Abstract
In Bacillus subtilis the anti-TRAP protein (AT) is produced in response to the accumulation of uncharged tRNATrp. AT regulates expression of genes involved in tryptophan biosynthesis and transport by binding to the tryptophan-activated trp RNA-binding attenuation protein (TRAP) and preventing its interaction with several mRNAs. Here, we report the x-ray structure of AT at 2.8 Å resolution, showing that the protein subunits assemble into tight trimers. Four such trimers are further associated into a 12-subunit particle in which individual trimers are related by twofold and threefold symmetry axes. Twelve DnaJ-like, cysteine-rich zinc-binding domains form spikes on the surface of the dodecamer. Available data suggest several possible ways for AT to interact with the 11-subunit TRAP. Interaction between the two symmetry-mismatching molecules could be assisted by the flexible nature of AT zinc-binding domains.
Keywords: DnaJ, trp RNA-binding attenuation protein, tryptophan biosynthesis, protein–protein interactions
In Bacillus subtilis, both the level of intracellular free tryptophan and the level of uncharged tRNATrp are used as regulatory signals for modulation of trp operon expression (1–4). The first regulatory mechanism involves the trp RNA-binding attenuation protein (TRAP), an 11-subunit oligomer that is activated by free tryptophan to bind to a specific segment of trp mRNA. The second mechanism involves the anti-TRAP protein (AT), which binds to tryptophan-activated TRAP and prevents its interaction with RNA; elevated levels of uncharged tRNATrp induce synthesis of AT.
Tryptophan biosynthesis requires expression of seven genes. Six of these genes are arranged in the trpECDFBA suboperon of the aro supraoperon, and the seventh, trpG, is located in the folate operon (1). When cellular levels of tryptophan are elevated TRAP becomes activated, the apparent dissociation constant of tryptophan binding to TRAP being 5–7.5 μM (5, 6). Tryptophan-activated TRAP binds to a region in the 5′ leader segment of the trpECDFBA operon transcript containing 11 UAG and GAG triplets with a Kd of ≈1 nM (7). This binding prevents formation of an antiterminator hairpin, thus permitting formation of a transcription terminator hairpin, which halts transcription of the operon (1, 8–11). TRAP also regulates translation of trpE (10, 11), trpG (pabA) (12, 13), trpP (yhaG) (14, 15), and ycbK (16) by binding to specific triplet repeat segments of these mRNAs and inhibiting translation initiation (1, 2).
B. subtilis AT is the product of the rtpA gene, which is located in the at operon (16). Expression of the at operon is controlled by tandem transcription and translation regulatory mechanisms based on sensing the level of charged/uncharged tRNATrp (17). Transcription of the structural genes of the at operon is regulated via the T-box transcription antitermination mechanism, where elevated levels of uncharged tRNATrp induce transcription of rtpA (18), leading to AT production and increased trp operon expression and tryptophan biosynthesis (19). Translational control of at operon expression is provided by the ability of a translating ribosome to move along the mRNA region encoding a tryptophan-rich leader peptide. Such movement is controlled by the level of charged tRNATrp (3, 20).
AT is a small oligomeric metalloprotein composed of identical 53-residue polypeptides (21). It binds one zinc atom per monomer; removal of zinc converts AT into inactive monomers (22). The amino acid sequence of AT contains two cysteine-rich motifs CXXCXGXG (where X represents a variable amino acid) with sequence homology to DnaJ (21). Substitution of any cysteine residue by alanine results in rapid degradation of the mutant protein in vivo (22).
