Abstract
The reliability of visual perception is thought to reflect the quality of the sensory information. However, we show that subjects' performance can be predicted, trial-by-trial, by neural activity that precedes the onset of a sensory stimulus. Using functional MRI (fMRI), we studied how neural mechanisms that mediate spatial attention affect the accuracy of a motion discrimination judgment. The amplitude of blood oxygen level-dependent (BOLD) signals after a cue directing spatial attention predicted subjects' accuracy on 60-75% of the trials. Widespread predictive signals, which included dorsal parietal, visual extra-striate, prefrontal and sensory-motor cortex, depended on whether the cue correctly specified the stimulus location. Therefore, these signals indicate the degree of utilization of the cued information and play a role in the control of spatial attention. We conclude that variability in perceptual performance can be partly explained by the variability in endogenous, preparatory processes and that BOLD signals can be used to forecast human behavior.
Keywords: functional MRI, performance variability, cue utilization, reward
Visual perception depends on the quality of sensory information and the fidelity of its neural representation. Recent experiments have emphasized the importance of endogenous processes such as attention, working memory, and motor planning, in modulating sensory activity (1-4). Furthermore, current theories and empirical results suggest that the brain maintains an on-line internal representation of the world that is modulated rather than determined by sensory information (5-7).
To study the influence of endogenous processes on visual perception, we examined whether preparatory signals related to the voluntary allocation of spatial attention are predictive of subjects' performance in a motion discrimination task. For correlational methods such as functional MRI (fMRI) and single unit recordings, the strongest and most direct link between brain activity and behavior is the trial-to-trial relation between neural signals and choice. The feasibility of this approach has been demonstrated repeatedly in single unit studies (8-12), where the neuronal response in areas sensitive to motion during a motion discrimination task correlates, on a trial-by-trial basis, with the animal's report. Importantly, this correlation persisted even when there was no net directional signal in the stimulus, suggesting that choice can depend on purely endogenous signals.
However, it is largely unknown to what degree endogenous signals that precede stimulus analysis are predictive of performance. Single-unit studies in extra-striate visual areas have failed thus far to show that trial-by-trial variations in neural signals preceding the stimulus bear on performance accuracy (12). In higher-order parietal cortex, the magnitude of the neuronal response correlates on average with the locus of attention, but it is unknown whether it is predictive of performance on a trial-by-trial basis (13). Although several studies in humans have examined the relation between blood oxygen level-dependent (BOLD) signals and behavior (14-18), we focus on purely endogenous preparatory signals that clearly precede the stimulus and decision.
To isolate endogenous sources of behavioral variability and to separate predictive signals from signals evoked by the stimulus, we measured cue-related activity in a paradigm in which a cue indicated the likely location of a motion signal whose direction subjects discriminated (12, 19). Cues have systematic effects on performance (20). Although there is no evidence that cues are used variably from trial to trial, thus contributing to performance variability, we expected that variations would occur for two reasons. First, during the long interval that separated cue and target, maintenance of attention may lapse, and attentional biases at the cued location may consequently vary. Second, shifts of attention are voluntary (21) and depend on a decision whether to use the cue. It is known that choices among alternatives associated with a probabilistic outcome vary from trial to trial (22). Because the cue sometimes does not indicate the target location, the decision to use it should vary. Critically, both sources of variation are purely endogenous.
Several processes could generate BOLD responses that are predictive of performance, such as alertness, effort, motivation, and spatial attention. To determine the nature of predictive signals, we measured BOLD on trials where the cue correctly (valid trials) or incorrectly (invalid trials) specified the subsequent target location. If BOLD amplitude reflects the use of the cue, which improves performance when it is valid but hinders performance when it is invalid, then larger BOLD signals should predict correct responses on valid trials but incorrect responses on invalid trials. In contrast, for signals unrelated to spatial attention, the difference between correct and incorrect trials should not depend on cue validity.
The relation between average BOLD signals and behavior does not answer how accurately BOLD can track the fluctuation of processes on a trial-by-trial basis, because it may capture only a small fraction of the neural activity that determines performance variability. A critical aspect of this work was to quantify the proportion of behavioral responses accounted for by trial-to-trial variations in the BOLD signal. We present data that indicate that BOLD is sensitive enough to account for performance variability.
Materials and Methods
Four subjects took part in the experiment. Each subject was scanned in seven to eight separate 3-h sessions and completed between 814 and 990 trials. Written consent was obtained before each behavioral and imaging session. The experimental protocol was approved by the Washington University Human Studies Review Board.
Experimental Stimuli and Design. Stimuli were generated with an Apple G4 by using graphic routines for matlab (23, 24) and were presented on a translucent screen by a Sharp liquid crystal display (LCD) projector (800 × 600-pixel resolution). The subjects viewed the screen by means of a mirror placed above the head coil.
