Abstract
Strains of Shiga toxin-producing Escherichia coli (STEC) have been associated with outbreaks of diarrhea, hemorrhagic colitis, and hemolytic-uremic syndrome in humans. Most clinical signs of disease arise as a consequence of the production of Shiga toxin 1 (Stx1), Stx2 or combinations of these toxins. Other major virulence factors include enterohemorrhagic E. coli hemolysin (EHEC hlyA), and intimin, the product of the eaeA gene that is involved in the attaching and effacing adherence phenotype. In this study, a series of multiplex-PCR assays were developed to detect the eight most-important E. coli genes associated with virulence, two that define the serotype and therefore the identity of the organism, and a built-in gene detection control. Those genes detected were stx1, stx2, stx2c, stx2d, stx2e, stx2f, EHEC hlyA, and eaeA, as well as rfbE, which encodes the E. coli O157 serotype; fliC, which encodes the E. coli flagellum H7 serotype; and the E. coli 16S rRNA, which was included as an internal control. A total of 129 E. coli strains, including 81 that were O157:H7, 10 that were O157:non-H7, and 38 that were non-O157 isolates, were investigated. Among the 129 samples, 101 (78.3%) were stx positive, while 28 (21.7%) were lacked stx. Of these 129 isolates, 92 (71.3%) were EHEC hlyA positive and 96 (74.4%) were eaeA positive. All STEC strains were identified by this procedure. In addition, all Stx2 subtypes, which had been initially identified by PCR-restriction fragment length polymorphism, were identified by this method. A particular strength of the assay was the identification of these 11 genes without the need to use restriction enzyme digestion. The proposed method is a simple, reliable, and rapid procedure that can detect the major virulence factors of E. coli while differentiating O157:H7 from non-O157 isolates.
Many strains of Escherichia coli produce a variety of potent toxins, including Shiga-like toxins (Stx). Indeed it is often by virtue of these toxins that Shiga toxin producing E. coli (STEC) isolates express virulence for humans (10, 12). These strains are often referred to as verotoxin-producing E. coli due to the effect these toxins have on Vero cells in culture (12, 15). Such toxigenic isolates have been identified as a worldwide cause of serious human gastrointestinal disease, often with severe complicating problems that include bloody diarrhea, hemorrhagic colitis (HC), and the life-threatening condition hemolytic-uremic syndrome (HUS) (10).
Using in vitro and animal model studies, several groups have reported that a number of factors account for the virulence of STEC isolates, and prominent among these is the Stx group of toxins (4, 12, 15). Based on serological methods and DNA sequence analysis, these Stx toxins have been divided in to two major subclasses, Stx1 and Stx2 (16; S. C. Head, M. A. Karmali, M. E. Roscoe, M. Petric, N. A. Strockbine, and I. K. Wachsmuth, Letter, Lancet ii:751, 1988). Since there is no currently available specific treatment for HUS, there is an urgent need for effective preventive measures based on a detailed understanding of the epidemiology of STEC infections. Such measures will also be dependent on the availability of rapid, sensitive, simple, and reproducible procedures for the detection of these pathogens and for the characterization of their toxins both in samples from human specimens and those of nonhuman origin such as food and water.
Although Stx1 is relatively homogeneous, five subtypes of Stx2 have been identified, and these include Stx2, Stx2c (15, 20), Stx2d (27, 28, 31), Stx2e (11, 21), and Stx2f (7, 35). Indeed, based on both restriction fragment length polymorphisms (RFLP) of the B-subunit encoding the DNA fragments obtained by PCR and digoxigenin oligonucleotide labeling, DNA probes specific for stx2 B-subunit genes encoding Stx2, Stx2c, and Stx2d have been further confirmed (4, 6, 8, 9, 14, 15, 24-26, 31, 37, 38). Other major virulence factors ascribed to the pathogen include a plasmid-encoded enterohemolysin from STEC (enterohemorrhagic E. coli [EHEC] hlyA) that is often associated with severe clinical disease in humans (33, 34) as well as intimin, the product of the eaeA gene involved in the bacterial attaching and effacing adherence phenomenon and clustered in a pathogenicity island termed the locus for enterocyte effacement (22, 23). In addition, the presence of the ehl1 gene, which encodes an enterohemolysin that is unrelated to the EHEC hlyA genotype, has been associated with a severe outbreak of E. coli disease among neonates (2).
For detection purposes, a number of multiplex-PCR assays have been developed for the various virulence genes associated with E. coli strains. Such assays are usually aimed at detecting the genes stx1; stx2; stx2e; rfbEO157, which encodes the E. coli somatic antigen O157; fliCH7, which encodes the E. coli structural flagella antigen H7; uidA, which encodes β-glucuronidase; EHEC hlyA and eaeA; and the genes for the cytotoxic necrotizing factors, heat-labile toxin; heat-stable toxin; enteroinvasive toxin, and the enteroaggregative protein (4, 6, 8, 9, 14, 24, 26, 39).
In this study, a multiplex-PCR assay is described that uses three primer sets to detect the genes for 10 E. coli O157:H7 genes simultaneously. The genes detected were the eight virulence genes stx1, stx2, stx2c, stx2d, stx2e, stx2f, EHEC hlyA, and eaeA and the two genes rfbEO157 and fliCH7 to provide genotypic identification of the O157:H7 serotype most commonly associated with disease. As an internal positive control for each reaction, primers were also designated to amplify the E. coli 16S rRNA. Validation of the multiplex-PCR primers was performed and interpreted using individual primers by PCR-RFLP analysis (31, 38). In addition, a total of 129 isolates of E. coli O157 and non-O157 were characterized for the presence of the various virulence genes.
