G/4G homozygous, 83 (43%) were 4G/5G heterozygous, and 64 (33%) were 5G/5G homozygous. The frequencies of the PAI-1 promoter genotypes 4G/4G, 4G/5G, and 5G/5G did not differ significantly between the 1996–1997 project, the 2001–2003 project and the control group (P = 0.520). The proportion of deaths among patients with the 4G/4G, 4G/5G and 5G/5G genotype did not differ significantly (1996–1997 project: P = 0.979; 2001–2003 project: P = 0.986; two projects combined: P = 0.781). The genotype frequencies among patients with respect to final clinical diagnosis according to the criteria set by the WHO are summarised in Table 3. Results indicate that the PAI-1 promoter genotypes are not associated with dengue disease severity (P = 0.508).
Table 1.
Clinical characteristics and laboratory findings
| Characteristic | |
| Age, median (IQR), years | 6 (4–10) |
| Male sex, n (%) | 92 (47) |
| Duration of symptoms, median (range), days | 4 (1–7) |
| Systolic blood pressure, median (IQR), mmHg | 90 (80–100) |
| Pulse pressure <20 mmHg, n (%) | 22 (11) |
| Spontaneous bleeding, n (%) | 118 (62) |
| Haematocrit, median (IQR), % | 41 (36–45) |
| Platelet count, median (IQR), cells *103/mm3 | 58 (37–85) |
° IQR denotes 25th and 75th interquartile range, n denotes number of patients.
Table 2.
Clinical outcome of dengue virus infected patients classified by PAI-1 genotype
| 1996–1997 | 2001–2003 | |||||
| Genotype | All patients | Survivors | Non-survivors | All patients | Survivors | Non-survivors |
| G4/G4 | 8 (18%) | 6 (14%) | 2 (5%) | 33 (22%) | 32 (21%) | 1 (1%) |
| G4/G5 | 15 (34%) | 11 (25%) | 4 (9%) | 62 (41%) | 60 (40%) | 2 (1%) |
| G5/G5 | 21 (48%) | 15 (34%) | 6 (14%) | 55 (37%) | 53 (35%) | 2 (1%) |
| Total | 44 (100%) | 32 (73%) | 12 (27%) | 150 (100%) | 145 (97%) | 5 (3%) |
Table 3.
Dengue disease severity and PAI-1 genotype
| Genotype | DF | DHF | DSS |
| G4/G4 | 6 (3%) | 13 (7%) | 22 (11%) |
| G4/G5 | 6 (3%) | 35 (18%) | 36 (19%) |
| G5/G5 | 8 (4%) | 27 (14%) | 41 (21%) |
| Total | 20 (10%) | 75 (39%) | 99 (51%) |
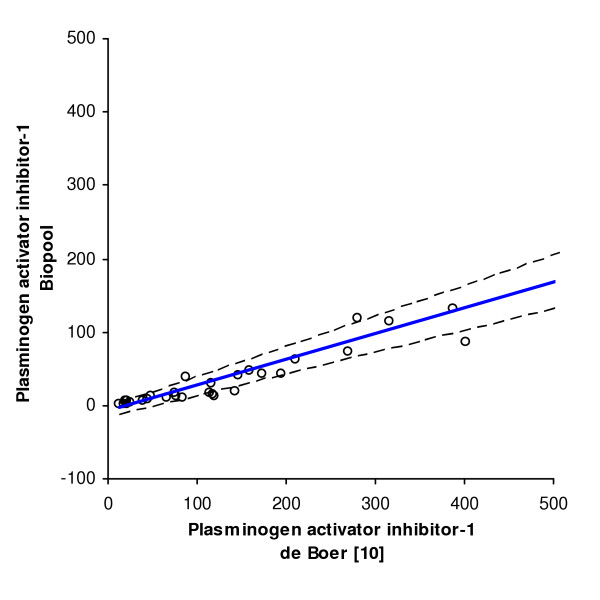
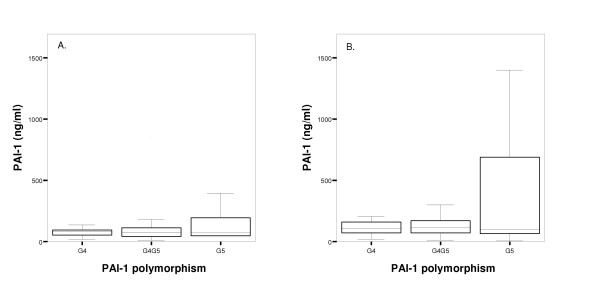
PAI-1 plasma levels were measured in 124 patients from whom sufficient frozen plasma samples was available with the use of two separate ELISA's. Thirty-nine random plasma samples were used for the comparison of the two assays. PAI-1 level measurements are known to be dependent on the assay employed, since not all antibodies used have the same specificity with respect to the various molecular forms of PAI-1 in blood (active PAI-1, PAI-1 complexed with vitronectin, inactive or latent PAI-1 and PAI-1 complexed with its target proteases tissue-type and urokinase-type plasminogen activator) [15]. Passing & Bablok regression analysis (Figure 1), however, clearly showed that the two assays employed in this study have a straightforward linear relationship between their outcomes. This allowed us to convert the PAI-1 levels measured in the 1996–1997 cohort to levels that would have been obtained with the assay used in the second cohort, using the Passing and Bablok regression equation (an intercept of -7.42 ng/ml (95% CI, -16.39 to -3.24 ng/ml) and slope of 0.35 (95% CI, 0.30 to 0.42)). Concentrations of PAI-1 on admission (71 ng/ml [42–118]) and peak values during admission (96 ng/ml [64–199]) were higher than the concentrations measured in the healthy control group (30–60 ng/ml). Patients included in the 2001–2003 project and diagnosed with DSS had higher PAI-1 plasma levels on admission (P = 0.002) and during admission (P < 0.001) than those diagnosed with DF or DHF (Table 4). However, no statistical significant difference was present between patients with different dengue disease severities when both projects were combined (PAI-1 plasma levels on admission: P = 0.212 and during admission: P = 0.089). Survival was significantly worse in patients with PAI-1 concentrations in the highest tertile (Table 5). As shown by multiple logistic regression analysis, the odds ratio for the risk of death – adjusted for age, sex, year project and plasminogen activator inhibitor-1 genotype – of PAI-1 levels at admission in the highest tertile was 7.57 (95%CI, 1.16–49.30). The adjusted odds ratio for the risk of death of peak PAI-1 levels during admission in the highest tertile was 7.41 (95%CI, 1.30–42.26). Figure 2 illustrates the relation between PAI-1 plasma concentrations and the PAI-1 genotypes. Differences were not statistically significant (values at admission: P = 0.919; peak values during admission: P = 0.470).
Figure 1.
Comparison of two PAI-1 assays. Comparison of PAI-1 plasma levels obtained by a Biopool assay and an assay previously described by de Boer and colleagues [10]). The Passing & Bablok regression equation is: y = 0,3505x - 7,4227 ng/ml; n = 39. Solid line, regression line; dashed lines, 95% CI for the regression line.
Table 4.
Relation between PAI-1 plasma levels and disease severity°
| Project | PAI-1 | DF | DHF | DSS |
| (ng/ml) | Median (IQR) | Median (IQR) | Median (IQR) | |
| 1996–1997¶ | Admission | - | - | 56.7 (33.0–130.2) |
| Peak values | - | - | 91.6 (37.7–343.6) | |
| 2001–2003 | Admission | 77.0 (69.0–99.0) | 60.0 (42.3–82.5) | 107.0 (52.0–670.0) |
| Peak values | 82.0 (69.0–129.0) | 78 (59.3–118.3) | 264.0 (117.0–844.0) | |
| Total | Admission | 77.0 (69.0–99.0) | 60.0 (42.3–82.5) | 87.0 (35.0–180.8) |
| Peak values | 82.0 (69.0–129.0) | 78 (59.3–118.3) | 130.7 (53.7–531.1) |
° IQR denotes 25th and 75th interquartile range.