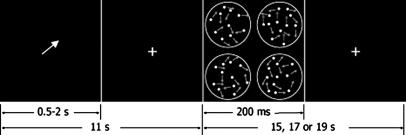
At the onset of each trial (Fig. 1), an arrow was presented at the center of the screen for 2.165 (subject 1) or 0.5 s (subjects 2, 3, and 4), which was followed by a fixation cross. Four random-dot kinematograms (RDKs) were presented for 200 ms, 4, 8, or 11 s after cue onset. Only data from trials with the longest delay are reported. Each RDK contained 100 dots in positive contrast placed within a circular window of 3.5° in diameter, centered in one visual quadrant at an eccentricity of 5.7°. Dot lifetime was two frames. Frame refresh rate was 72 Hz. In one of the RDKs, a fixed proportion of the dots was displaced coherently in a given direction (left, right, up, or down) at a speed of 6.5°/s. Subjects indicated the direction of motion by pressing one of four keys. The percentage of dots moving coherently was determined before scanning in multiple behavioral sessions by using a QUEST procedure (25). The cue indicated the visual quadrant containing the coherent motion on 80% of the trials (valid trials). On the remaining 20% (invalid trials), the coherent motion occurred in one of the uncued locations. A new trial started 15, 17, or 19 s after the onset of the previous target, allowing the target-evoked BOLD response to return to baseline.
Fig. 1.
Motion discrimination task, valid trial. The circles, which were not visible in the display, show the areas occupied by the RDKs. Each arrow within the circles indicates the direction of motion of a dot.
Eye position was monitored with an ASL 504 (Applied Science Laboratories, Bedford, MA) eye tracker. Acceptable recordings were obtained on 30% of the trials in subject 1 and in 32% of the trials in subject 4. Accurate fixation (i.e., eye deviation <1.5°) occurred in >99% of the trials. In the other two subjects, the quality of the recordings did not allow offline analysis of the eye position traces. Eye position was nevertheless monitored online. Fixation was encouraged regularly between scans, and subjects were unaware of interruptions in recordings.
MRI. Data were collected on a Siemens (Iselin, NJ) 1.5-Tesla vision system. Anatomical images were acquired by using a magnetization prepared rapid gradient echo (MP-RAGE) sequence [repetition time (TR) = 97 ms, echo time (TE) = 4 ms, flip angle = 12°, inversion time T1 = 300 ms]. Functional scans consisted of 132 frames acquired with an asymmetric spin-echo, echo-planar sequence sensitive to BOLD contrast over the whole brain (TR = 2.165 s, TE = 37 ms, flip angle = 90°, 16 contiguous 8-mm axial slices, 3.75 × 3.75-mm in-plane resolution). Images were realigned within and across runs, and across sessions, to correct for head motion by using six degrees of freedom, rigid-body realignment.
Analysis. Voxel-wise BOLD time courses were computed by fitting the BOLD time series with a general linear model containing a set of delta functions, one for each frame of the average BOLD response and nuisance variables, which included a constant, a linear term, and low-frequency sinusoids. The average BOLD response for each trial type was estimated over 14 MR frames. When the intertrial interval was <8 frames (i.e., 15 or 17 s), estimates of the last 1 or 2 frames were subtracted from the first frames of the next trial. This procedure allowed us to discount the effects of the trailing edge of the target-evoked response on the cue-evoked response of the subsequent trial. Residual images, obtained after subtracting the nuisance parameters, were treated as modulations of the BOLD signal due to changes in neural activity. A randomized, unbalanced design, voxel-wise ANOVA was used to identify regions in which the BOLD signal showed significant modulations by task factors (e.g., validity and performance).
Conjunction maps were obtained in the following way: individual statistical z maps were smoothed with a 12-mm-diameter sphere and thresholded at z ≥ 2 (P < 0.046) for the interaction and z ≥ 3 (P < 0.0027) for the main effect. Voxels in the conjunction map were those above the threshold in at least three subjects.
The relation between cue-period BOLD signals and performance was studied on a trial-by-trial basis by computing the predictive waveform over the first six MR frames. The template was estimated by using three methods. First, the predictive waveform was estimated as the difference of the average BOLD time courses preceding correct and incorrect responses. Second, the predictive component was estimated as a set of weights, β, by fitting a multivariate logistic regression (MLR) model:
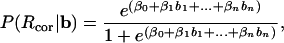 |
[1] |
where P(Rcor) is the probability of a correct response and b is the BOLD signal over n frames (here 6) counting all trials. The difference of the log-likelihood functions (the λ-statistic) for the complete model (βi, 0 ≤ i ≤ 6) vs. one containing only β0 was used to ascertain the significance of the multivariate logistic regression. This statistic has a χ2 distribution with n degrees of freedom (26). Finally, the predictive component was obtained by searching in β space for a set of weights, which maximized the area under the receiver operator characteristic (ROC, see below) curve. The search was implemented by using an unconstrained optimization algorithm (27). Each of the three methods yielded a waveform template that was used to predict the subject's accuracy trial by trial. The predictive value, α, was computed as the inner product of the BOLD signal and the template. Then, the two conditional probabilities, P(α ≥ crit|Rcor) and P(α ≥ crit|Rinc) were systematically evaluated as function of crit to obtain an ROC curve. To obtain an unbiased estimate of the predictive value of each of the templates, a repeated splitting procedure was implemented. Half of the data was used to estimate the templates whereas the other half was used to compute the αs. This procedure was repeated 100 times and yielded boot-strapped estimates of the area under the characteristic curve (AUC).