MATERIALS AND METHODS
Bacterial strains and culture media.
A total of 129 E. coli isolates derived from the culture collection of the National Laboratory for Enteric Pathogens were used in this study, and these included 79 E. coli O157:H7 isolates, five O157:NM (nonmotile) isolates, seven O157:non-H7 isolates (one each of O157:H10, H19, H21, H43, and H45 and two of H16), 12 non-O157:H7 isolates (two isolates of O27:H7, three of O18:H7, five of O55:H7, and one each of O156:H7 and O83:H7), six non-O157:NM isolates (one each of O1:NM, O7:NM, O91:NM, and O rough:NM and two isolates of O111:NM), 14 non-O157:non-H7 isolates (one isolate of O6:H1; two of O103:H2; one each of O146:H21, O26:H11, O70:H11, O91:H21, O139:K82, O128:B12, and O15:H27; two of O128:H untypeable, 1 of O113:H21, and 1 of O rough:H21), three isolates of O untypeable:H7, 1 of O untypable:H8, and 2 of O untypeable:H untypeable. Of these, 101 strains were STEC and 28 were negative for Stx. The control strains had been previously defined in terms of virulence factors and toxigenicity with respect to the genes stx1, stx2, stx2c, stx2-stx2c, stx2d, stx2e, stx2f, EHEC hlyA, and eaeA (Table 1).
TABLE 1.
Characteristics of reference strains used in this study
| Strain | Serotype | Stx gene(s)a | EHEC genotype | Reference(s) or source |
|---|---|---|---|---|
| H19 | O26:H11 | stx1 (SLT-I; VT1) | 3 | |
| 933W | O157:H7 | stx2 (SLT-II; VT2) | 16 | |
| 87-1215 | O157:H7 | stx1, stx2 | This study | |
| B2F1 | O91:H21 | stx2c (SLT-IIvha, SLT-IIvhb; VT2c) | 15 | |
| 86-704 | O15:H27 | stx2c (SLT-IIvhb; VT2c) | This study | |
| E32511 | O157:NM | stx2, stx2c (SLT-II, SLT-IIc) | 32 | |
| 91-126 | O128:H? | stx1 + stx2d (Stx1 + Stx2d) | 31; this study | |
| 412 | O139:K82 | stx2e (SLT-IIe; VTe) | 11, 40 | |
| H.I.8 | O128:B12 | stx2f (SLT-IIva; VTeV) | 7 | |
| 90-2380 | O157:H7 | stx2 | hlyA eaeA | This study |
| 25922 | O6:H1 | Lacking | American Type Culture Collection | |
| 92-3136 | O157:H21 | Lacking | This study | |
| 91-0575 | O55:H7 | Lacking | This study |
Data in parentheses are Shiga-like toxin genes, Stx toxins, and/or verotoxins.
DNA isolation.
Total DNA was isolated from 0.5 ml of brain heart infusion broth culture grown overnight for all the bacterial strains used in the study. The procedure used for DNA isolation was as described previously (38). DNA samples were dissolved in Tris-EDTA buffer (10 mM Tris, 1 mM EDTA at pH 8.0), and the DNA concentration was determined in micrograms per milliliter at an optical density reading of A260. The template DNA concentration used was 2 μg/ml.
Primer design.
Oligonucleotides ranging from 19- to 25-mers were selected from the published DNA sequences of E. coli using Oligo software (version 3.4). Synthesis of oligonucleotides was carried out at the DNA Core Facility at the National Microbiology Laboratory, Winnipeg, Canada. For multiplex PCR, three primers sets were prepared. Set A was designed to amplify stx1, stx2, stx2f, and 16S rRNA; set B was designed to amplify stx2c, stx2e, eaeA, and 16S rRNA; and set C was designed to amplify stx2d, EHEC hlyA, rfbEO157, and fliCH7 as well as 16S rRNA. The primer sequences used in the multiplex PCR are outlined in Table 2.
TABLE 2.
Primers used in this study
| Primer set | Primer | Sequence (5′ to 3′) | Target gene | Location within gene | Size of PCR amplicon (bp) | GenBank accession no. |
|---|---|---|---|---|---|---|
| A | Stx1-a | TCTCAGTGGGCGTTCTTATG | stx1 | 777-796 | 338 | M17358 |
| Stx1-b | TACCCCCTCAACTGCTAATA | 1114-1095 | ||||
| Stx2f-a | TGTCTTCAGCATCTTATGCAG | stx2f | 300-320 | 150 | M29153 | |
| Stx2f-b | CATGATTAATTACTGAAACAGAAAC | 449-425 | ||||
| Stx2-a | GCGGTTTTATTTGCATTAGC | stx2 | 1228-1247 | 115 | X07865 | |
| Stx2-b | TCCCGTCAACCTTCACTGTA | 1342-1323 | ||||
| B | Stx2c-a | GCGGTTTTATTTGCATTAGT | stx2c | 1186-1205 | 124 | M59432 |
| Stx2c-b | AGTACTCTTTTCCGGCCACT | 1309-1290 | ||||
| Stx2e-a | ATGAAGTGTATATTGTTAAAGTGGA | stx2e | 204-228 | 303 | M36727 | |
| Stx2e-b | AGCCACATATAAATTATTTCGT | 506-485 | ||||
| EAE-a | ATGCTTAGTGCTGGTTTAGG | eaeA | 132-151 | 248 | Z11541 | |
| EAE-b | GCCTTCATCATTTCGCTTTC | 379-360 | ||||
| C | Stx2d-a | GGTAAAATTGAGTTCTCTAAGTAT | stx2d | 1221-1244 | 175 | AF043627 |
| Stx2d-b | CAGCAAATCCTGAACCTGACG | 1395-1375 | ||||
| HlyA-a | AGCTGCAAGTGCGGGTCTG | EHEC hlyA | 867-885 | 569 | X79839 | |
| HlyA-b | TACGGGTTATGCCTGCAAGTTCAC | 1435-1412 | ||||
| RfbE-a | CTACAGGTGAAGGTGGAATGG | rfbEO157 | 673-693 | 327 | S83460 | |
| RfbE-b | ATTCCTCTCTTTCCTCTGCGG | 999-979 | ||||
| FliC-a | TACCATCGCAAAAGCAACTCC | fliCH7 | 1068-1088 | 247 | AF228488 | |
| FliC-b | GTCGGCAACGTTAGTGATACC | 1314-1294 | ||||
| Alla | E16S-a | CCCCCTGGACGAAGACTGAC | E. coli 16S rRNA | 1682-1701 | 401 | AB035924 |
| E16S-b | ACCGCTGGCAACAAAGGATA | 2082-2063 |
Used in all sets as the internal control.
