Abstract
The gene encoding Babesia bovis rhoptry-associated protein 1 (RAP-1) was used to develop an enzyme-linked immunosorbent assay (ELISA) to measure specific antibodies against B. bovis. The B. bovis RAP-1 gene was subcloned into a baculovirus transfer vector, and the RAP-1 protein was expressed in insect cells infected with a recombinant baculovirus. The recombinant B. bovis RAP-1 of 65 kDa was detected with anti-RAP-1 mouse serum by Western blotting, and this recombinant RAP-1 was used as an antigen in the ELISA. The ELISA was able to differentiate between B. bovis-infected sera and B. bigemina-infected sera or noninfected normal bovine sera. The results demonstrate that the recombinant RAP-1 expressed in insect cells might be a useful antigen for the detection of antibodies to B. bovis.
Babesia bovis is a tick-borne hemoprotozoan parasite of cattle causing babesiosis, a disease of economic importance to the livestock industry in subtropical and tropical regions of the world since at least several million cattle globally are at risk (17). Hence, it is essential to be able to ascertain the serological status of cattle with regard to B. bovis and have a rapid, inexpensive, and reliable test for the detection of the anti-B. bovis-specific antibody. Such a test would have great benefits in large-scale epidemiological surveys and lead to eradication. Until now, many serological tests have been used for the diagnosis of Babesia infection in cattle, such as the indirect immunofluorescent-antibody test (IFAT) and the enzyme-linked immunosorbent assay (ELISA) (3, 26). IFAT has been widely used for the detection of the anti-B. bovis antibody; however, besides not being particularly sensitive, IFAT is unsuitable for use with a large number of serum samples. Furthermore, the results of IFAT may be influenced by the subjective judgment of the operator (3, 30). In contrast, ELISA is quite sensitive and may be easily used to test large numbers of samples (3, 26). ELISA has previously been evaluated for the detection of antibodies to B. bovis by use of a native antigen. Its potential ability has been demonstrated to be a powerful tool for serological surveys (2, 8, 16, 27), but the poor quality of antigens and the cross-reaction with B. bigemina have impeded its application (3, 9, 26). Recently, an ELISA based on a recombinant antigen has been significantly developed (2, 10, 31) because it offers two major advantages: there is a negligible batch-to-batch variation in the antigen and there is no need to kill experimental animals for preparation of the native antigen (2).
The B. bovis rhoptry-associated protein 1 (RAP-1) gene encoding a 60-kDa merozoite apical membrane polypeptide was identified by Suraez et al. (23). The function of RAP-1 is poorly understood, but it is believed that rhoptry proteins play an important role in host cell invasion (21, 22). The major immunogenic B-cell and T-cell epitopes on RAP-1 are conserved among all strains tested, but they are not conserved between different species (5, 24). The lack of extensive differences in RAP-1 among geographically distinct isolates of B. bovis suggests that RAP-1 should be considered a candidate antigen in the development of a diagnostic reagent and subunit vaccine (4, 7, 19). In this study, the gene encoding B. bovis RAP-1 was expressed in insect cells by using a baculovirus expression system. Then, the ELISA based on the recombinant antigen was developed, and its potential use for the detection of antibodies to B. bovis in cattle was evaluated.
MATERIALS AND METHODS
Parasites.
B. bovis strain Texas was continuously cultured with bovine erythrocytes by using a microaerophilous stationary-phase culturing system (15). When the level of parasitemia reached 5 to 10%, the infected erythrocytes were washed three times with phosphate-buffered saline (PBS), and the pellets were stored at −80°C.
Cloning of RAP-1 gene.