¶ Only DSS patients were included in the 1996 project.
Table 5.
Mortality risk for plasminogen activator-1 plasma levels.*
| PAI-1 | ng/ml | Number of patients | Number of deaths | OR (95% CI) | ORadj (95% CI)° |
| Value at admission | <50 | 41 | 2 | 1 | 1 |
| 50–94 | 40 | 3 | 1.6 (0.3–10.0) | 3.3 (0.4–25.0) | |
| >94 | 41 | 8 | 4.7 (0.9–23.8) | 7.6 (1.2–49.3) | |
| Maximum value during admission | <72 | 41 | 2 | 1 | 1 |
| 72–151 | 42 | 3 | 1.5 (0.2–9.5) | 2.1 (0.2–18.2) | |
| >151 | 41 | 10 | 6.3 (1.3–30.8) | 7.4 (1.3–42.3) |
* CI, denotes confidence interval; OR, denotes odds ratio; ORadj, denotes adjusted odds ratio
° Risk of death adjusted for age, sex, year project and plasminogen activator inhhibitor-1 polymorphism. Lowest tertile was used as reference category.
Figure 2.
Relation between PAI-1 plasma concentrations and PAI-1 genotype in dengue virus infected patients. Box-and-whisker plots of PAI-1 plasma concentrations on admission (A.) and peak values during admission (B.). The central line in the box plot represents the median, the boxed areas represent the interquartile ranges. Whiskers at the ends of the box show the distance from the end of the box to the largest and smallest observed values that are less than 1.5 box lengths from either end of the box.
Discussion
This study was undertaken to investigate the relationship between PAI-1 plasma concentrations and clinical outcome of dengue virus infections, and to establish whether PAI-1 plasma concentrations in dengue virus infected individuals are associated with the 4G/5G promotor polymorphism in the PAI-1 gene. We and others have previously found increased PAI-1 plasma concentrations in patients with severe dengue in particular in those with a poor clinical outcome [5,7]. Since a genetic predisposition to produce high PAI-1 plasma concentrations appears to be associated with poor clinical outcome in Neisseria meningitides infections [8,9], we hypothesised that dengue virus infected individuals carrying the 4G/4G genotype have higher PAI-1 plasma concentrations and are therefore at increased risk of death. Consistent with previous studies, we found increased PAI-1 concentrations in dengue virus infected individuals. However, PAI-1 plasma concentrations were not related to dengue disease severity, but were significantly associated with death from dengue. No significant association between PAI-1 plasma concentrations and carriage of the 4G/4G genotype was observed. The frequencies of the three genotypes between survivors and non-survivors, and between patients with different disease severities were not different. These findings suggest that increased PAI-1 plasma concentrations, and dengue disease severity and mortality are not dependent on the 4G polymorphism in the PAI-1 gene in this population.
An increased risk of death in dengue virus infected patients with high PAI-1 plasma concentrations adds to findings of PAI-1 levels being able to predict lethality in patients with bacterial sepsis in a very sensitive way [16-20]. One of the primary roles of PAI-1 in vivo is to inhibit tissue-type plasminogen activator, the major proteolytic activator of plasminogen [21,22]. By inhibiting fibrinolytic activity, increased PAI-1 concentrations may contribute to a procoagulant state leading to an increased deposition of fibrin and formation of microthrombi with subsequent multiorgan failure and death. A variety of cells, including endothelial cells, hepatocytes and platelets, synthesize and secrete PAI-1 in response to inflammatory stimuli such as interleukin-1 and tumour necrosis factor [10,22-24]. The release of these inflammatory mediators by monocytes and T lymphocytes activated by dengue virus may well contribute to the over-production of this inhibitor of fibrinolysis [25-27]. Dawson and colleagues showed that the common insertion/deletion (4G/5G) polymorphism in the promotor region of the PAI-1 gene affects the response of the gene to acute phase stimuli [23]. The 4G allele produced six times more mRNA than the 5G allele in response to interleukin-1 [23]. Eriksson and colleagues, however, were unable to reproduce these findings and based on their study results they concluded that the insertion/deletion (4G/5G) polymorphism is not related to an allele-specific response to interleukin-1 [28]. Instead, they found that the insertion/deletion (4G/5G) polymorphism influences basal PAI-1 transcription only [28].