Results
Accuracy was higher on valid than invalid trials, indicating that subjects used the spatial information provided by the cue (see Table 1, which is published as supporting information on the PNAS web site).
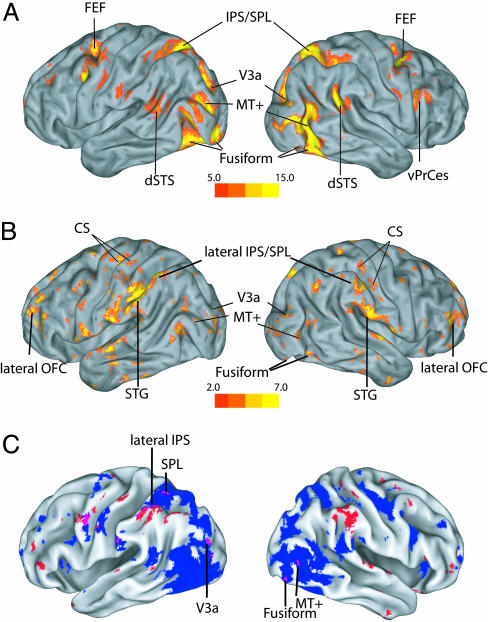
A random-factor ANOVA was used to identify regions active between cue and target presentation (i.e., the first six MR frames). Fig. 2A displays regions of significant BOLD modulation after the cue in subject 1. The map includes regions, identified on the basis of anatomical landmarks (28-31) as follows: frontal [frontal eye field (FEF) and ventral precentral sulcus (vPrCes)], parietal [intra-parietal sulcus (IPS)/superior parietal lobule (SPL)], and temporal [dorsal superior temporal sulcus (dSTS) and occipital cortex (V3a, MT+, fusiform)]; the map is similar to maps reported in group studies of spatial cueing (32). The effect of performance and cue validity on the BOLD signal was assessed by using the validity-by-performance interaction. Fig. 2B shows this statistic mapped onto the subject's brain. Regions whose relation with performance was modulated by cue validity were found within the lateral orbito-frontal cortex (OFC), the central sulcus (CS), the superior temporal gyrus (STG), the lateral IPS and inferior parietal lobule (IPL), V3a, MT+, and fusiform. Fig. 2C shows the group conjunction maps of performance-related BOLD signals modulated by cue validity (red) and of the overall BOLD signal (blue). In a few visual extra-striate and posterior parietal regions, BOLD activity showed both an overall modulation and a correlation with subsequent discrimination performance that depended on cue validity. Conversely, several performance-related regions did not show a systematic modulation of the BOLD response during the cue period, such as OFC and CS. To quantify the degree of anatomical congruency between overall and performance-related signals, we computed the voxel-wise correlation between their z scores. We found that, on average, there was a small negative correlation, which in individual subjects was small, not significant or negative (see Table 2, which is published as supporting information on the PNAS web site), indicating that the overlap between overall and performance-related signals is no greater than expected by chance.
Fig. 2.
Individual and group statistical maps. (A) Map of significant BOLD modulation between cue onset and target onset for subject 1. (B) Map of validity by performance interaction. Performance-related signals that depended on cue validity were observed in the lateral OFC (59), along the CS, STG, the lateral IPS/IPL, V3a, MT+, and fusiform. (C) Conjunction maps of the main effect of time (in blue) and the validity and performance interaction (in red) are superimposed on a volumetric rendition of a standard brain. The highlighted voxels have a z score higher than 3.0, for the main effect, or 2.0 for the interaction, in at least three of the four subjects. The respective uncorrected P values are 10-7 and 0.0005. Overlap between overall and performance-related BOLD that modulate with cue validity is mostly observed in occipital (right fusiform, right MT+, and left V3a) and dorsal parietal regions (left SPL and left lateral IPS/IPL), whereas anatomical segregation was observed in frontal regions.
Fig. 6, which is published as supporting information on the PNAS web site, shows the conjunction map of the validity and performance interaction. Performance-related signals that were modulated by cue validity were found also in anterior and posterior cingulate gyrus.
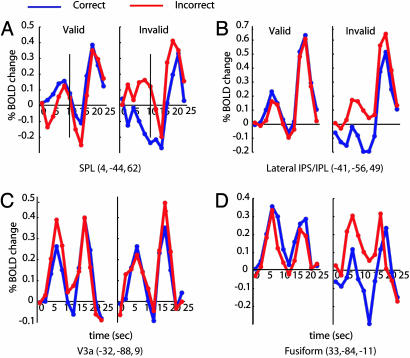
Figs. 3 and 4 show average time courses obtained from the highlighted voxels, which demonstrate differences in the BOLD response preceding correct and incorrect discriminations that depended on cue validity. For all but one region, the BOLD response preceding the stimulus was greater on correct than incorrect trials when the cue was valid. When the cue was invalid, the opposite pattern was observed. The reversal of the BOLD-performance relation with validity indicates that predictive activity was related specifically to the use of the cue and, by extension, spatial attention.
Fig. 3.