B. bovis-infected erythrocyte pellets were suspended in a DNA extraction buffer (100 mM Tris-HCl [pH 8.0], 1% sodium dodecyl sulfate [SDS], 0.1 M NaCl, 10 mM EDTA) and digested with proteinase K (100 μg/ml) for 2 h at 55°C. The genomic DNA was then extracted with phenol-chloroform and precipitated with ethanol. The DNA pellets were suspended in TE buffer (10 mM Tris-HCl [pH 8.0], 1 mM EDTA) and used as a DNA template for a PCR. The entire RAP-1 gene was amplified by PCR with two primers, 5′-ACGGATCCGACAATGAGAATCATT-3′ and 5′-ACGGATCCAAACGCATCTCATCAG-3′, both of which contained a BamHI site at the 5′ end (23). The PCR was performed in 100 μ1 of a reaction mixture containing 100 pmol of each primer, 0.5 μg of template DNA, 20 μM of a mixture of deoxynucleoside triphosphates, 10 μl of a 10× buffer, and 2.5 U of Taq Gold polymerase (Perkin-Elmer, Foster City, Calif.). The PCR amplification was carried out for 30 cycles under the following conditions. Each cycle consisted of 1 min at 95°C for denaturation (10 min for the first cycle), 1 min at 55°C for annealing, and 2 min at 73°C for extension. After the PCR was completed, the amplified DNA products were digested with BamHI. The DNA fragment containing the RAP-1 gene was gel purified by using a MinElute gel extraction kit (Qiagen Inc., Valencia, Calif.) and ligated into the BamHI site of a pBluescript SK(+) cloning vector. The resulting plasmid was designated pBS/RAP-1. DNA sequencing of the RAP-1 gene was performed by using an ABI PRISM 377 DNA sequencer (Perkin-Elmer) with a dye primer cycle sequencing ready-reaction kit (Perkin-Elmer).
Preparation of anti-RAP-1 mouse serum.
The RAP-1 gene fragment was recovered from pBS/RAP-1 after the digestion with BamHI and inserted into the BamHI site of the Escherichia coli expression vector pGEMEX-2 (Promega Corp., Madison, Wis.). The vector was designated pGEMEX/RAP-1 and was used to express the RAP-1 polypeptide as a fusion protein with the bacteriophage T7 gene 10 leader peptide in E. coli. Eight-week-old female BALB/c mice were intraperitoneally immunized with 10 μg of the RAP-1 fusion protein in complete Freund's adjuvant. On days 14 and 28, the mice were immunized with the same antigen in incomplete Freund's adjuvant by intraperitoneal injection. Sera from the immunized mice were collected 10 days after the final immunization.
Construction of recombinant baculovirus.
The RAP-1 gene fragment from pBS/RAP-1 was inserted into the BamHI site of Bac-to-Bac donor plasmid pFastBac Ht (Life Technologies, Grand Island, N.Y.). The recombinant donor plasmid, pFB/RAP-1-Ht, was transformed into DH10Bac competent cells (Life Technologies). The resultant transposed bacmid containing the RAP-1 gene was used to cotransfect insect (Spodoptera frugiperda) cells (Sf9 cells) with baculovirus DNA by using liposome reagent (Cellfectin; Life Technologies). After 3 days of incubation at 27°C, the culture supernatant containing recombinant viruses expressing the RAP-1 gene, AcRAP-1-Ht, was collected and used to transfect High five insect cells. The technical methods were in agreement with the instruction manual of the Bac-to-Bac baculovirus expression system (Life Technologies). The expression of the RAP-1 gene was confirmed by IFAT and Western blotting analysis with anti-RAP-1 mouse serum or B. bovis- or B. bigemina-infected bovine serum.
IFAT.
IFAT was performed as described by Avarazed et al. (1).
SDS-polyacrylamide gel electrophoresis (PAGE) and Western blotting analysis.
After 3 days of incubation, High five insect cells infected with recombinant baculovirus were harvested and centrifuged. The cell pellets were suspended in PBS, sonicated, and mixed 1:1 with an SDS sample buffer (62.5 mM Tris-HCl [pH 6.8], 2% SDS, 5% β-mercaptoethanol, 10% glycerol, 0.02% bromophenol blue). The samples were boiled for 5 min prior to electrophoresis in an SDS-10% polyacrylamide gel, and the gel was then subjected to a Coomassie blue staining or Western blotting analysis after electrophoresis. For Western blotting analysis, the proteins were transferred to nitrocellulose membranes (Immobilon transfer membrane; Millipore) with a semidry blotting apparatus. The membrane was incubated in a blocking solution (3% skim milk in PBS) for 1 h at room temperature and then with bovine or mouse serum for 1 h. The membranes were washed three times with 0.05% Tween 20 in PBS (PBST) and incubated with horseradish peroxidase-labeled goat anti-mouse or anti-bovine immunoglobulin G (ICN Biomedicals, Inc., Aurora, Ohio) for 1 h. The membrane was washed three times with PBST and placed into a substrate solution containing 0.5 mg of diabinobenzidine per ml and 0.005% H2O2 to visualize the antigen-antibody complexes.