Apparently other underlying mechanisms not related to the 4G/5G polymorphism must be involved in the increase in PAI-1 levels found in dengue virus infected individuals. This might include clearance impairment rather than or in addition to stimulation of synthesis. It is interesting to note that PAI-1 is cleared from the circulation by the liver [29]. Indeed in patients with severe liver disease, PAI-1 has been shown to be increased as a result of a decrease in hepatic clearance [30,31]. Since hepatic dysfunction is a relatively common finding in severe dengue virus infections, it is possible that a less efficient clearance contributes to increased PAI-1 levels in dengue virus infected individuals [32-34]. Previous studies investigated factors that could potentially influence PAI-1 levels, including environmental factors, metabolic determinants, ethnicity and a variety of other polymorphisms within the PAI-1 gene [35-38]. It remains unclear whether and to what extent these factors contribute to the variability in PAI-1 levels in dengue virus infected individuals. Previously studied individuals were either healthy, were patients with coronary artery disease, or were patients with diabetes mellitus. Clearly these study populations cannot be compared to patients suffering from a severe infectious disease that is characterised by an overwhelming inflammatory response.
Several potential limitations of the present study should be noted. The 1996–1997 cohort was characterized with a high mortality rate of 27%. Although the exact reason for this high mortality remains to be determined, it is likely that it results from a combination of factors. Our study was performed in a Tertiary Hospital that serves a large part of Middle-Java. Patients may travel long distances to be treated in this hospital and it could well be that they are presented late in the course of disease. Initial fluid resuscitation according to WHO guidelines is generally insufficient in these cases and patients usually end up in profound shock. Despite admission at the Pediatric Intensive Care Unit mortality rate is high. In addition, the 1996–1997 rainy season was characterised by high numbers of patients admitted to hospitals because of DHF/DSS and high number of non-survivors. It is believed that an unusually virulent virus circulated that year although microbiological sampling could not be performed at that time because of limited resources.
Study size is an important issue in the establishment of an association between the insertion/deletion (4G/5G) polymorphism in the PAI-1 gene and clinical outcome. Hermans and colleagues previously found an association between the homozygous 4G deletion polymorphism and mortality from Neisseria meningitides infection among 129 patients from two different cohort groups [8]. This association was also observed when the largest of the two cohort groups was studied separately, but was not seen in the smallest group in which only 37 patients were included. In order to obtain a sufficient number of patients, we therefore decided to combine the results of two different projects. This decision was based on the fact that these two projects included patients with the same ethnic background, used similar trial procedures and applied uniform diagnostic and clinical management procedures. Although mortality rates between the 1996–1997 cohort and the 2001–2003 cohort differed considerably, one must realise that in the 1996–1997 cohort only DSS patients were included. In the 2001–2003 cohort also included patients who had no evidence of circulatory failure were included. Mortality rate among DSS patients included in the 2001–2003 cohort was 18%. Our findings of similar frequencies of the PAI-1 genotypes within the 1996–1997 project and the 2001–2003 project supports our decision to combine both groups.
In conclusion, this study demonstrates that high PAI-1 plasma levels are associated with an increased risk of death from dengue without the 4G/5G polymorphism in the promotor of the gene for PAI-1 playing a role. Additional studies are needed to explore the possibility of other polymorphisms within the PAI-1 gene and factors, like ethnicity or environmental factors, contributing to the variability of PAI-1 plasma concentrations in patients with dengue.
Competing interests
The author(s) declare that they have no competing interests.