BOLD time courses within visual and attention-related regions from the validity by performance group conjunction map. BOLD response for correct (blue) and incorrect discriminations (red) are shown for valid (left column) and invalid trials (right column). The abscissa is the time from the onset of the cue. The vertical line on the first time course marks the time of the onset of the target.
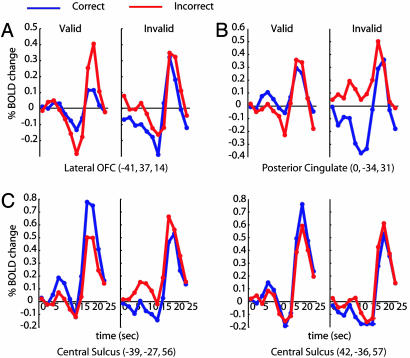
Fig. 4.
BOLD time courses in regions outside the attention network that showed performance-related activity that depended on cue validity. The figure format is identical to that of Fig. 3.
Different regions had idiosyncratic BOLD waveforms. SPL (Fig. 3A) showed sustained BOLD signal during the cue period. Previous studies reported sustained cue BOLD responses in dorsal parietal regions, suggesting that they are involved in maintaining attention at the cued location (33-35). Lateral IPS/IPL (see data for left IPS/IPL in Fig. 3B) showed much larger performance-related modulations for invalid than valid trials, replicating a pattern that to a lesser degree was found across most performance-related regions. Three regions in visual extra-striate cortex, MT+ (time courses in Fig. 5), V3a, and fusiform, showed differential BOLD signals preceding correct and incorrect discriminations that depended on cue validity. Fusiform and V3a (Fig. 3 C and D, respectively) showed large cue responses, probably reflecting the sensory effect of the cue. The finding of performance-related activity preceding motion discrimination in the fusiform is consistent with previous studies, which have found signals in this area related to working memory for motion direction (36) and to motion-direction discrimination (37). V3a was unique in showing greater cue responses preceding incorrect than correct discrimination on valid trials, with no reliable difference on invalid trials. Single-unit data obtained in a memory saccade task from area V3a of nonhuman primates (38) showed a suppression of firing rate below baseline during the delay period in most units, together with greater responses to a saccadic target than a non-target. This finding and the transient nature of the cue-evoked BOLD response may indicate that V3a is involved in target selection but not maintenance of spatial information and suggest that performance-related signal in this region reflected suppression during the cue period.
Fig. 5.
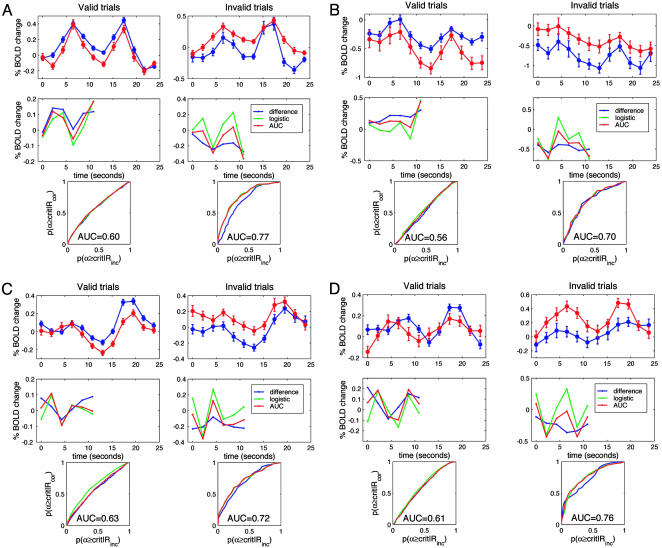
Predictive signals in MT+. Average BOLD time courses on valid and invalid trials, predictive BOLD components, and ROCs are shown from MT+ of all subjects. For each subject, the Top panel shows the average BOLD response for correct (blue) and incorrect (red) discriminations on valid (Left column) and invalid (Right column) trials. The abscissa is the time from cue onset. The error bars are standard errors. (Middle) The predictive component computed according to three different methods. The blue line is the difference of the BOLD signals for correct and incorrect discriminations; the red line is the BOLD component whose magnitude, trial-by-trial, best discriminates correct from incorrect trials; the green line is the BOLD component estimated by the multivariate logistic regression. (Bottom) ROC curves. The abscissa and the ordinate are the probability that the predictive component is greater than a criterion when the subject is incorrect and correct, respectively. The diagonal from the left lower to the right upper corner represent chance predictability. The area under the characteristic curve (AUC) represents the strength of the predictive signal.
Several regions outside the attentional network (32) showed performance-related signals that were modulated by cue validity. Lateral OFC (Fig. 4A) showed deactivations preceding the stimulus, which nevertheless differentiated between correct and incorrect discriminations. Previous studies suggest that lateral OFC is involved in error detection and processes related to reward history that affect subsequent behavior (39, 40). Consistently with this hypothesis, larger target BOLD activity was found after incorrect than correct discriminations. Posterior cingulate (Fig. 4B) involvement in cue utilization is consistent with previous suggestions that it acts as an interface between high-level reward signals and the attentional system (41). Sensory-motor cortices (Fig. 4C) also showed performance-related modulations during the cue period that depended on cue validity, which suggest that the evaluation of the sensory information, which is affected by the cue, is undertaken throughout the brain. According to this speculative, but not novel, viewpoint (42), motor cortex is not a passive recipient of a decision elaborated somewhere else. This finding is consistent with the role of premotor structures in representing the perceptual decision variable (e.g., refs. 11 and 43).