Multiplex-PCR conditions.
Three sets of primer mixtures were prepared with slight modification according to the instructions supplied with the AmpliTaq Gold kit (Applied Biosystems, Foster City, Calif). In general, all of the multiplex primer sets contained 200 μM deoxynucleoside triphosphates, 2.5 μl of 10× reaction buffer (100 mM Tris-HCl at pH 8.3, 500 mM KCl), 1.5 mM MgCl2, and a 0.1 μM concentration of the E. coli 16S rRNA (E16S) primers. In addition to these, set A contained a 0.5 μM concentration of each of the primers Stx1, Stx2, and Stx2f together with 2.5 U of Taq DNA polymerase (AmpliTaq Gold; Applied Biosystems) and 5 ng of template DNA. The volume of this mix was adjusted to 25 μl with sterile water. In addition to the common constituents, the multiplex primer set B included primers at the following concentrations: 1.5 μM Stx2c, 0.4 μM Stx2e, and 0.75 μM EAE. Multiplex primer set C comprised common components plus primers at the following concentrations: 1.5 μM Stx2d, 1.0 μM (each) HlyA and RfbE, and 0.4 μM FliC (Table 2). DNA amplification was carried out in a Perkin-Elmer thermocycler 2400 using an initial denaturation step at 95°C for 8 min, followed by 30 cycles of amplification with denaturation at 95°C for 30 s, annealing at 58°C for 30 s, and extension at 72°C for 30 s, ending with a final extension at 72°C for 7 min.
RESULTS
Multiplex PCR for the detection of E. coli virulence genes and O157:H7 serotype genes.
The reaction conditions for the multiplex-PCR assay were optimized to ensure that all of the target gene sequences were satisfactorily amplified. Initially, equimolar primer concentrations of 0.5 μM each were used in the multiplex PCR, but there was uneven amplification, and some of the products were barely visible even after the reaction was optimized for the cycling conditions. Overcoming this problem required changing the proportions of the various primers in the reaction mixture to give an increase in the concentration of primers for the “weak” loci and a decrease in the primer concentration for the “strong” loci. The final concentration of the primers (0.1 to 1.5 μM) varied considerably among the loci and was established empirically.
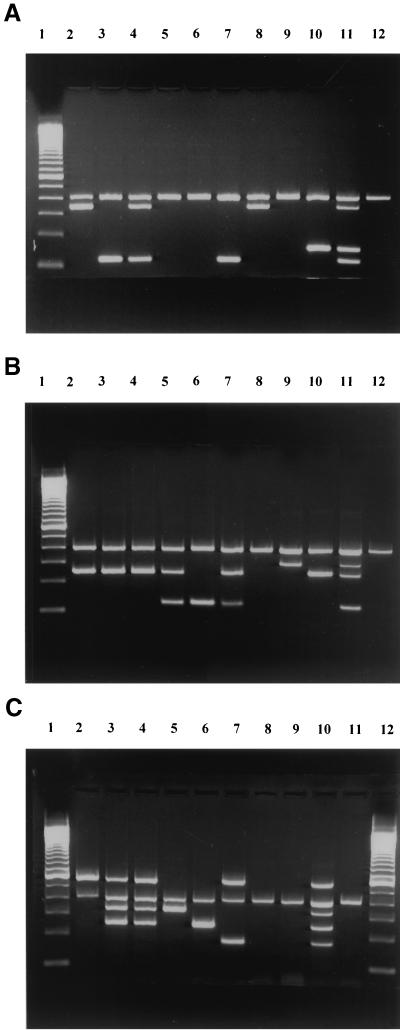
The primers were designed to target the coding regions of the genes, and care was taken to avoid areas of homology within the structural genes encoding the Stx2 family of toxins. The primers used in each set had identical annealing temperatures, which reduced the possibility of the occurrence of unwanted bands originating from nonspecific amplification. Figure 1 shows the presence of the amplified product profiles after agarose gel electrophoresis, when DNA extracted from a reference E. coli strain (positive control) was used as the template in the PCR using the multiplex primer sets. The four bands in set A, stx1, stx2, stx2f, and 16S rRNA, were amplified consistently even when mixtures of DNA derived from the same strains were tested (Fig. 1A). Similarly, four bands were obtained in set B when a mixture of DNA extracts from the corresponding strains that carried the genes stx2c, stx2e, eaeA, and 16S rRNA was tested (Fig. 1B). For set C, which contained primers to amplify the genes stx2d, EHEC hlyA, rfbEO157, fliCH7, and 16S rRNA, a total of five bands was obtained from the positive-control DNA (Fig. 1C). The various control strains corresponded to the predicted sizes (Table 2). As a negative control, all sets were tested with E. coli strain ATCC 25922, and only the 16S rRNA band was observed (lanes 12 in Fig. 1A and B; lane 11 in Fig. 1C). Genomic DNA from Aeromonas hydrophila and Campylobacter jejuni was also tested using these three primer sets, and none gave PCR amplification bands (data not shown).