Little is known about the specific interactions of TRAP with AT. TRAP is a toroid-shaped molecule composed of 11 identical subunits with 11 tryptophan-binding sites located at subunit–subunit interfaces (5, 6). In TRAP/RNA complexes, the 11 (G/U)AG repeats of the RNA chain bind to 11 binding sites on the surface of the protein and form a belt around the protein ring (23–25). A study with mutant TRAP proteins revealed that AT forms a complex only with tryptophan-activated TRAP and appears to recognize a region that is also involved in RNA binding (19). These observations led to the proposal that AT prevents the TRAP/RNA interaction by masking RNA-binding sites of TRAP (19). Interestingly, after TRAP has bound to RNA, AT is unable to disrupt the TRAP/RNA complex (19). In analytical ultracentrifugation experiments, a complex containing 12 AT subunits per each TRAP 11-mer was observed (26). Here, we describe the crystal structure of B. subtilis AT and discuss several possible models for its interaction with TRAP.
Materials and Methods
Protein Production, Crystallization, and X-Ray Data Collection. AT was expressed by using a T7 expression system in Escherichia coli from pET17b (Novagen) and purified as described (27). AT was crystallized as described (27). The best crystals were grown from a 15-mg/ml protein solution containing 1 mM DTT, 20 mM NaCl, and 5 mM triethanolamine (pH 8.0). The reservoir contained 21–23% (wt/vol) of 5,000 monomethyl ether polyethylene glycol (PEG), 0.1 M sodium cacodylate (pH 6.5), and 50 mM magnesium acetate. Crystals were flash-frozen in cryosolution containing crystallization ingredients with a higher concentration of a precipitating agent (30% PEG) as well as 16% (vol/vol) glycerol.
Diffraction data were collected at 120 K from a single crystal at three wavelengths near the zinc absorption edge. One data set at a remote wavelength was collected by using synchrotron radiation at the 9.6 beamline at the Synchrotron Radiation Source (SRS, Daresbury, U.K.). Two additional data sets from the same crystal (peak and inflection) were collected at the BM14 beamline at the European Synchrotron Radiation Facility (ESRF, Grenoble, France). Diffraction data sets were integrated by using mosf lm (28) and scaled/merged with scala (29). The main statistics of data collection are shown in Table 1.
Table 1. Data collection statistics.
| λpeak | λinf-point | λremote | |
|---|---|---|---|
| Wavelength, Å | 1.2826 | 1.2830 | 0.87 |
| Resolution, Å | 20-2.9 (2.95-2.9) | 20-3.08 (3.13-3.08) | 20-2.8 (2.95-2.8) |
| Unique reflections | 13,694 (661) | 11,073 (402) | 14,370 (1,869) |
| Completeness, % | 98.9 (92.8) | 96.7 (87.8) | 93.4 (83.0) |
| Rmerge,* % | 8.9 (51.2) | 8.9 (55.2) | 10.8 (18.1) |
| Average I/σ(I) | 15.2 (2.5) | 11.3 (1.3) | 5.7 (2.4) |
| Multiplicity | 6.6 (6.1) | 4.0 (4.0) | 1.9 (1.8) |
Values in parentheses are for the highest-resolution shell.
Rmerge = Σ|I — 〈I 〉|/Σ I.
Structure Determination and Refinement. A heavy atom search performed by shelxd (30) using anomalous differences located twelve zinc atoms. Their positions, occupancies, and B values were refined by using sharp (31). Initial phases were improved in resolve (31, 32), and several protein helices were located in the starting electron density maps by using the automated model building option. These helices allowed deduction of the noncrystallographic symmetry matrices relating 12 subunits in the P1 cell. The phases were further improved by using dm (33) by applying solvent flattening and twelvefold noncrystallographic averaging. The initial model was built by using x-autofit, implemented within quanta (Accelrys, San Diego). Crystallographic refinement was performed by refmac (34) with the TLS option (35) by using the data set collected at λremote. Noncrystallographic symmetry restraints were imposed throughout the refinement, and the distances between the zinc atoms and coordinating sulfur atoms of cysteines were constrained to 2.34 Å. The resulting crystallographic R factor (∑||Fobs – 0Fcalc||/ ∑ Fobs) was 20.5%. The value of Rfree, calculated over 5% of randomly chosen reflections that were excluded from the refinement, was 30.6%. The rms deviations from ideal geometry were 0.014 Å for bond length and 1.7° for bond angles. All figures were generated by using pymol (36). Multiple sequence alignment was performed by using clustalw (37).