We next quantified how well the BOLD signal predicted performance. Fig. 5 shows predictive signals in MT+ and the accuracy of the prediction for each subject (see Fig. 7, which is published as supporting information on the PNAS web site, for predictive analysis in lateral IPS/IPL). The Top row of each panel shows the average BOLD responses on valid and invalid trials. The Middle row shows the predictive components of the BOLD response, computed according to three different criteria (see Materials and Methods). Similar predictive components were obtained with the three methods, suggesting robust estimates, although the predictive waveform obtained from the difference of correct and incorrect average time courses (blue line) was flatter than the other two.
The predictive components for valid and invalid trials were similar in shape but reversed in sign, indicating that the same processes that improve performance on valid trials hamper performance on invalid trials. Two other aspects of the shape of the predictive component are worth emphasizing. First, in three subjects, differences between BOLD responses on correct and incorrect trials seemed already present at the onset of the cue (Fig. 5 B-D), suggesting that cue utilization may depend on processes that precede the cue. Secondly, the average time course of the BOLD response was seldom identical to the time course of the predictive component. For example, in subject 1 (Fig. 5A), the average BOLD response showed a unimodal response with a peak on the fourth frame, whereas the predictive component showed a bimodal modulation. This result indicates that the BOLD response reflects multiple processes, but only some vary from trial to trial and hence predict performance.
An ROC analysis was used to quantify the degree to which preparatory signals predicted accuracy. As shown in the Bottom panels of Fig. 5, the area under the characteristic curve (AUC) was 0.6-0.75, indicating that, at least on 60-75% of the trials, performance could be predicted on the basis of BOLD. Performance probabilities (i.e., the probability of predicting whether the subject will be correct or incorrect) estimated from signals that precede the decision compare favorably with choice probabilities (i.e., the probability of correctly predicting the actual response) estimated from single-unit activity recorded at decision time (9, 11, 44). Although the upper bound for choice probability is 1.0, the upper bound for performance probability is <1.0. Because the BOLD response on trials in which subjects guessed should not differ for correct and incorrect responses, a certain proportion of correct trials will be indistinguishable from incorrect trials.
Several regions showed a significant effect of performance and lack of interaction with cue validity, suggesting that these signals were not related to spatial attention. Interestingly, the cue BOLD signal was mostly greater on incorrect than correct trials, indicating that activity in these regions interfered with performance. Fig. 8, which is published as supporting information on the PNAS web site, shows average time courses from a left medial orbito-frontal region that demonstrates this activity pattern.
Discussion
Accuracy was predicted, trial-by-trial, by the BOLD signal that preceded the stimulus and the subjects' decision. Predictive activity was widespread but overlapped only a few regions that showed overall modulations of the BOLD response (see Fig. 2), including motion and shape selective extra-striate areas such as MT+, V3a, and fusiform gyrus, and dorsal parietal areas such as SPL and lateral IPS/IPL, thought to be involved in the control of spatial attention. Moreover, predictive signals were also found in several regions that did not demonstrate overall modulation of the BOLD signal during the cue period, such as lateral OFC, CS, and STG. In both sets of regions, larger BOLD activity was mostly associated with higher accuracy on valid trials and lower accuracy on invalid trials, indicating that the predictive activity was related to the use of the cue. These findings indicate that performance signals related to cue utilization were generated beyond the attentional network (32), for example in regions involved in regulating behavior based on reward. Finally, we found that, at least on 60-75% of the trials, the subject's accuracy could be forecast on the basis of the preceding BOLD signal.
Significance of Endogenous Sources of Performance Variability. This experiment was designed to study purely endogenous sources of performance variability. First, we found BOLD signals that preceded the stimulus and the subject's response and were predictive of accuracy. Second, because the cue was a simple, highly visible foveal stimulus, predictive signals could not reflect variability in cue detection or identification. Rather, predictive signals reflected endogenous processes unrelated to the sensory quality of the cue. Third, because the cue indicated the likely location of the target and not its direction of motion, these predictive signals affected perceptual rather than response processes. Fourth, because the BOLD-accuracy relationship depended on the validity of the cue, predictive activity was specifically linked to spatial processes rather than arousal, effort, or perceptual difficulty.
The presence of a substantial endogenous predictive activity is inconsistent with the view that performance variability reflects only noise in the image luminance pattern, sensory processes, and limited capacity to analyze sensory information (45-47). Previous psychophysical work on the factors limiting motion detection indicates that stimulus and sensory factors cannot account for >30% of performance variability (48). Our results suggest that a large proportion of performance variability depends on variations in spatial attention and other endogenous signals that precede the stimulus. This conclusion rests on quantitative estimates of trial-by-trial predictability (i.e., ROC curves) rather than methods that compare only mean activity across correct and incorrect trials.