FIG. 1.
Shown are multiplex-PCR amplification profiles from reference E. coli strains with primer set A (A), B (B), and C (C). (A) Lane 1, 100-bp DNA ladder (Bethesda Research Laboratories Inc., Gaithersburg, Md.); lane 2, stx1 and 16S rRNA (E. coli strain H19); lane 3, stx2 and 16S rRNA (strain 90-2380); lane 4, stx1, stx2, and 16S rRNA (strain 87-1215); lane 5, 16S rRNA (formerly stx2Va, strain 91-2245); lane 6, 16S rRNA (formerly stx2Vb, strain 86-704); lane 7, stx2 (stx2-stx2c,strain E32511); lane 8, stx1 and 16S rRNA (stx1-stx2d, strain 91-126); lane 9, 16S rRNA (stx2e, strain 412); lane 10, stx2f and 16S rRNA (stx2f, strain H.I.8); lane 11, stx1, stx2, stx2f, and 16S rRNA; lane 12, stx negative control (ATCC 25922). (B) Lane 1, 100-bp DNA ladder (Bethesda Research Laboratories Inc.); lane 2, amplification products of eaeA and 16S rRNA (stx1, strain H19); lane 3, eaeA and 16S rRNA(stx2, strain 90-2380); lane 4, eaeA and 16S rRNA(stx1-stx2, strain 87-1215); lane 5, eaeA, stx2c, and 16S rRNA (formerly stx2Va, strain 91-2245); lane 6, stx2c and 16S rRNA (formerly stx2Vb, strain 86-704); lane 7, eaeA, stx2c and 16S rRNA (stx2-stx2c, strain E32511); lane 8, 16S rRNA(stx1-stx2d, strain 91-126); lane 9, stx2e and 16S rRNA (stx2e, strain 412); lane 10, eaeA and 16S rRNA (stx2f, strain H.I.8); lane 11, eaeA, stx2c, stx2e, and 16S rRNA; lane 12, stx negative control (ATCC 25922). (C) Lanes 1 and 12, 100-bp DNA ladder (Bethesda Research Laboratories Inc.); lane 2, hlyA and 16S rRNA (strain H19); lane 3, hlyA, rfbEO157, fliCH7, and 16S rRNA(strain 90-2380, O157:H7); lane 4, hlyA, rfbEO157, fliCH7, and 16S rRNA (strain E32511, O157: NM); lane 5, rfbEO157 and 16S rRNA (O157: H21); lane 6, fliCH7 and 16S rRNA (O55: H7); lane 7, hlyA, stx2d, and 16S rRNA (stx1-stx2d, strain 91-126); lane 8, 16S rRNA (strain 412); lane 9, 16S rRNA (strain H.I.8); lane 10, positive control of hlyA, rfbEO157, fliCH7, stx2d, and 16S rRNA; lane 11, stx negative control (ATCC 25922).
To substantiate the multiplex-PCR technique, 129 strains of E. coli that were tested by multiplex PCR were also screened for the presence of individual toxin genes by using the methods described previously (14, 17, 18, 24, 30). stx2 subtype, stx2c, and stx2d genes were confirmed by PCR-RFLP analysis (31, 38). The serotype identity of the E. coli O157:H7 isolates and the non-O157:H7 strains was determined at the National Laboratory for Enteric Pathogens. Agreement between the toxigenic profile and O157:H7 serotype results and the multiplex-PCR data was observed (Table 3). However, 3 of 11 phenotypically NM strains showed gene-positive results for H7 by the PCR assay, and one of these was the reference stain E32511, previously shown to be genotypically H7. An internal control of E. coli 16S rRNA was present in all of the E. coli samples, thus confirming the presence and the quality of E. coli DNA amplification as well as validating the PCR conditions.
TABLE 3.