ELISA.
High five insect cells infected with AcRAP-1-Ht were washed with PBS and lysed in a lysis buffer (40 mM Tris-HCl [pH 7.5], 150 mM NaCl, 5 mM β-mercaptoethanol, 20% glycerol, 0.1% Triton X-100, 1 μg each of pepstatin A and lenpeptine per ml). The mixture was placed on ice for 5 min and centrifuged at 1,500 × g for 20 min at 4°C. The supernatant was centrifuged again at 18,000 × g for 30 min and diluted with a coating buffer (50 mM carbonate-bicarbonate buffer [pH 9.6]) as an ELISA antigen to a final concentration of 10 μg/ml. Each well of 96-well plates (Nalge Nunc International, Roskilde, Denmark) was coated with 50 μ1 of antigen overnight at 4°C. On the following day, the plates were washed once with PBST and incubated with 100 μ1 of a blocking solution (3% skim milk in PBS) for 1 h at 37°C. After one wash with PBST, 50 μ1 of an individual test serum sample diluted to 1:200 with the blocking solution was added to each well and the plate was incubated for 1 h at 37°C. The plates were washed six times with PBST and then incubated for 1 h at 37°C with 50 μ1 of horseradish peroxidase conjugate (ICN Biomedicals) that had been diluted to 1:4,000 with the blocking solution. The plates were washed as described above, and then 50 μ1 of a substrate solution [0.1 M citric acid, 0.2 M sodium phosphate, 0.003% H2O2, 0.3 mg of 2,2′-azide-bis(3-ethylbenzthiazoline-6-sulfonic acid) (Sigma Chemical) per ml] was added to each well. After 1 h of incubation at room temperature the optical density (OD) was measured at a wavelength of 415 nm.
Sera.
Serum samples from cattle experimentally infected with B. bovis or B. bigemina and negative serum samples from healthy cattle were kindly provided by individuals from Washington State University (Pullman, Wash.) and Texas A&M University (College Station, Tex.). Field serum samples from 201 cattle in Brazil and 283 cattle in Mongolia were also examined.
RESULTS
Cloning of RAP-1 gene from B. bovis.
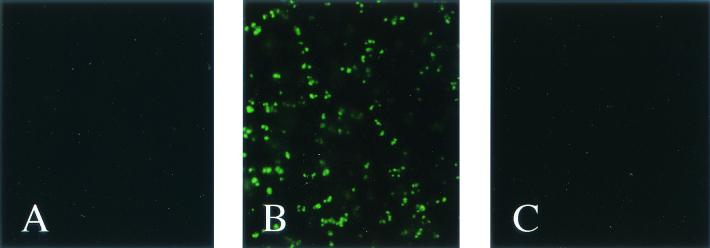
The RAP-1 gene of B. bovis was amplified from the Texas strain by the PCR method. As a result, a 1,695-bp DNA fragment was amplified from the B. bovis genomic DNA and inserted into pBluescript SK(+). The identify of the PCR product was proved by sequencing. The DNA sequence data for the fragment were compared with the original RAP-1 sequence data (GenBank accession no. AF027149), and there was no variation in the nucleotide sequence. The RAP-1 gene was expressed as a gene 10 fusion protein in E. coli, and anti-RAP-1 mouse serum was prepared from the immunized mice with the recombinant gene 10 fusion protein. The antiserum reacted with B. bovis but not with either B. bigemina or normal bovine erythrocytes (Fig. 1).
FIG. 1.
IFAT analysis of mouse anti-B. bovis RAP-1 antibody. Noninfected bovine erythrocytes (A) B. bovis-infected bovine erythrocytes (B), and B. bigemina-infected bovine erythrocytes (C) were reacted with mouse anti-B. bovis RAP-1 antibody.