Results and Discussion
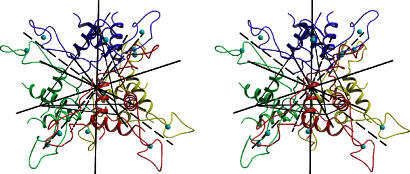
AT Monomer. Crystals of B. subtilis AT belong to the space group P1, with a = 51.55 Å, b = 60.12 Å, c = 60.35 Å, α = 113.99°, β = 101.37°, and γ = 100.50°, and contain 12 protein subunits in the asymmetric part. The structure was determined by multiwavelength anomalous dispersion by using data collected at Zn-absorption edge. Initial electron density maps were improved by twelvefold averaging. The final model was refined at 2.8 Å resolution. In the crystal, four AT trimers are arranged at the corners of a tetrahedron to form a dodecamer (Fig. 1) with overall dimensions of ≈65 × 65 × 65 Å.
Fig. 1.
Dodecamer of AT. Stereo diagram with AT trimers drawn in different colors. The four trimers are arranged in the corners of a tetrahedron. Continuous black lines show the threefold symmetry axes, whereas dashed lines show the twofold axes.
Each AT monomer is L-shaped, with an angle of ≈100° between the two “wings” (Fig. 2A). The zinc-binding domain is formed by the middle portion of the polypeptide chain, residues 9–36, and is located on one wing of the monomer. The other wing is composed of two α-helical regions: a short N-terminal helix α1 formed by residues 5–8 and a longer C-terminal helix α2 formed by residues 38–50. A two-stranded antiparallel β-sheet, formed by residues 9–11 (β1) and 34–36 (β4) in the center of the monomer connects the two wings. Residues 20–21 (β2) and 24–25 (β3) form a β-hairpin at the far end of the zinc-binding domain. Residues 51–53 at the C termini are exposed to the solvent and are not clearly visible in the electron density of several subunits.
Fig. 2.
Comparison of AT with DnaJ. (A) AT monomer is drawn as a ribbon with cysteine residues participating in zinc coordination represented as sticks and zinc atom as a cyan sphere. (B) E. coli DnaJ segment containing two zinc-binding domains (residues 139–209) is shown in a similar orientation to A.
AT exhibits sequence similarity with the type I family of Hsp40 molecular chaperones (see Fig. 6, which is published as supporting information on the PNAS web site). The middle part of the polypeptide chain (residues 9–39) of AT is 32% identical to the E. coli DnaJ protein (21). The 3D structure is available for two representatives of this family of molecular chaperones. dali (38) indicates the highest score of 3.2 for the zinc-binding fragment of E. coli DnaJ protein (39), and a lower score of 2.2 for the Ydj1 protein from Saccharomyces cerevisiae (40). In AT, the cysteine-rich motif CXXCXGXG appears twice whereas, in the type I family of Hsp40 molecular chaperones, this motif is repeated four times (41). In all cases, two cysteine-rich motifs are involved in binding one zinc atom with four cysteine side chains coordinated to the metal. Two zinc-binding domains of DnaJ have identical folds with an rms difference between their backbones of 0.18 Å (39). The zinc-binding domain of AT most closely resembles the second zinc-binding domain of DnaJ, with a 1.1 Å rms difference between the Cα atoms of residues 9–22 and 23–36 of AT and residues 28–41 and 50–63 of DnaJ. In DnaJ, the sequence separating the two cysteine-rich segments is longer (Fig. 6), resulting in an extended and flexible antiparallel β-hairpin exposed to the solvent (ref. 39 and Fig. 2B). The N-terminal (residues 1–8) and C-terminal (residues 40–53) segments of AT have no significant sequence homology with the type I family of Hsp40 molecular chaperones, and indeed these segments have a different 3D structure.