The presence of endogenous predictive signals is the strongest evidence that can be obtained by using correlational methods, for a causal relation between neural activity and behavior. An alternative explanation that predictive signals are a consequence of behavior is unlikely, because the behavior (motion discrimination) had yet to take place at the time these signals were recorded. However, because our inferences are based on correlational observations, it is possible that the correlation is accounted for by unobserved factors that affect both BOLD and behavior.
Cue-related performance probabilities were as large as the choice probabilities estimated from single-units data obtained at the time of stimulus presentation (44, 49). Similar values for choice probability using fMRI have been recently found during a perceptual decision involving facial expressions (17). One explanation for this surprising degree of reliability is that, whereas the BOLD signal is degraded by a number of noise sources (50), it represents the cumulative effects of synaptic activity of large populations of afferent and intra-cortical fibers (51, 52), similar to local field potentials, which have been shown to be more accurate than single-unit spike rates in predicting aspects of behavior (53).
The Nature of Endogenous Sources of Performance Variability. We separated predictive activity related to the use of a spatial cue from predictive activity reflecting other processes that also vary from trial to trial. The dependence of the BOLD-behavior relationship on cue validity clearly demonstrates that the predictive signals index cue utilization and introduces a direct method for establishing the nature of performance-related signals. This interaction is important, because other experiments have relied on the anatomical colocalization (14, 15) or the temporal coincidence of predictive activity with specific psychological processes (18) to infer its nature. We found that, although the topographical distribution of mean cue-related activity was identical to that associated with spatial attention in other experiments (32, 54, 55), only a few of those regions actually contained activity that predicted performance. Furthermore, even in areas that showed predictive activity, only some of the temporal components of the BOLD response were predictive. We also found predictive signals time-locked to cue onset but did not depend on cue validity and therefore cannot be related to spatial attention. Interestingly, these signals were generally greater on incorrect than correct trials. A speculative interpretation is that these signals reflect task-irrelevant processes and that their suppression may improve performance by freeing resources for task-relevant processes.
Although predictive signals that depend on cue validity must reflect the use of the cued information, we note two interpretations. First, these signals may reflect maintenance of attention at the cued location. Single-unit data in parietal cortex have shown that cue-evoked neural activity across units with local receptive fields correlates with contrast sensitivity across the visual field (13), suggesting that they control the allocation of spatial attention. A similar view of the role of parietal cortex was proposed in fMRI studies, which reported sustained BOLD activity in frontal and parietal regions during the delay between a spatial cue and a target. It was suggested that sustained activity represented maintenance of attention at the cued location (34, 35). Our results partly support this hypothesis because some parietal regions with more sustained BOLD responses to the cue showed predictive signals that interacted with cue validity. Sustained, preparatory activity has been reported in visual cortex after spatial cues (16, 34, 56) and interpreted as a correlate of a top-down attentional bias at the cued location. However, it is unknown whether these signals reflect functionally significant activity. Because predictive signals were found in extra-striate areas with transient responses, such as fusiform and V3a, one should infer that sustained increases are not necessary for attentional effects on visual cortex. In our paradigm, which used a very long delay interval, not even parietal regions with sustained responses showed increased activity up to the time of the target-evoked response.
A second interpretation is that predictive signals reflect the subject's ongoing estimate of the cue validity, which determines the probability that the subject will pay attention to the cued location. For example, after a long sequence of invalid cues, subjects may expect a valid cue, increasing the probability of attending to the cued location. If expected cue validity is reflected in the amplitude of the BOLD response, then larger target BOLD responses will be associated with greater cue utilization and accuracy on valid trials and greater cue utilization but lower accuracy on invalid trials. According to this hypothesis, predictive signals are similar to expected “utility” or “reward” value signals (57, 58). The finding in lateral OFC of larger BOLD responses after incorrect than correct discriminations as well as performance-related signals preceding the target is consistent with the view that the estimation of cue validity depends on performance in previous trials. Also, the presence of performance-related signals in posterior cingulate is consistent with the view that cue utilization depends on an estimate of its behavioral value. Our results suggest that, even in a simple visual discrimination task, performance is continuously monitored to update internal estimates of regularities in the environment and that the mechanisms that are involved in appetitive behavior may contribute to the evaluation of internal representations of the external world.
Supplementary Material
Acknowledgments
We thank F. Miezin and A. Snyder for technical support. We also thank M. C. Morrone, D. Burr, and A. Snyder for useful comments on an early draft of this manuscript. This work was supported by National Institutes of Health Grant MH 71920-06 and by the J. S. McDonnell Foundation (to M.C.).
Author contributions: A.S., G.dA., G.L.S., and M.C. designed research; A.S. performed research; A.S. and G.dA. analyzed data; G.dA. and M.M. contributed new reagents/analytic tools; and A.S., G.dA., G.L.S., and M.C. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: fMRI, functional MRI; BOLD, blood oxygen level-dependent; RDK, random-dot kinematogram; ROC, receiver operator characteristic; OFC, orbito-frontal cortex; CS, central sulcus; STG, superior temporal gyrus; IPS, intra-parietal sulcus; IPL, inferior parietal lobule; SPL, superior parietal lobule.
Data deposition: The neuroimaging data have been deposited with the fMRI Data Center, www.fmridc.org (accession no. 2-2005-120EE).