Construction of recombinant baculovirus.
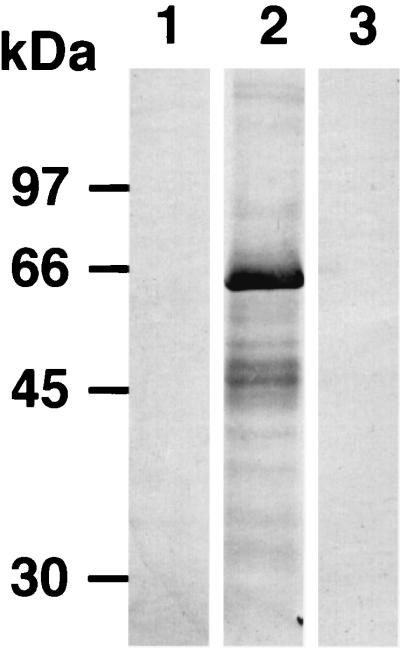
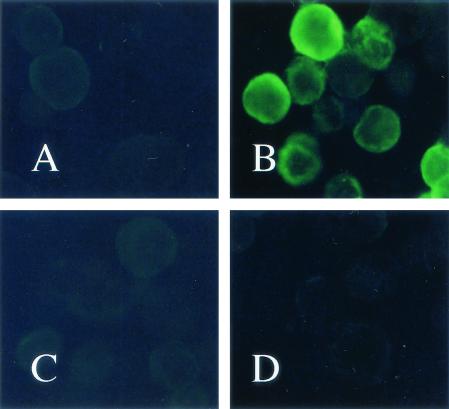
To obtain sufficient amounts of the soluble RAP-1 antigen for ELISA, we constructed a recombinant baculovirus capable of expressing the B. bovis RAP-1 gene. High five insect cells were infected with recombinant baculovirus AcRAP-1-Ht. After incubation for 3 days, the entire cell extract was analyzed by SDS-PAGE (10% polyacrylamide). A major polypeptide band with a molecular mass of 65 kDa was identified (data not shown). The molecular mass of the recombinant protein was larger than that of the native RAP-1 protein due to the six-histidine affinity tag (data not shown). A 65-kDa polypeptide was overproduced by the AcRAP-1-Ht-infected cells, and the protein expressed was identified as the B. bovis RAP-1 gene product by its reaction with anti-RAP-1 mouse serum by Western blotting analysis (Fig. 2). In contrast, no band was detected from noninfected cell extracts. The AcRAP-1-Ht-infected cells were also examined by IFAT with serum experimentally infected with B. bovis or B. bigemina. Specific fluorescence was observed in AcRAP-1-Ht-infected cells with B. bovis-infected bovine serum but not in mock-infected cells (Fig. 3).
FIG. 2.
Western blot analysis of B. bovis RAP-1 expressed in High five insect cells by using mouse anti-B. bovis RAP-1 antibody or bovine anti-B. bigemina antibody. High five insect cells (lane 1) and High five insect cells infected with AcRAP-1-Ht (lane 2) were reacted with mouse anti-B. bovis RAP-1 antibody. High five insect cells infected with AcRAP-1-Ht (lane 3) were reacted with bovine anti-B. bigemina antibody.
FIG. 3.
IFAT analysis of B. bovis RAP-1 expressed in High five insect cells by using bovine anti-B. bovis antibody or bovine anti-B. bigemina antibody. High five insect cells (A) and High five insect cells infected with AcRAP-1-Ht (B) were reacted with bovine anti-B. bovis antibody. High five insect cells (C) and High five insect cells infected with AcRAP-1-Ht (D) were reacted with bovine anti-B. bigemina antibody.
Detection of antibody to B. bovis in cattle by ELISA with recombinant RAP-1 as antigen.