Authors' contributions
A.M., T.S. and P.K. wrote the first draft of the study protocol. H.C., D.B., A.O. and E.G. contributed to the writing of the study protocol. A.M. and T.S. were responsible for implementation of the study. T.S. was responsible for management of patients, and data collection at the study site. C.H., A.L., S.F. and P.R. were responsible for the measurement of PAI-1 plasma levels and for typing of the polymorphism. P.K. and A.O. were responsible for all diagnostic procedures. A.M. and A.L. did all statistical analyses. A.M., A.L., H.C., and E.G. wrote the first draft of the report, and all other authors contributed at subsequent stages.
Acknowledgments
Acknowledgements
This project was supported by a grant from the Royal Netherlands Academy of Arts and Sciences.
H. ten Cate is a Clinical Established Investigator of the Netherlands Heart Foundation.
Besides the authors, the following investigators participated in this study: Indonesia: C. Suharti, R. Djokomoeljanto (Department of Internal Medicine, dr. Kariadi Hospital, University of Diponegoro, Semarang); A. Soemantri (Department of Paediatrics, dr. Kariadi Hospital, University of Diponegoro, Semarang); K. Djamiatun (Medical Faculty, University of Diponegoro, Semarang, Indonesia). The Netherlands: J.W.M. van der Meer, W.M.V. Dolmans (Department of Internal Medicine, University Medical Centre St. Radboud, Radboud University Nijmegen, Nijmegen); Y.T. van der Heide (Clinical Chemistry and Hematology Laboratory, Slotervaart Hospital, Amsterdam); K. Stittelaar (Institute of Virology, Erasmus Medical Centre, Rotterdam); E. Vogels (Laboratory for Experimental Medicine, Academic Medical Centre, University of Amsterdam, Amsterdam); K. Joop (Hematology and Clinical Chemistry Laboratory, Onze Lieve Vrouwe Gasthuis, Amsterdam).
Contributor Information
ATA Mairuhu, Email: igrmr@slz.nl.
TE Setiati, Email: tatty@smg.melsa.net.id.
P Koraka, Email: p.koraka@erasmusmc.nl.
CE Hack, Email: e.hack@crucell.nl.
A Leyte, Email: a.leyte@olvg.nl.
SMH Faradz, Email: sultana@indosat.net.id.
H ten Cate, Email: h.tencate@bioch.unimaas.nl.
DPM Brandjes, Email: igtso@slz.nl.
ADME Osterhaus, Email: a.osterhaus@erasmusmc.nl.
PH Reitsma, Email: p.h.reitsma@amc.uva.nl.
ECM van Gorp, Email: ecmvangorp@yahoo.co.uk.
References
- Rigau-Perez JG, Clark GG, Gubler DJ, Reiter P, Sanders EJ, Vorndam AV. Dengue and dengue haemorrhagic fever. Lancet. 1998;352:971–977. doi: 10.1016/S0140-6736(97)12483-7. [DOI] [PubMed] [Google Scholar]
- Dengue Haemorrhagic Fever: Diagnosis, treatment, prevention and control. 2. World Health Organisation; 1997. [Google Scholar]
- Hales S, de Wet N, Maindonald J, Woodward A. Potential effect of population and climate changes on global distribution of dengue fever: an empirical model. Lancet. 2002;360:830–834. doi: 10.1016/S0140-6736(02)09964-6. [DOI] [PubMed] [Google Scholar]
- Mairuhu AT, Mac Gillavry MR, Setiati TE, Soemantri A, ten Cate H, Brandjes DP, van Gorp EC. Is clinical outcome of dengue-virus infections influenced by coagulation and fibrinolysis? A critical review of the evidence. Lancet Infect Dis. 2003;3:33–41. doi: 10.1016/S1473-3099(03)00487-0. [DOI] [PubMed] [Google Scholar]