The conformation of the AT monomer is stabilized by an extensive network of interactions within the zinc-binding domain (Fig. 3A). The side chains of four cysteine residues are coordinated to the zinc atom in a tetrahedral arrangement. In addition, the two loops around the zinc atom, residues 12–19 and 26–33, are stabilized by four main chain/main chain hydrogen bonds and by four unusual weak hydrogen bonds between main chain nitrogen and sulfur atoms (Sγ) of cysteine residues, which are atypical for regular secondary structure.
Fig. 3.
Zinc-binding domain of AT. (A) Stereoview with residues drawn as sticks and with the zinc atom shown as cyan sphere and hydrogen bonds shown by dashed lines. Regular hydrogen bonds and hydrogen bonds between main chain nitrogen and sulfur atoms of cysteine residues are shown in red and gray, respectively. (B) Superposition of 12 monomers drawn as Cα backbones and shown in different colors. The view is along the threefold symmetry axis of the AT trimer, depicted by a triangle. Superposition is based on main-chain atoms of residues 5–8 and 37–50 (rms deviation ≈0.35 Å).
The electron density corresponding to the zinc-binding domains is less clear than that corresponding to the N- and C-terminal parts of the polypeptide chain. In particular, in one subunit, electron density is absent for the loop connecting the two β-strands of the β-hairpin (residues 20–24). In accordance, atoms of zinc-binding domains have higher temperature factors. For example, in one subunit, the average temperature factor is ≈100 Å2 for the main chain atoms of the zinc-binding domain and ≈70 Å2 for the main chain atoms of the C-terminal α2 helix. These data suggest flexibility of the zinc-binding domains. Moreover, superposition of the 12 monomers reveals a hinge between the zinc-binding domain and the rest of the structure (Fig. 3B). Rotation around the hinge results in maximum observed displacement of residues at the far end of the zinc-binding domain by up to 5 Å.
AT Trimer and Association of Trimers into a Dodecamer. The trimer has a diameter of 60 Å (Fig. 4A), with the zinc atoms located at the radius of 23 Å. The distance between pairs of individual zinc atoms is ≈40 Å. Each monomer has a solvent-accessible area of 4,400 Å2 of which 950 Å2 are buried in intersubunit contacts. Within the trimer, three C-terminal helices, one from each monomer, form a coil-coil. Each helix is inclined by ≈30° relative to the axis of the trimer. The three helices interact with each other through a hydrophobic core formed by side chains of Val-2, Ile-3, Leu-8, Leu-36, Leu-43, Leu-44, Phe-46, Ile-47, and Leu-51, (Fig. 4B). Apart from the hydrophobic interactions, the trimer is stabilized through direct intersubunit hydrogen bonds formed between main chain atoms of Val-2 and Ile-3 with the side chain of Gln-39, and between side chains of pairs of residues Glu-9-His-50 and Gln-48-Asn-52 (data not shown).
Fig. 4.
AT trimer. (A) Ribbon diagram of the trimer shown together with molecular surface in two orthogonal views. Each subunit is colored differently, and zinc atoms are shown as cyan spheres. (B) Hydrophobic core of the trimer. Shown is a stereoview with ribbons corresponding to residues 1–9 and 35–53 of three monomers shown in different colors. Side chains of residues that form stabilizing hydrophobic interactions are shown in sticks. Residues from one monomer are labeled.
Zinc is essential, not only for stabilization of the zinc-binding domain, but also for maintenance of the quaternary structure of the trimer. Removal of zinc atoms converts the AT oligomer into inactive monomers, and substitution of any of the zinc-bound cysteine residues with alanine results in rapid degradation of the resulting mutant protein in vivo (22). Zinc-binding domains are not involved in formation of direct contacts between adjacent trimers. Stabilization of the trimer by bound zinc suggests that its binding not only influences the conformation of AT locally, but also has a profound effect on areas outside the zinc-binding cluster. In particular, we expect that loss of bound zinc would lead to conformational changes within the segment containing residues 10–35 and result in disruption of monomer–monomer interactions between residues 1–8 and 39–50 that stabilize the trimer.