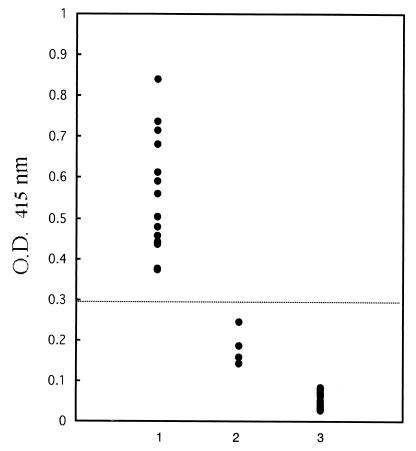
In order to assess the ability of ELISA to detect anti-B. bovis-specific antibody on the basis of recombinant RAP-1 expression in infected cells, a total of 31 reference bovine serum samples were tested by ELISA. All 14 positive samples with antibodies against B. bovis had ODs >0.3, whereas all 4 positive samples with antibodies against B. bigemina and 13 negative control samples had ODs <0.3 (Fig. 4). Field serum samples from 201 cattle in Brazil and 283 cattle in Mongolia were tested by ELISA; 19 of 201 (9.4%) samples from Brazil (Table 1) and 101 of 283 (35.7%) samples from Mongolia (Table 2) showed positive responses (the cutoff was chosen as an OD >0.3). The ages of the cattle varied from 1 to 10 years. The seroprevalence of antibodies to B. bovis in Brazil was low in animals of all age groups (Table 1), whereas the majority of the cattle in Mongolia in which antibodies to B. bovis were the most seroprevalent were in the 1- to 2-year-old age group (58.7%) (Table 2).
FIG. 4.
Value from ELISA with recombinant RAP-1 with experimentally infected bovine sera. Lane 1, B. bovis-infected bovine sera; lane 2, B. bigemina-infected bovine sera; lane 3, noninfected bovine sera.
TABLE 1.
Prevalence of antibodies against B. bovis in Brazil at various ages
| Age (yr) | No. of samples | No. (%) of seropositive samples |
|---|---|---|
| 1-2 | 130 | 14 (10.8) |
| 3-4 | 17 | 1 (5.9) |
| 5-6 | 26 | 1 (3.8) |
| 7-10 | 28 | 3 (10.7) |
| Total | 201 | 19 (9.4) |
TABLE 2.
Prevalence of antibodies against B. bovis in Mongolia at various ages
| Age (yr) | No. of samples | No. (%) of seropositive samples |
|---|---|---|
| 1-2 | 75 | 44 (58.7) |
| 3-4 | 90 | 29 (32.2) |
| 5-6 | 59 | 17 (28.8) |
| 7-10 | 49 | 11 (22.4) |
| Total | 283 | 101 (35.7) |
DISCUSSION
RAP-1 is a strong immunogenic protein of B. bovis (5). Vaccination of target animals with recombinant RAP-1 had been shown to give protection against a virulent heterologous strain (29). Bovine B. bovis-immune serum can immunoprecipitate the whole recombinant RAP-1 or a product of the RAP-1 deletion clone (25). Therefore, it was considered a candidate antigen for use in vaccine development (4, 7, 19) and to have potential as a diagnostic antigen for the detection of anti-B. bovis antibodies in cattle. In the present study, we expressed recombinant RAP-1 in High five insect cells by using a baculovirus expression system and evaluated it to determine its diagnostic potential in an ELISA for the detection of antibodies to B. bovis in cattle.
The ELISA based on recombinant RAP-1 was able to differentiate clearly between B. bovis-infected sera and B. bigemina-infected sera or noninfected normal bovine sera at an OD at 415 nm (OD415) of 0.3, which was the cutoff, but B. bigemina-infected sera cross-reacted with the recombinant RAP-1 protein at an OD415 of about 0.2. However, the number of bovine serum samples tested was small, and further evaluation with a large number of bovine serum samples will be necessary. This cross-reaction may be due to the high degree of sequence identity in the first 300 amino acids of B. bovis RAP-1 and B. bigemina p58 (24). Suraez et al. (25) reported that antibodies in serum from cattle immune to B. bigemina did not react with whole RAP-1 or the product of RAP-1 deletion clone F2 (amino acids 235 to 565) but reacted with the product of RAP-1 deletion clone F1 (amino acids 1 to 235). This is similar to our result that B. bigemina-infected bovine serum did not react with recombinant RAP-1 protein by Western blot analysis or IFAT. The cross-reactive epitopes are poorly immunogenic and inaccessible in whole RAP-1 (25).