- van Gorp EC, Minnema MC, Suharti C, Mairuhu AT, Brandjes DP, ten Cate H, Hack CE, Meijers JC. Activation of coagulation factor XI, without detectable contact activation in dengue haemorrhagic fever. Br J Haematol. 2001;113:94–99. doi: 10.1046/j.1365-2141.2001.02710.x. [DOI] [PubMed] [Google Scholar]
- van Gorp EC, Setiati TE, Mairuhu AT, Suharti C, Cate HH, Dolmans WM, van der Meer JW, Hack CE, Brandjes DP. Impaired fibrinolysis in the pathogenesis of dengue hemorrhagic fever. J Med Virol. 2002;67:549–554. doi: 10.1002/jmv.10137. [DOI] [PubMed] [Google Scholar]
- Wills BA, Oragui EE, Stephens AC, Daramola OA, Dung NM, Loan HT, Chau NV, Chambers M, Stepniewska K, Farrar JJ, Levin M. Coagulation Abnormalities in Dengue Hemorrhagic Fever: Serial Investigations in 167 Vietnamese Children with Dengue Shock Syndrome. Clin Infect Dis. 2002;35:277–285. doi: 10.1086/341410. [DOI] [PubMed] [Google Scholar]
- Hermans PW, Hibberd ML, Booy R, Daramola O, Hazelzet JA, de Groot R, Levin M. 4G/5G promoter polymorphism in the plasminogen-activator-inhibitor-1 gene and outcome of meningococcal disease. Meningococcal Research Group. Lancet. 1999;354:556–560. doi: 10.1016/S0140-6736(99)02220-5. [DOI] [PubMed] [Google Scholar]
- Westendorp RG, Hottenga JJ, Slagboom PE. Variation in plasminogen-activator-inhibitor-1 gene and risk of meningococcal septic shock. Lancet. 1999;354:561–563. doi: 10.1016/S0140-6736(98)09376-3. [DOI] [PubMed] [Google Scholar]
- de Boer JP, Abbink JJ, Brouwer MC, Meijer C, Roem D, Voorn GP, Lambers JW, van Mourik JA, Hack CE. PAI-1 synthesis in the human hepatoma cell line HepG2 is increased by cytokines--evidence that the liver contributes to acute phase behaviour of PAI-1. Thromb Haemost. 1991;65:181–185. [PubMed] [Google Scholar]
- Mansfield MW, Stickland MH, Grant PJ. Plasminogen activator inhibitor-1 (PAI-1) promoter polymorphism and coronary artery disease in non-insulin-dependent diabetes. Thromb Haemost. 1995;74:1032–1034. [PubMed] [Google Scholar]
- Groen J, Koraka P, Velzing J, Copra C, Osterhaus AD. Evaluation of six immunoassays for detection of dengue virus-specific immunoglobulin M and G antibodies. Clin Diagn Lab Immunol. 2000;7:867–871. doi: 10.1128/CDLI.7.6.867-871.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koraka P, Burghoorn-Maas CP, Falconar A, Setiati TE, Djamiatun K, Groen J, Osterhaus AD. Detection of immune-complex-dissociated nonstructural-1 antigen in patients with acute dengue virus infections. J Clin Microbiol. 2003;41:4154–4159. doi: 10.1128/JCM.41.9.4154-4159.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passing H, Bablok W. Comparison of several regression procedures for method comparison studies and determination of sample sizes. Application of linear regression procedures for method comparison studies in Clinical Chemistry, Part II. J Clin Chem Clin Biochem. 1984;22:431–445. doi: 10.1515/cclm.1984.22.6.431. [DOI] [PubMed] [Google Scholar]
- Meijer P, Pollet DE, Wauters J, Kluft C. Specificity of antigen assays of plasminogen activator inhibitor in plasma: Innotest PAI-1 immunoassay evaluated. Clin Chem. 1994;40:110–115. [PubMed] [Google Scholar]