In the dodecamer, the twofold and threefold internal symmetry axes intersect in the center of the tetrahedron (Fig. 1). Each threefold symmetry axis relating subunits within a trimer also relates the remaining three trimers. Each trimer of AT has a solvent-accessible surface area of 9,400 Å2 of which 2,340 Å2 (≈780 Å2 from each monomer) are contributed to the formation of the dodecamer. Trimer–trimer association is stabilized largely by van der Waals interactions in two areas. The first is a hydrophobic cavity in the center of the molecule lined by residues 1–3 from all 12 monomers. A second region of stabilizing interactions is generated along the twofold molecular axes by residues Val-10, Pro-13, and Ile-35 (Fig. 3A), which form hydrophobic contacts between pairs of adjacent zinc-binding domains. There are only two direct hydrogen-bonding interactions, formed between pairs of residues, Met-1 and Asp-7 from contacting subunits of the two trimers (not shown). These observations suggest weak interactions and potential flexibility at the trimer–trimer interface.
Analytical ultracentrifugation experiments (26) showed that, in vitro, AT exists in reversible equilibrium between trimeric and dodecameric forms. (AT)12 species appeared at 4 μM (AT)3 concentrations but were not detected below 3 μM. At ≈20 μM (AT)3, equal weight fractions of the (AT)3 and (AT)12 species were observed (26). There are ≈200–400 TRAP molecules per B. subtilis cell, corresponding to ≈0.08 μM of 11-mers (42). The level of TRAP is relatively constant and independent of tryptophan level. In contrast, the level of AT depends on the concentration of uncharged tRNATrp and therefore indirectly on the concentration of free tryptophan. However, only tryptophan-activated TRAP is able to interact with AT. Recently, concentrations of AT in the cell were estimated at different physiological conditions (43). The highest ratios of (AT)3 to (TRAP)11 were observed during severe tryptophan starvation to be 0.83 for normal B. subtilis cells and ≈2 for mutant B. subtilis cells containing alterations in the at operon regulatory leader region that result in maximal AT production. Under these conditions, it appears that much of the TRAP in the cell is inactive (43). According to these observations, the maximal concentration of (AT)3 in the cell would be ≈0.2 μM, which is significantly lower than the 4 μM concentration required for association of trimers into the dodecamer. These data therefore suggest that AT may exist predominantly as trimers in vivo. However, because AT synthesis is highly regulated and its function depends on tryptophan activation of TRAP, we cannot exclude the possibility that the observed 12-mer structure forms in vivo. In particular, under normal physiological conditions, there could be cooperativity of trimer–trimer interactions favoring formation of the dodecamer.
Functional Implications: Zinc-Binding Domains. The role of zinc-binding domains in protein–protein interactions has been extensively studied in DnaJ, a type I Hsp40 molecular chaperone that contains two zinc-binding domains similar to that of AT. The first zinc-binding domain of DnaJ (Zn1 in Fig. 2B) interacts with unfolded proteins to prevent their aggregation (41). Indeed, substitution of any cysteine residue by serine in this domain resulted in mutant proteins with reduced ability to bind to denatured proteins (44). The second zinc-binding domain (Zn2 in Fig. 2B) is important for interaction of DnaJ with another chaperone protein, DnaK (44, 45). Substitution of any cysteine residue by serine in this domain reduced DnaK-dependent chaperone activity (44). Similar results were obtained for Ydj1, a close homologue of DnaJ in yeast (46). Complete deletions of zinc-binding domains of Ydj1 significantly compromised its activity (47). Taken together, the data suggest involvement of zinc-binding domains in protein–protein interactions and indicate that the cysteine residues are crucial for zinc binding and ultimately for maintaining the proper fold of the zinc-binding domain, including the two surrounding β-sheets.