A baculovirus expression system has been used to express proteins of protozoan parasites (6, 18, 31). It has many advantages over other expression systems, such as a high level of expression efficacy and the ability to preserve the biological properties of the recombinant protein (12, 14). We demonstrated that the recombinant RAP-1 expressed in High five insect cells by the baculovirus expression system can be used as an antigen in an ELISA for the detection of the anti-B. bovis antibodies in cattle, but we still need to consider the cross-reaction with B. bigemina. Next, we are going to construct a deletion clone of B. bovis RAP-1 in order to get a more specific recombinant antigen with no cross-reactivity with B. bigemina. Several recent studies performed to develop an ELISA with a recombinant antigen for the detection of anti-Babesia antibodies have shown satisfactory results (2, 10, 13, 31). In the present study, the cost of the assay for each sample tested in duplicate was estimated to be less than US$0.08, and this low cost of performance may result in a promising serodiagnostic tool for the detection of B. bovis infections in developing countries. Therefore, the use of a recombinant antigen in an ELISA may lead to the development of highly standardized diagnostic tests based on well-defined, reproducible, and inexpensive antigens for the detection of babesiosis.
Although bovine babesiosis is widespread in Brazil (20), the mean prevalence of samples positive for antibodies to B. bovis was only 9.4% in this study. The seroprevalence of antibodies to B. bovis was low in all age groups. This low prevalence is probably due to some differences in the distribution of the disease among the different regions of the country, which is attributed to the distribution of the tick vector and tick control programs. On the contrary, the mean prevalence of samples for positive B. bovis in Mongolia was found to be high (35.7%), especially in the 1- to 2-year-old age group (58.7%). The results from this serological survey suggest that bovine babesiosis caused by B. bovis is probably endemic in Mongolia. In areas of endemicty, an antibody to B. bovis can be detected in neonatal calves and considered of maternal origin (11, 28). A prominent feature of the pattern of antibodies against this parasite was a fall in antibody levels during the first months of life and a rise in antibody titers in the first year of life, which is probably in response to tick-transmitted infection (28). However, further surveys with more samples from additional populations will be necessary to evaluate the status of B. bovis infection in Brazil and Mongolia.
Acknowledgments
This work was supported by a Grant-in-Aid for Scientific Research (A) from the Japan Society for the Promotion of Science.
REFERENCES
- 1.Avarazed, A., D. T. de Waal, I. Igarashi, A. Saito, T. Oyamada, Y. Toyoda, and N. Suzuki. 1997. Prevalence of equine piroplasmosis in central Mongolia. Ondersterpoort J. Vet. Res. 64:141-145. [PubMed] [Google Scholar]
- 2.Bose, R., R. H. Jacobson, K. R. Gale, D. J. Waltisbuhl, and I. G. Wright. 1990. An improved ELISA for detection of antibodies against Babesia bovis using either a native or a recombinant B. bovis antigen. Parasitol. Res. 76:648-652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bose, R., W. K. Jorgensen, R. J. Dalgliesh, K. T. Friedhoff, and A. J. de Vos. 1995. Current state and future trends in diagnosis of babesiosis. Vet. Parasitol. 57:61-74. [DOI] [PubMed] [Google Scholar]
- 4.Brown, W. C., and G. H. Palmer. 1999. Designing a blood-stage vaccine against Babesia bovis and B. bigemina. Parasitol. Today 15:275-281. [DOI] [PubMed] [Google Scholar]