- Brandtzaeg P, Joo GB, Brusletto B, Kierulf P. Plasminogen activator inhibitor 1 and 2, alpha-2-antiplasmin, plasminogen, and endotoxin levels in systemic meningococcal disease. Thromb Res. 1990;57:271–278. doi: 10.1016/0049-3848(90)90326-8. [DOI] [PubMed] [Google Scholar]
- Kornelisse RF, Hazelzet JA, Savelkoul HF, Hop WC, Suur MH, Borsboom AN, Risseeuw-Appel IM, van V, de Groot R. The relationship between plasminogen activator inhibitor-1 and proinflammatory and counterinflammatory mediators in children with meningococcal septic shock. J Infect Dis. 1996;173:1148–1156. doi: 10.1093/infdis/173.5.1148. [DOI] [PubMed] [Google Scholar]
- Mesters RM, Florke N, Ostermann H, Kienast J. Increase of plasminogen activator inhibitor levels predicts outcome of leukocytopenic patients with sepsis. Thromb Haemost. 1996;75:902–907. [PubMed] [Google Scholar]
- Paramo JA, Perez JL, Serrano M, Rocha E. Types 1 and 2 plasminogen activator inhibitor and tumor necrosis factor alpha in patients with sepsis. Thromb Haemost. 1990;64:3–6. [PubMed] [Google Scholar]
- Pralong G, Calandra T, Glauser MP, Schellekens J, Verhoef J, Bachmann F, Kruithof EK. Plasminogen activator inhibitor 1: a new prognostic marker in septic shock. Thromb Haemost. 1989;61:459–462. [PubMed] [Google Scholar]
- Kruithof EK, Tran-Thang C, Ransijn A, Bachmann F. Demonstration of a fast-acting inhibitor of plasminogen activators in human plasma. Blood. 1984;64:907–913. [PubMed] [Google Scholar]
- Pannekoek H, Veerman H, Lambers H, Diergaarde P, Verweij CL, van Zonneveld AJ, van Mourik JA. Endothelial plasminogen activator inhibitor (PAI): a new member of the Serpin gene family. EMBO J. 1986;5:2539–2544. doi: 10.1002/j.1460-2075.1986.tb04532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson SJ, Wiman B, Hamsten A, Green F, Humphries S, Henney AM. The two allele sequences of a common polymorphism in the promoter of the plasminogen activator inhibitor-1 (PAI-1) gene respond differently to interleukin-1 in HepG2 cells. J Biol Chem. 1993;268:10739–10745. [PubMed] [Google Scholar]
- Ryan MP, Kutz SM, Higgins PJ. Complex regulation of plasminogen activator inhibitor type-1 (PAI-1) gene expression by serum and substrate adhesion. Biochem J. 1996;314 ( Pt 3):1041–1046. doi: 10.1042/bj3141041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bethell DB, Flobbe K, Cao XT, Day NP, Pham TP, Buurman WA, Cardosa MJ, White NJ, Kwiatkowski D. Pathophysiologic and prognostic role of cytokines in dengue hemorrhagic fever. J Infect Dis. 1998;177:778–782. doi: 10.1086/517807. [DOI] [PubMed] [Google Scholar]
- Green S, Vaughn DW, Kalayanarooj S, Nimmannitya S, Suntayakorn S, Nisalak A, Lew R, Innis BL, Kurane I, Rothman AL, Ennis FA. Early immune activation in acute dengue illness is related to development of plasma leakage and disease severity. J Infect Dis. 1999;179:755–762. doi: 10.1086/314680. [DOI] [PubMed] [Google Scholar]
- Nguyen TH, Lei HY, Nguyen TL, Lin YS, Huang KJ, Le BL, Lin CF, Yeh TM, Do QH, Vu TQ, Chen LC, Huang JH, Lam TM, Liu CC, Halstead SB. Dengue hemorrhagic fever in infants: a study of clinical and cytokine profiles. J Infect Dis. 2004;189:221–232. doi: 10.1086/380762. [DOI] [PubMed] [Google Scholar]