Major virulence genes detected by the three primer sets in the multiplex PCR analysis
| Toxin(s) confirmed by PCR or PCR-RFLP analysis (n)a | Serotype (n)b | No. of genes detected by PCR with primer set:
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| A
|
B
|
C
|
|||||||||
| stx1 | stx2 | stx2f | stx2c | stx2e | eaeA | stx2d | hlyA | rfbEO157 | fliCH7 | ||
| Stx1 (13) | Non-O157:non-H7 (5) | 5 | —c | — | — | — | 4 | — | 5 | — | — |
| O111:NM (1) | 1 | — | — | — | — | 1 | — | 1 | — | — | |
| O91:NM (1) | 1 | — | — | — | — | — | — | 1 | — | — | |
| O157:H7 (5) | 5 | — | — | — | — | 5 | — | 5 | 5 | 5 | |
| O157:NM (1) | 1 | — | — | — | — | 1 | — | 1 | 1 | 1 | |
| Stx1 + Stx2 (27) | O UT:H UTd (1) | 1 | 1 | — | — | — | — | — | — | — | — |
| O157:H7 (26) | 26 | 26 | — | — | — | 26 | — | 26 | 26 | 26 | |
| Stx1 + Stx2c (2) | O157:H7 (1) | 1 | — | — | 1 | — | 1 | — | 1 | 1 | 1 |
| O111:NM (1) | 1 | — | — | 1 | — | 1 | — | — | — | — | |
| Stx1 + Stx2d (3) | O128:H? (2) | 2 | — | — | — | — | — | 2 | 2 | — | — |
| O rough:NM (1) | 1 | — | — | — | — | — | 1 | — | — | — | |
| Stx2 (16) | O157: H7 (16) | — | 16 | — | — | — | 16 | — | 16 | 16 | 16 |
| Stx2 + Stx2c (27) | O157:NM (1) | — | 1 | — | 1 | — | 1 | — | 1 | 1 | 1 |
| O157:H7 (26) | — | 26 | — | 26 | — | 26 | — | 26 | 26 | 26 | |
| Stx2c (10) | O91:H21 (1) | — | — | — | 1 | — | — | — | 1 | — | — |
| Non-O157:non-H7 (3) | — | — | — | 3 | — | — | — | 1 | — | — | |
| O157:H7 (5) | — | — | — | 5 | — | 5 | — | 5 | 5 | 5 | |
| O55:H7 (1) | — | — | — | 1 | — | 1 | — | — | — | 1 | |
| Stx2d (1) | O UT:H8 (1) | — | — | — | — | — | — | 1 | — | — | — |
| Stx2e (1) | O139:K82 (1) | — | — | — | — | 1 | — | — | — | — | — |
| Stx2f (1) | O128:B12 (1) | — | — | 1 | — | — | 1 | — | — | — | — |
| Stx negative (28) | O157:non-H7 (7) | — | — | — | — | — | 6 | — | — | 7 | — |
| O157:NM (3) | — | — | — | — | — | — | — | — | 3 | — | |
| Non-O157:H7 (11) | — | — | — | — | — | 1 | — | — | — | 11 | |
| O UT:H7 (3) | — | — | — | — | — | — | — | — | — | 3 | |
| Non-O157:NM (2) | — | — | — | — | — | — | — | — | — | 1 | |
| Non-O157:non-H7 (1) | — | — | — | — | — | — | — | — | — | — | |
| O UT:H UT (1) | — | — | — | — | — | — | — | — | — | — | |
| Total | 101/129 | 45 | 70 | 1 | 39 | 1 | 96 | 4 | 92 | 91 | 106 |
Primary validation of amplicons.
The sizes of the amplicons obtained from the multiplex primer sets were identical to those predicted from the design of the primers (Table 2). The amplicons from the control strains were subjected to further confirmation and characterization by digestion with restriction endonucleases with cleavage sites within each of the amplicons. The restriction enzymes used and the predicted product sizes are given in Table 4. Enzyme fragments with the anticipated sizes were obtained in all cases (data not shown).
TABLE 4.
Predicted sizes of restriction fragments and enzymes used for RFLP analysis of amplified products of multiplex PCR
| Genes | PCR amplicon size (bp) | Multiplex primer set(s) | Enzyme | Expected sizes of restriction fragments (bp) |
|---|---|---|---|---|
| stx1 | 338 | A | BglI | 136, 202 |
| stx2 | 115 | A | BsrDI | 37, 78 |
| stx2f | 150 | A | AluI | 54, 96 |
| stx2e | 303 | B | TaqI | 51, 87, 165 |
| stx2c | 124 | B | HhaI | 48, 76 |
| eaeA | 248 | B | AluI | 109, 139 |
| stx2d | 175 | C | RsaI | 66, 109 |
| EHEC hlyA | 569 | C | ApaI | 299, 270 |
| rfbEO157 | 327 | C | AluI | 80, 93, 154 |
| fliCH7 | 247 | C | AluI | 40, 207 |
| E. coli 16S rRNA | 401 | A, B, and C | RsaI | 156, 245 |
Analysis of results.
Among the 129 strains tested, 101 (78.3%) were positive for Stx toxins. All of O157:H7 strains were eaeA and EHEC hlyA positive. A total of 96 (74.4%) E. coli isolates were positive for the eaeA gene, while seven eaeA positives were detected among strains otherwise lacking stx. The ability of the C set of primers to identify O157:H7 from other E. coli strains was determined by analyzing 79 O157:H7, 5 O157:NM, 7 O157:non-H7, and 38 non-O157 E. coli isolates. Two of the five O157:NM strains and one of six non-O157:NM strains were fliCH7 gene positive, indicating that these isolates were genetically H7 with flagellum antigens that were either not expressed or not detectable in serotyping tests (Table 3) (5). All of the 129 samples tested contained the E. coli 16S rRNA gene.
DISCUSSION
STEC strains have been associated with outbreaks of disease that included cases of HC and HUS in humans. The two major categories of E. coli Stx toxins are Stx1 and Stx2. Stx1 is a relatively homogeneous family of toxins that show identity with the Shiga toxins of Shigella dysenteriae. Stx2 toxins, however, are a more heterogeneous group that are serologically distinct from Stx1. Within the Stx2 toxin family, Stx2c was formerly subdivided into Stx2-Va and Stx2-Vb (15, 38). These are only partially neutralized by antiserum to Stx2 (14; Head et al., letter). Stx2d shows a low cytotoxicity in Vero cells (27, 28, 31), while Stx2e is cytotoxic only in Vero cells and has been associated with porcine edema disease (11, 21). Stx2f (also called VTeV) has low-level cytotoxicity for Vero cells and is readily neutralized by antisera that are raised against Stx2 and Stx2e (7, 35).