References
- 1.Moran, J. & Desimone, R. (1985) Science 229, 782-784. [DOI] [PubMed] [Google Scholar]
- 2.Corbetta, M., Miezin, F. M., Dobmeyer, S., Shulman, G. L. & Petersen, S. E. (1990) Science 248, 1556-1559. [DOI] [PubMed] [Google Scholar]
- 3.Zhang, M. & Barash, S. (2000) Nature 408, 971-975. [DOI] [PubMed] [Google Scholar]
- 4.Miller, E. K., Li, L. & Desimone, R. (1993) J. Neurosci. 13, 1460-1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kenet, T., Bibitchkov, D., Tsodyks, M., Grinvald, A. & Arieli, A. (2003) Nature 425, 954-956. [DOI] [PubMed] [Google Scholar]
- 6.Freeman, W. T. (1994) Nature 368, 542-545. [DOI] [PubMed] [Google Scholar]
- 7.Kersten, D., Mamassian, P. & Yuille, A. (2004) Annu. Rev. Psychol. 55, 271-304. [DOI] [PubMed] [Google Scholar]
- 8.Britten, K. H., Shadlen, M. N., Newsome, W. T. & Movshon, J. A. (1992) J. Neurosci. 12, 4745-4765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Britten, K. H., Newsome, W. T., Shadlen, M. N., Celebrini, S. & Movshon, J. A. (1996) Vis. Neurosci. 13, 87-100. [DOI] [PubMed] [Google Scholar]
- 10.Newsome, W. T., Britten, K. H. & Movshon, J. A. (1989) Nature 341, 52-54. [DOI] [PubMed] [Google Scholar]
- 11.Shadlen, M. N. & Newsome, W. T. (2001) J. Neurophysiol 86, 1916-1936. [DOI] [PubMed] [Google Scholar]
- 12.Cook, E. P. & Maunsell, J. H. (2002) Nat. Neurosci. 5, 985-994. [DOI] [PubMed] [Google Scholar]
- 13.Bisley, J. W. & Goldberg, M. E. (2003) Science 299, 81-86. [DOI] [PubMed] [Google Scholar]
- 14.Wagner, A. D., Schacter, D. L., Rotte, M., Koustaal, W., Maril, A., Dale, A. M., Rosen, B. R. & Buckner, R. L. (1998) Science 281, 1188-1191. [DOI] [PubMed] [Google Scholar]
- 15.Brewer, J. B., Zhao, Z., Desmond, J. E., Glover, G. H. & Gabrieli, J. D. (1998) Science 281, 1185-1187. [DOI] [PubMed] [Google Scholar]
- 16.Ress, D., Backus, B. T. & Heeger, D. J. (2000) Nat. Neurosci. 3, 940-945. [DOI] [PubMed] [Google Scholar]
- 17.Pessoa, L. & Padmala, S. (2005) Proc. Natl. Acad. Sci. USA 102, 5612-5617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pessoa, L., Gutierrez, E., Bandettini, P. & Ungerleider, L. (2002) Neuron 35, 975-987. [DOI] [PubMed] [Google Scholar]
- 19.Cook, E. P. & Maunsell, J. H. (2002) J. Neurosci. 22, 1994-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Posner, M. I., Snyder, C. R. R. & Davidson, B. J. (1980) J. Exp. Psychol. Gen. 109, 160-174. [PubMed] [Google Scholar]
- 21.Jonides, J. (1981) in Attention and Performance, eds. Posner, M. I. & Marin, O. (Lawrence Erlbaum Associates, Hillsdale, NJ), Vol. XI, pp. 187-205. [Google Scholar]
- 22.Herrnstein, R. J. (1961) J. Exp. Anal. Behav. 4, 267-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brainard, D. H. (1997) Spat. Vis. 10, 433-436. [PubMed] [Google Scholar]
- 24.Pelli, D. G. (1997) Spat. Vis. 10, 437-442. [PubMed] [Google Scholar]
- 25.Watson, A. B. & Pelli, D. G. (1983) Percept. Psychophys. 33, 113-120. [DOI] [PubMed] [Google Scholar]
- 26.Cox, D. R. & Snell, E. J. (1989) Analysis of Binary Data (Chapman & Hall, London).