All residues, except the four cysteine residues of the AT zinc-binding domain have their side chains exposed and could therefore be involved in protein–protein interactions. However, none of these residues are conserved among the type I Hsp40 molecular chaperones (Fig. 6). Indeed, in these domains, only the cysteine and glycine residues of the CXXCXGXG motifs are conserved. The absence of sequence conservation in residues with exposed side chains suggests that the zinc-binding domains of DnaJ may interact with other proteins via their main-chain atoms. In agreement, inspection of the DnaJ structure (47) showed that more than half of the residues constituting zinc-binding domain have their main chain atoms exposed at the surface.
The structure of the zinc-binding domain of AT is highly similar to the structures of DnaJ zinc-binding domains (Fig. 2). The highest similarity is with the Zn2 domain of DnaJ, although in AT the β-hairpin is shorter by four residues. This structural homology suggests potential involvement for the AT zinc-binding domains in protein–protein interactions. In both the trimer and the dodecamer structures of AT, zinc-binding domains point outward, making these domains ideal candidates for interacting with TRAP.
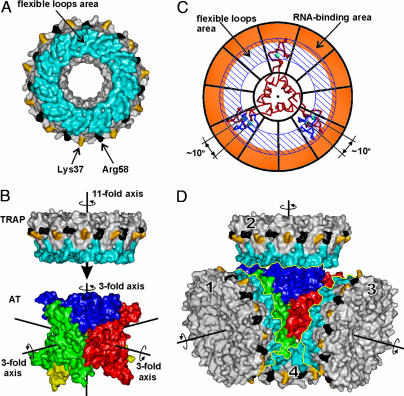
TRAP/AT Interaction. There are several similarities in the ways that TRAP binds to AT and to RNA. In each case, the interaction takes place only with tryptophan-activated TRAP (19). Furthermore, mutant TRAP proteins deficient in RNA binding (Lys37Ala and Arg58Ala) have low affinity toward AT. Each bound tryptophan molecule makes eight stabilizing hydrogen-bonding interactions with two loops of TRAP, formed by residues 25–33 and 49–52. NMR studies have shown that these two loops become conformationally disordered in the absence of tryptophan (48). These two flexible loops define a substantial area on the surface of TRAP (Fig. 5A); the requirement of bound tryptophan for interaction with AT suggests that this area could be directly involved in the AT/TRAP interaction.
Fig. 5.
AT/TRAP interaction. (A) Molecular surface of TRAP. Surfaces corresponding to tryptophan-binding loops are in blue, and those corresponding to Lys-37 and Arg-58 are in yellow and black, respectively. (B) Molecular surfaces of AT and TRAP are aligned so that one of the four threefold rotational axes of AT 12-mer coincides with the elevenfold rotational axis of TRAP. The AT 12-mer is roughly in the same orientation as in Fig. 1 with its trimers shown in different colors. The three threefold symmetry axes are shown by black lines; the fourth axis (not shown) is roughly perpendicular to the view. (C) Symmetry adjustment. AT trimer is shown as a Cα model (red), and TRAP is shown schematically as a wheel with its RNA-binding area colored orange and the area of flexible trp-binding loops highlighted by diagonal lines. Symmetry adjustment involves rotation of two zinc-binding domains of AT trimer by 10°, to fit the symmetry of the TRAP; these alternate positions are shown in blue. (D) Model for the complex of AT 12-mer with four molecules of TRAP. TRAP and AT are shown as in A and B.
Ultracentrifugation studies performed in the range of 12–48 μM (AT)3 and molar ratios of (AT)3:(TRAP)11 of 1:1 to 16:1 indicate that, in the TRAP/AT complex, four trimers of AT bind per TRAP 11-mer (26). At these conditions, a significant proportion of AT exists in the trimeric form. Nevertheless, no complexes corresponding to one, two, or three trimers of AT bound to TRAP were detected, suggesting either that TRAP interacts with a preassembled AT dodecamer or that its interaction with trimers of AT is cooperative, promoting formation of a dodecamer assembly. Because physiological concentrations of AT (43) are more than an order of magnitude lower than required for dodecamer formation in vitro (26), it is likely that the interaction is cooperative. Analytical ultracentrifugation studies showed that, at molar ratios of (TRAP)11:(AT)12 greater than 1:1, complexes consistent with multiple TRAP 11-mers interacting with single AT dodecamer were present (26). In contrast, when (AT)12 was in excess of (TRAP)11, no complexes corresponding to multiple (AT)12 binding to single TRAP11 were observed. These data are consistent with a model in which each TRAP 11-mer can interact with only one dodecamer of AT, whereas a single AT dodecamer can bind several TRAP molecules. In agreement, at the highest levels of tryptophan starvation in the cell, the molar ratio of (AT)12 to (TRAP)11 is close to 1:4 (43).