Among STEC isolates, certain strains appear to have a greater degree of virulence for humans, while some data suggest that toxin type is important in determining the probability of developing HUS. Indeed, it has been shown epidemiologically that Stx2 is more critical than Stx1 for the development of HUS, in that strains producing Stx2 were more frequently associated with cases of HUS than were those isolates expressing Stx1 only (10). In addition to serological differences, the Stx2 group of toxins may differ in terms of their in vitro or in vivo properties. Experiments with clones carrying chimeric O48/OX3b stx2 operons indicated that the increased virulence was a function of the A subunit of stx2/OX3b. This differs in the A-subunit structure from that of stx2/O48 by only two amino acids (Met-4→Thr and Gly-102→Asp, respectively). These findings raise the possibility that naturally occurring Stx2 sequence variations may directly impact the capacity of a given Stx-producing E. coli strain to cause severe disease (29).
The use of multiplex PCR or PCR-RFLP analysis to characterize the various subtypes of the stx2 genes has been well documented (4, 6, 8, 9, 14, 24-26, 31, 38). Lin et al. (19) introduced common primers for PCR-RFLP analysis in order to detect the genes for various Stx toxins. However, all the PCR-related methods require restriction digestion to achieve identification. A multiplex PCR-based diagnostic protocol is described for the detection of those genes encoding the various Stx toxins, including Stx1, Stx2, Stx2c, Stx2d, Stx2e, and Stx2f, EHEC hlyA, and eaeA, together with the genes governing the serotype O157 (rfbE) and H7 (fliC) in the absence of restriction enzyme digestion. Compared to the individual primers and PCR-RFLP analysis results, the multiplex-PCR primer sets proved to be highly specific, they gave consistent results, and they were effective in detecting all 11 genes, including the internal control gene. All primers were gene specific, as demonstrated by restriction fragment lengths obtained after specific restriction endonuclease digestion of the amplicons.
In this study, the toxin genotype and O157:H7 serotype of a range of E. coli strains was demonstrated (Table 3). Of 81 STEC O157:H7 clinical isolates (including the 2 that were serotypically O157:NM but were PCR positive for fliCH7), all were positive for the EHEC hlyA and eaeA genes. These finding were in agreement with previous reports (1, 33). Interestingly, among 20 of the non-O157 STEC isolates, 11 (55%) were EHEC hlyA positive and 8 (40%) were eaeA positive. Three of 10 strains positive for the stx2c gene only were negative for EHEC hlyA, while the reference stx2c gene-positive strain (serotype O91:H21, an isolate from a patient with HUS) was EHEC hlyA positive and lacked eaeA, suggesting that the eaeA may not be an essential major virulence factor for the acquisition of HUS. All four strains that possessed the stx2d genotype lacked eaeA. Of these four strains, three possessed both the stx1 and stx2d genotype, while one was positive for stx2d alone. Two of these were hlyA positive. This suggested that STEC strains without EHEC hlyA may possess reduced pathogenicity or may even be nonpathogenic in humans (36). Furthermore, among the 28 non-STEC isolates (Stx negative), 7 were eaeA positive and none possessed the EHEC hlyA gene. This suggested that EHEC hlyA may be a more critical virulence factor for disease than eaeA (Table 3). Further studies are required on EHEC hlyA, eaeA, and the various Stx components in order to elucidate the role that these toxins play in STEC disease in general and in HUS and HC in particular.
In total 11 phenotypic nonmotile E. coli isolates were analyzed. Of these, three were fliCH7 positive. Two of these were O157:NM strains (including reference strain E32511), and one was an O1:NM strain. All three were confirmed as H7 positive using primer FLICh7-F and FLICh7-R (14). An E. coli fliCH7 sequence comparison (GenBank accession no. AF228487 for O157:H7, AF228495 for O19ab:H7, AF228496 for O53:H7, AF228489 for O55:H7, and U47614 for O157:NM) also confirmed that fliC is highly conserved among different serogroups. Therefore, it would appear that some of E. coli strains that are serologically NM are genetically H7 (5). The factors that govern fliC expression are unclear; however, environmental factors or other related genes may play key roles.
From these data we conclude that the multiplex primer sets described are specific and give highly consistent results. The use of this three-tube assay should allow simultaneous detection of the major virulence factors associated with E. coli O157 and non-O157 strains and avoids the need for endonuclease digestion steps.
Acknowledgments
We thank David Woodward, Richard Caldeira, Jennifer Campbell, David Spreitzer, Kelly Robinson, Kevin Hill, and Julie Walsh for valuable technical assistance.