- 27.Nelder, J. A. & Mead, R. (1965) Comput. J. 7, 308-315. [Google Scholar]
- 28.Corbetta, M., Akbudak, E., Conturo, T. E., Snyder, A. Z., Ollinger, J. M., Drury, H. A., Linenweber, M. R., Petersen, S. E., Raichle, M. E., Van Essen, D. C. & Shulman, G. L. (1998) Neuron 21, 761-773. [DOI] [PubMed] [Google Scholar]
- 29.Watson, J. D., Myers, R., Frackowiak, R. S., Hajnal, J. V., Woods, R. P., Mazziotta, J. C., Shipp, S. & Zeki, S. (1993) Cereb. Cortex 3, 79-94. [DOI] [PubMed] [Google Scholar]
- 30.Huk, A. C., Dougherty, R. F. & Heeger, D. J. (2002) J. Neurosci. 22, 7195-7205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tootell, R. B. H., Mendola, J. D., Hadjikhani, N. K., Ledden, P. J., Liu, A. K., Reppas, J. B., Sereno, M. I. & Dale, A. M. (1997) J. Neurosci. 15, 7060-7078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Corbetta, M. & Shulman, G. L. (2002) Nat. Rev. Neurosci. 3, 201-215. [DOI] [PubMed] [Google Scholar]
- 33.Shulman, G. L., Ollinger, J. M., Akbudak, E., Conturo, T. E., Snyder, A. Z., Petersen, S. E. & Corbetta, M. (1999) J. Neurosci. 19, 9480-9496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kastner, S., Pinsk, M. A., De Weerd, P., Desimone, R. & Ungerleider, L. G. (1999) Neuron 22, 751-761. [DOI] [PubMed] [Google Scholar]
- 35.Corbetta, M., Kincade, J. M., Ollinger, J. M., McAvoy, M. P. & Shulman, G. L. (2000) Nat. Neurosci. 3, 292-297. [DOI] [PubMed] [Google Scholar]
- 36.Ferrera, V. P., Rudolph, K. K. & Maunsell, J. H. (1994) J. Neurosci. 14, 6171-6186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cornette, L., Dupont, P., Rosier, A., Sunaert, S., Van Hecke, P., Michiels, J., Mortelmans, L. & Orban, G. A. (1998) J. Neurophysiol. 79, 2749-2765. [DOI] [PubMed] [Google Scholar]
- 38.Nakamura, K. & Colby, C. L. (2000) J. Neurophysiol. 84, 677-692. [DOI] [PubMed] [Google Scholar]
- 39.O'Doherty, J., Kringelbach, M. L., Rolls, E. T., Hornak, J. & Andrews, C. (2001) Nat. Neurosci. 4, 95-102. [DOI] [PubMed] [Google Scholar]
- 40.Kringelbach, M. L. & Rolls, E. T. (2004) Prog. Neurobiol. 72, 341-372. [DOI] [PubMed] [Google Scholar]
- 41.Small, D. M., Gitelman, D., Simmons, K., Bloise, S. M., Parrish, T. & Mesulam, M. M. (2005) Cereb. Cortex, in press. [DOI] [PubMed]
- 42.Knill, D. C. & Pouget, A. (2004) Trends Neurosci. 27, 712-719. [DOI] [PubMed] [Google Scholar]
- 43.Horwitz, G. D., Batista, A. P. & Newsome, W. T. (2004) J. Neurophysiol. 91, 2281-2296. [DOI] [PubMed] [Google Scholar]
- 44.Dodd, J. V., Krug, K., Cumming, B. G. & Parker, A. J. (2001) J. Neurosci. 21, 4809-4821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barlow, H. (1977) in Vertebrate Photoreception, eds. Barlow, H. B. & Fatt, P. (Academic, London), pp. 337-358.
- 46.Pelli, D. G. (1990) in Vision: Coding and Efficiency, ed. Blakemore, C. (Cambridge Univ. Press, Cambridge, U.K.), pp. 3-24.
- 47.Geisler, W. S. (1989) Psychol. Rev. 96, 267-314. [DOI] [PubMed] [Google Scholar]
- 48.Barlow, H. & Tripathy, S. P. (1997) J. Neurosci. 17, 7954-7966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shadlen, M., Britten, K. H., Newsome, W. T. & Movshon, J. A. (1996) J. Neurosci. 16, 1486-1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aguirre, G. K., Zarahn, E. & D'Esposito, M. (1998) NeuroImage 8, 360-369. [DOI] [PubMed] [Google Scholar]
- 51.Logothetis, N. K., Pauls, J., Augath, M., Trinath, T. & Oeltermann, A. (2001) Nature 412, 150-157. [DOI] [PubMed] [Google Scholar]
- 52.Lauritzen, M. & Gold, L. (2003) J. Neurosci. 23, 3972-3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pesaran, B., Pezaris, J. S., Sahani, M., Mitra, P. P. & Andersen, R. A. (2002) Nat. Neurosci. 5, 805-811. [DOI] [PubMed] [Google Scholar]
- 54.Kastner, S. & Ungerleider, L. G. (2001) Neuropsychologia 39, 1263-1276. [DOI] [PubMed] [Google Scholar]
- 55.Kanwisher, N. & Wojciulik, E. (2000) Nat. Rev. Neurosci. 1, 91-100. [DOI] [PubMed] [Google Scholar]
- 56.Hopfinger, J. B., Buonocore, M. H. & Mangun, G. R. (2000) Nat. Neurosci. 3, 284-291. [DOI] [PubMed] [Google Scholar]
- 57.Platt, M. L. & Glimcher, P. W. (1999) Nature 400, 233-238. [DOI] [PubMed] [Google Scholar]
- 58.Sugrue, L. P., Corrado, G. S. & Newsome, W. T. (2004) Science 304, 1782-1787. [DOI] [PubMed] [Google Scholar]
- 59.Dumoulin, S. O., Bittar, R. G., Kabani, N. J., Baker, C. L., Jr., Le Goualher, G., Bruce Pike, G. & Evans, A. C. (2000) Cereb. Cortex 10, 454-463. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.