- Eriksson P, Kallin B, 't Hooft FM, Bavenholm P, Hamsten A. Allele-specific increase in basal transcription of the plasminogen-activator inhibitor 1 gene is associated with myocardial infarction. Proc Natl Acad Sci U S A. 1995;92:1851–1855. doi: 10.1073/pnas.92.6.1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing LR, Hawksworth GM, Bennett B, Booth NA. Clearance of t-PA, PAI-1, and t-PA-PAI-1 complex in an isolated perfused rat liver system. J Lab Clin Med. 1991;117:109–114. [PubMed] [Google Scholar]
- Hayashi T, Kamogawa A, Ro S, Yamaguchi K, Kobayashi Y, Takahashi Y, Murayama M. Plasma from patients with cirrhosis increases tissue plasminogen activator release from vascular endothelial cells in vitro. Liver. 1998;18:186–190. doi: 10.1111/j.1600-0676.1998.tb00148.x. [DOI] [PubMed] [Google Scholar]
- Tran-Thang C, Fasel-Felley J, Pralong G, Hofstetter JR, Bachmann F, Kruithof EK. Plasminogen activators and plasminogen activator inhibitors in liver deficiencies caused by chronic alcoholism or infectious hepatitis. Thromb Haemost. 1989;62:651–653. [PubMed] [Google Scholar]
- Mohan B, Patwari AK, Anand VK. Hepatic dysfunction in childhood dengue infection. J Trop Pediatr. 2000;46:40–43. doi: 10.1093/tropej/46.1.40. [DOI] [PubMed] [Google Scholar]
- Pancharoen C, Rungsarannont A, Thisyakorn U. Hepatic dysfunction in dengue patients with various severity. J Med Assoc Thai. 2002;85 Suppl 1:S298–S301. [PubMed] [Google Scholar]
- Souza LJ, Alves JG, Nogueira RM, Gicovate NC, Bastos DA, Siqueira EW, Souto Filho JT, Cezario TA, Soares CE, Carneiro RC. Aminotransferase changes and acute hepatitis in patients with dengue fever: analysis of 1,585 cases. Braz J Infect Dis. 2004;8:156–163. doi: 10.1590/s1413-86702004000200006. [DOI] [PubMed] [Google Scholar]
- Festa A, D'Agostino RJ, Rich SS, Jenny NS, Tracy RP, Haffner SM. Promoter (4G/5G) plasminogen activator inhibitor-1 genotype and plasminogen activator inhibitor-1 levels in blacks, Hispanics, and non-Hispanic whites: the Insulin Resistance Atherosclerosis Study. Circulation. 2003;107:2422–2427. doi: 10.1161/01.CIR.0000066908.82782.3A. [DOI] [PubMed] [Google Scholar]
- Henry M, Chomiki N, Scarabin PY, Alessi MC, Peiretti F, Arveiler D, Ferrieres J, Evans A, Amouyel P, Poirier O, Cambien F, Juhan-Vague I. Five frequent polymorphisms of the PAI-1 gene: lack of association between genotypes, PAI activity, and triglyceride levels in a healthy population. Arterioscler Thromb Vasc Biol. 1997;17:851–858. doi: 10.1161/01.atv.17.5.851. [DOI] [PubMed] [Google Scholar]
- Henry M, Tregouet DA, Alessi MC, Aillaud MF, Visvikis S, Siest G, Tiret L, Juhan-Vague I. Metabolic determinants are much more important than genetic polymorphisms in determining the PAI-1 activity and antigen plasma concentrations: a family study with part of the Stanislas Cohort. Arterioscler Thromb Vasc Biol. 1998;18:84–91. doi: 10.1161/01.atv.18.1.84. [DOI] [PubMed] [Google Scholar]
- Mansfield MW, Stickland MH, Grant PJ. Environmental and genetic factors in relation to elevated circulating levels of plasminogen activator inhibitor-1 in Caucasian patients with non-insulin-dependent diabetes mellitus. Thromb Haemost. 1995;74:842–847. [PubMed] [Google Scholar]