REFERENCES
- 1.Boerlin, P., S. A. McEwen, B. P. Franziska, J. B. Wilson, R. P. Johnson, and C. L. Gyles. 1999. Associations between virulence factors of Shiga toxin-producing Escherichia coli and disease in humans. J. Clin. Microbiol. 37:497-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chinen, I., M. Rivas, V. Soriano, E. Miliwebsky, G. F. Galvez, G. Chillemi, A. Baschkier, G. Wang, R. Caldeira, D. L. Woodward, and F. G. Rodgers. 2002. Escherichia coli ehl1 gene-positive serotype O18ac:H31 associated with an outbreak of diarrhea in a neonatal nursery in Neuquen City, Argentina. J. Clin. Microbiol. 40:1225-1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Grandis, S., J. Ginsberg, M. Toone, S. Climie, J. Friesen, and J. Brunton. 1987. Nucleotide sequence and promoter mapping of the Escherichia coli Shiga-like toxin operon of bacteriophage H-19B. J. Bacteriol. 169:4313-4319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feng, P., and S. R. Monday. 2000. Multiplex PCR for detection of trait and virulence factors in enterohemorrhagic Escherichia coli serotypes. Mol. Cell. Probes 14:333-337. [DOI] [PubMed] [Google Scholar]
- 5.Fields, P. I., K. Blom, H. J. Hughes, L. O. Helsel, P. Feng, and B. Swaminathan. 1997. Molecular characterization of the gene encoding H antigen in Escherichia coli and development of a PCR-restriction fragment length polymorphism test for identification of E. coli O157:H7 and O157:NM. J. Clin. Microbiol. 35:1066-1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fratamico, P. M., L. K. Bagi, and T. Pepe. 2000. A multiplex polymerase chain reaction assay for rapid detection and identification of Escherichia coli O157:H7 in foods and bovine feces. J. Food Prot. 63:1032-1037. [DOI] [PubMed] [Google Scholar]
- 7.Gannon, V. P., C. Teerling, S. A. Masri, and C. L. Gyles. 1990. Molecular cloning and nucleotide sequence of another variant of the Escherichia coli Shiga-like toxin II family. J. Gen. Microbiol. 136:1125-1135. [DOI] [PubMed] [Google Scholar]
- 8.Gannon, V. P., S. D'Souza, T. Graham, R. K. King, K. Rahn, and S. Read. 1997. Use of the flagella H7 gene as a target in multiplex PCR assays and improved specificity in identification of enterohemorrhagic Escherichia coli strains. J. Clin. Microbiol. 35:656-662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gannon, V. P., S. D'Souza, T. Graham, and R. K. King. 1997. Specific identification of Escherichia coli O157:H7 using a multiplex PCR assay. Adv. Exp. Med. Biol. 412:81-82. [DOI] [PubMed] [Google Scholar]
- 10.Griffin, P. M., and R. V. Tauxe. 1991. The epidemiology of infections caused by Escherichia coli O157:H7, other enterohemorrhagic E. coli, and the associated hemolytic uremic syndrome. Epidemiol. Rev. 13:60-98. [DOI] [PubMed] [Google Scholar]
- 11.Gyles, C. L., S. A. De Grandis, C. MacKenzie, and J. L. Brunton. 1988. Cloning and nucleotide sequence analysis of the genes determining verocytotoxin production in a porcine edema disease isolate of Escherichia coli. Microb. Pathog. 5:419-426. [DOI] [PubMed] [Google Scholar]
- 12.Gyles, C. L. 1992. Escherichia coli cytotoxins and enterotoxins. Can. J. Microbiol. 38:734-746. [DOI] [PubMed] [Google Scholar]
- 13.Hii, J. H., C. Gyles, T. Morooka, M. A. Karmali, R. Clarke, S. DeGrandis, and J. L. Brunton. 1991. Development of verotoxin 2- and verotoxin 2 variant (VT2v)-specific oligonucleotide probes on the basis of the nucleotide sequence of the B cistron of VT2v from Escherichia coli E32511 and B2F1. J. Clin. Microbiol. 29:2704-2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu, Y., Q. Zhang, and J. C. Meitzler. 1999. Rapid and sensitive detection of Escherichia coli O157:H7 in bovine faeces by a multiplex PCR. J. Appl. Microbiol. 87:867-876. [DOI] [PubMed] [Google Scholar]
- 15.Ito, H., A. Terai, H. Kurazono, Y. Takeda, and M. Nishibuchi. 1990. Cloning and nucleotide sequencing of Vero toxin 2 variant genes from Escherichia coli O91:H21 isolated from a patient with the hemolytic uremic syndrome. Microb. Pathog. 8:47-60. [DOI] [PubMed] [Google Scholar]
- 16.Jackson, M. P., R. J. Neill, A. D. O'Brien, R. K. Holmes, and J. W. Newland. 1987. Nucleotide sequence analysis and comparison of the structural genes for Shiga-like toxin, I., and Shiga-like toxin II encoded by bacteriophages from Escherichia coli. FEMS Microbiol. Lett. 44:109-114. [DOI] [PubMed] [Google Scholar]
- 17.Johnson, W. M., D. R. Pollard, H. Lior, S. D. Tyler, and K. R. Rozee. 1990. Differentiation of genes coding for Escherichia coli verotoxin 2 and the verotoxin associated with porcine edema disease (VTe) by the polymerase chain reaction. J. Clin. Microbiol. 28:2351-2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson, W. M., S. D. Tyler, G. Wang, and H. Lior. 1991. Amplification by the polymerase chain reaction of a specific target sequence in the gene coding for Escherichia coli verotoxin (VTe variant). FEMS Microbiol Lett. 68:227-230. [DOI] [PubMed] [Google Scholar]
- 19.Lin, Z., H. Kurazono, S. Yamasaki, and Y. Takeda. 1993. Detection of various variant verotoxin genes in Escherichia coli by polymerase chain reaction. Microbiol. Immunol. 37:543-548. [DOI] [PubMed] [Google Scholar]