- 5.Brown, W. C., T. F. McElwain, B. J. Ruef, C. E. Suraez, V. Shkap, C. G. Chitko-Mckown, W. Tuo, A. C. Riece-Ficht, and G. H. Palmer. 1996. Babesia bovis: rhoptry-associated protein 1 is immunodominant for T helper cell epitopes conserved among geographically distant B. bovis strains. Infect. Immun. 64:3341-3350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang, S. P., S. P. Case, W. L. Gosnell, A. Hashimoto, K. J. Kramer, L. Q. Tam, C. Q. Hashiro, C. M. Nikaido, H. L. Gibson, C. T. Lee-Ng, F. J. Barr, B. T. Yokota, and G. S. N. Hui. 1999. A recombinant baculovirus 42-kilodalton C-terminal fragment of Plasmodium falciparum merozoite surface protein 1 protects Aotus monkeys against malaria. Infect. Immun. 64:253-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dalrymple, B. P. 1993. Molecular variation and diversity in candidate vaccine antigens from Babesia. Acta Trop. 53:227-238. [DOI] [PubMed] [Google Scholar]
- 8.de Echaide, S. T., I. E. Echaide, A. B. Gaido, A. J. Mangold, C. I. Lugaresi, V. R. Vancini, and A. A. Guglielmone. 1995. Evaluation of enzyme-linked immunosorbent assay kit to detect Babesia bovis antibodies in cattle. Prev. Vet. Med. 24:277-283. [Google Scholar]
- 9.Fuginaga, T., T. Minami, and T. Ishihara. 1980. Serological relationship between a large Babesia found in Japanese cattle and Babesia major, Babesia bigemina, and Babesia bovis. Res. Vet. Sci. 29:230.. [PubMed] [Google Scholar]
- 10.Ikadai, H., X. Xuan, I. Igarashi, S. Tanaka, T. Kanemaru, H. Nagasawa, K. Fujisaki, N. Suzuki, and T. Minami. 1999. Cloning and expression of a 48-kDa Babesia caballi merozoite rhoptry protein and potential use of the recombinant antigen in an enzyme-linked immunosorbent assay. J. Clin. Microbiol. 37:3475-3480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.James, M. A., A. Coronado, W. Lopez, R. Malendez, and M. Rustic. 1985. Seroepidemiology of bovine anaplasmosis and babesiosis in Venezuela. Trop. Anim. Health Prod. 17:9-18. [DOI] [PubMed] [Google Scholar]
- 12.Jiang, X., M. Wang, D. Y. Graham, and M. K. Estes. 1992. Expression, self-assembly, and antigenicity of the Norwalk virus capsid protein. J. Virol. 66:6527-6532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kappmeyer, L. S., L. E. Perryman, S. A. Hines, T. V. Baszler, J. B. Katz, S. G. Hennager, and D. P. Knowles. 1999. Detection of equine antibodies to Babesia caballi by recombinant B. caballi rhoptry-associated protein 1 in competitive inhibition enzyme-linked immunosorbent assay. J. Clin. Microbiol. 37:2285-2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laurent, S., J. F. Vautherot, M. F. Medelaine, G. le Gall, and D. Rasschaert. 1994. Recombinant rabbit hemorrhagic disease virus capsid protein expressed in baculovirus self-assembles into virus-like particles and induces protection. J. Virol. 68:6794-6798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levy, M. G., and M. Rustic. 1980. Babesia bovis: continuous cultivation in a microaerophilus stationary phase culture. Science 204:1218-1220. [DOI] [PubMed] [Google Scholar]
- 16.Machado, R. Z., H. J. Montassior, A. A. Pinto, E. G. Lemos, M. R. F. Machado, I. F. F. Valadao, E. B. Braci, and E. B. Malheiros. 1997. An enzyme-linked immunosorbent essay (ELISA) for the detection of antibodies against Babesia bovis in cattle. Vet. Parasitol. 71:17-26. [DOI] [PubMed] [Google Scholar]
- 17.McCosker, P. J. 1981. The global importance of babesiosis, p. 1-24. In M. Ristic and J. P. Kreier (ed.), Babesiosis. Academic Press, Inc., New York, N.Y.
- 18.Nene, V., S. Inumaru, D. McKeever, S. Mosaria, M. Shaw, and A. Musake. 1995. Characterization of an insect cell-derived Theileriaparva sporozoite vaccine antigen and immunogenicity in cattle. Infect. Immun. 63:503-508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Palmer, G. H., and T. F. McElwain. 1995. Molecular basis for vaccine development against anaplasmosis and babesiosis. Vet. Parasitol. 57:233-253. [DOI] [PubMed] [Google Scholar]
- 20.Patarroyo, J. H., M. I. Vagous, and P. L. Bicudo. 1982. Description of lesions in cattle in a natural outbreak of Babesia bovis infection in Brazil. Vet. Parasitol. 11:301-308. [DOI] [PubMed] [Google Scholar]
- 21.Perkin, M. E. 1992. Rhoptry organelles of apicomplexan parasites. Parasitol. Today 8:28-32. [DOI] [PubMed] [Google Scholar]
- 22.Sam-Yellowe, T. Y. 1996. Rhoptry organelles of apicomplexa: their role in host cell invasion and intracellular survival. Parasitol. Today 12:308-315. [DOI] [PubMed] [Google Scholar]
- 23.Suraez, C. E., G. H. Palmer, D. P. Jasmer, S. A. Hines, L. E. Perryman, and T. F. McElwain. 1991. Characterization of the gene encoding a 60-kilodalton Babesia bovis merozoite protein with conserved and surface-exposed epitopes. Mol. Biochem. Parasitol. 46:45-52. [DOI] [PubMed] [Google Scholar]
- 24.Suraez, C. E., T. F. McElwain, E. B. Stephens, V. S. Mishra, and G. H. Palmer. 1991. Sequence conservation among merozoite apical complex proteins of Babesia bovis, Babesia bigemina, and other apicomplexa. Mol. Biochem. Parasitol. 49:329-332. [DOI] [PubMed] [Google Scholar]
- 25.Suraez, C. E., G. H. Palmer, A. Hines, and T. F. McElwain. 1993. Immunogenic B-cell epitopes of Babesia bovis rhoptry-associated protein 1 are distinct from sequences conserved between species. Infect. Immun. 61:3511-3517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weiland, G., and I. Reiter. 1988. Methods for serological response to Babesia, p. 143-158. In M. Ristic. (ed.), Babesiosis of domestic animal and man. CRC Press, Inc., Boca Raton, Fla.
- 27.Wiltisbuhl, D. J., I. G. Goodger, I. G. Wright, M. A. Commins, and D. F. Mahoney. 1987. An enzyme-linked immunosorbent assay to diagnose Babesia bovis infection in cattle. Parasitol. Res. 73:126-131. [DOI] [PubMed] [Google Scholar]
- 28.Woodford, J. D., T. W. Jones, P. F. Rae, R. Boid, and L. Bell-Sakyi. 1990. Seroepidemiological studies of bovine babesiosis on Pemba Island, Tanzania. Vet. Parasitol. 37:175-184. [DOI] [PubMed] [Google Scholar]
- 29.Wright, I. G., R. Casu, M. A. Commins, B. P. Dalrymple, K. R. Gale, B. V. Goodger, P. W. Riddles, D. J. Waltisbuhl, I. Abetz, D. A. Berrie, Y. Bowles, C. Dimmock, T. Hayes, H. Kalnins, G. Leatch, R. McCrae, P. E. Montague, I. T. Nisbet, F. Parrodi, J. M. Peters, P. C. Scheiwe, W. Smith, K. Rode-Bramanis, and M. A. White. 1992. The development of a recombinant Babesia vaccine. Vet. Parasitol. 44:3-13. [DOI] [PubMed] [Google Scholar]
- 30.Wright, I. G. 1990. Immunodiagnosis of and immunoprophylaxis against the hemoparasites Babesia sp. and Anaplasma sp. in domestic animals. Rev. Sci. Tech. Off. Int. Epizoot. 9:345-356. [DOI] [PubMed] [Google Scholar]
- 31.Xuan, X., A. Larsen, H. Ikadai, T. Tanaka, I. Igarashi, H. Nagasawa, K. Fujisaki, Y. Toyoda, N. Suzuki, and T. Minami. 2001. Expression of Babesia equi merozoite antigen 1 in insect cells by recombinant baculovirus and evaluation of its diagnostic potential in enzyme-linked immunosorbent assay. J. Clin. Microbiol. 39:705-709. [DOI] [PMC free article] [PubMed] [Google Scholar]