- 20.Lindgren, S. W., J. E. Samuel, C. K. Schmitt, and A. D. O'Brien. 1994. The specific activities of Shiga-like toxin type II (SLT-II) and SLT-II-related toxins of enterohemorrhagic Escherichia coli differ when measured by Vero cell cytotoxicity but not by mouse lethality. Infect. Immun. 62:623-631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marques, L. R. M., J. S. M. Peiris, S. J. Cryz, and A. D. O'Brien. 1987. Escherichia coli strains isolated from pigs produce a variant of Shiga-like toxin II. FEMS Microbiol. Lett. 44:33-38. [Google Scholar]
- 22.McDaniel, T. K., K. G. Jarvis, M. S. Donnenberg, and J. B. Kaper. 1995. A genetic locus of enterocyte effacement conserved among diverse enterobacterial pathogens. Proc. Natl. Acad. Sci. USA 92:1664-1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McDaniel, T. K., and J. B. Kaper. 1997. A cloned pathogenicity island from enteropathogenic Escherichia coli confers the attaching and effacing phenotype on E. coli K12. Mol. Microbiol. 2:399-407. [DOI] [PubMed] [Google Scholar]
- 24.Meng, J., S. Zhao, M. P. Doyle, S. E. Mitchell, and S. Kresovich. 1997. A multiplex PCR for identifying Shiga-like toxin-producing Escherichia coli O157:H7. Lett. Appl. Microbiol. 24:172-176. [DOI] [PubMed] [Google Scholar]
- 25.Meng, J., S. Zhao, and M. P. Doyle. 1998. Virulence genes of Shiga toxin-producing Escherichia coli isolated from food, animals and humans. Int. J. Food Microbiol. 45:229-235. [DOI] [PubMed] [Google Scholar]
- 26.Pass, M. A., R. Odedra, and R. M. Batt. 2000. Multiplex PCRs for identification of Escherichia coli virulence genes. J. Clin. Microbiol. 38:2001-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paton, A. W., J. C. Paton, M. W. Heuzenroeder, P. N. Goldwater, and P. A. Manning. 1992. Cloning and nucleotide sequence of a variant Shiga-like toxin II gene from Escherichia coli OX3:H21 isolated from a case of sudden infant death syndrome. Microb. Pathog. 13:225-236. [DOI] [PubMed] [Google Scholar]
- 28.Paton, A. W., J. C. Paton, P. N. Goldwater, M. W. Heuzenroeder, and P. A. Manning. 1993. Sequence of a variant Shiga-like toxin type-I operon of Escherichia coli O111:H-. Gene 129:87-92. [DOI] [PubMed] [Google Scholar]
- 29.Paton, A. W., A. J. Bourne, P. A., Manning, and J. C. Paton. 1995. Comparative toxicity and virulence of Escherichia coli clones expressing variant and chimeric Shiga-like toxin type II operons. Infect. Immun. 63:2450-2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paton, A. W., and J. C. Paton. 1997. Detection and characterization of Shiga toxigenic Escherichia coli by using multiplex PCR assays for stx1, stx2, eaeA, enterohemorrhagic E. coli hlyA, rfb0111, and rfb0157. J. Clin. Microbiol. 36:598-602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pierard, D., G. Muyldermans, L. Moriau, D. Stevens, and S. Lauwers. 1998. Identification of new verocytotoxin type 2 variant B-subunit genes in human and animal. Escherichia coli isolates. J. Clin. Microbiol. 36:3317-3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schmitt, C. K., M. L. McKee, and A. D. O'Brien. 1991. Two copies of Shiga-like toxin II-related genes common in enterohemorrhagic Escherichia coli strains are responsible for the antigenic heterogeneity of the O157:H− strain E32511. Infect. Immun. 59:1065-1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmidt, H., L. Beutin, and H. Karch. 1995. Molecular analysis of the plasmid-encoded hemolysin of Escherichia coli O157:H7 strain EDL 933. Infect. Immun. 63:1055-1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schmidt, H., and H. Karch. 1996. Enterohemolytic phenotypes and genotypes of Shiga-toxin-producing Escherichia coli O111 strains from patients with diarrhea and hemolytic-uremic syndrome. J. Clin. Microbiol. 34:2364-2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schmidt, H., J. Scheef, S. Morabito, A. Caprioli, L. H. Wieler, and H. Karch. 2000. A new Shiga toxin 2 variant (Stx-2f) from Escherichia coli isolated from pigeons. Appl. Environ. Microbiol. 66:1205-1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stephan, R., and L. E. Hoelzle. 2000. Characterization of shiga toxin type 2 variant B-subunit in Escherichia coli strains from asymptomatic human carriers by PCR-RFLP. Lett. Appl. Microbiol. 31:139-142. [DOI] [PubMed] [Google Scholar]
- 37.Thomas, A., H. R. Smith, and B. J. Rowe. 1993. Use of digoxigenin-labelled oligonucleotide DNA probes for VT2 and VT2 human variant genes to differentiate Vero cytotoxin-producing Escherichia coli strains of serogroup O157. J. Clin. Microbiol. 31:1700-1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tyler, S. D., W. M. Johnson, H. Lior, G. Wang, and K. R. Rozee. 1991. Identification of verotoxin type 2 variant B-subunit genes in Escherichia coli by the polymerase chain reaction and restriction fragment length polymorphism analysis. J. Clin. Microbiol. 29:1339-1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang, L., D. Rothemund, H. Curd, and P. R. Reeves. 2000. Sequence diversity of the Escherichia coli H7 fliC genes: implication for a DNA-based typing scheme for E. coli O157:H7. J. Clin. Microbiol. 38:1786-1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weinstein, D. L., M. P. Jackson, J. E. Samuel, R. K. Holmes, and A. D. O'Brien. 1988. Cloning and sequencing of a Shiga-like toxin type II variant from Escherichia coli strain responsible for edema disease of swine. J. Bacteriol. 170:4223-4230. [DOI] [PMC free article] [PubMed] [Google Scholar]