The accumulated data provide the basis for proposing several different models for the AT/TRAP complex. Here, we discuss one possibility that is consistent with ultracentrifugation (26), biochemical (43), and structural data. The AT dodecamer observed in the x-ray structure (Fig. 1) may represent the functionally active state. Indeed, such a dodecamer contains four equivalent surfaces that could form equivalent contacts with up to four 11-mers of TRAP. We propose that each TRAP molecule binds with its rotational symmetry axis coinciding with one of the threefold axes of AT. In this way, the area defined by the two flexible tryptophan-binding loops would be opposing three zinc-binding domains of one particular trimer of AT (Fig. 5B). If the AT/TRAP interactions occur between these two opposing surfaces, the two critical residues of TRAP, Lys-37, and Arg-58, would not participate directly in the interaction. These two residues are situated further from the elevenfold axis of TRAP (Fig. 5 A and B), within the RNA-binding rim that is largely defined by extension of one of the two loops, residues 34–37. Like the two tryptophan-binding loop segments, residues 34–37 are also conformationally disordered in the absence of tryptophan, suggesting communication of a signal from tryptophan-binding sites to the RNA-binding surface (48). Moreover, recent studies have shown that substitutions of several residues within the RNA-binding surface alter tryptophan-binding properties of TRAP (V. Payal and P.G., unpublished results), suggesting that a signal can also be communicated in the opposite direction, i.e., from the RNA-binding area to the tryptophan-binding area. These observations suggest that Lys-37 and Arg-58 need not interact directly with AT to influence its binding; instead, they could influence the conformation of the tryptophan-binding loops or be involved in generating an electrostatic potential that favors complex formation.
The symmetry mismatch between the AT trimer and TRAP 11-mer suggests that complex formation may lead to breaking the symmetry of one molecule to fit the symmetry of the other; i.e., either the threefold symmetry of AT or the elevenfold symmetry of TRAP may become distorted during complex formation so that pairs of interacting surfaces will be related by rotational symmetry of one of the two molecular components. The zinc-binding domains of AT are flexible, suggesting that symmetry adjustments in the molecule of AT are more likely than in TRAP. To fit the elevenfold symmetry of TRAP, zinc-binding domains of two AT subunits would have to rotate around the threefold axis of the trimer by ≈10°, as shown on Fig. 5C. The observed displacement of zinc-binding domains relative the hinge by up to 5 Å (Fig. 3B) demonstrates that such movement is possible. We could therefore expect that, in the complex between the 12-mer of AT and the 11-mer of TRAP, zinc-binding domains of AT undergo small rotations to fit the symmetry of the TRAP molecule. TRAP oligomer is more rigid than AT because it is stabilized by multiple main chain inter-subunit hydrogen bonds. Nevertheless, we cannot completely exclude that AT/TRAP interaction generates a 12-mer of TRAP that fits the threefold symmetry of AT. Although 12-mer species of TRAP have never been observed in crystal structures or in ultracentrifugation studies, 12-subunit TRAP molecules were detected during MALDI mass spectrometry analysis of cross-linked TRAP samples (8). Moreover, recent tandem mass spectrometry analysis confirmed the presence of 12-mer species of TRAP under certain conditions (49).
In our crude model (Fig. 5D), four TRAP molecules are positioned with their tryptophan-binding loops facing zinc-binding domains of AT. In this arrangement, each 11-mer of TRAP interacts with three subunits from a single trimer of AT. Alternatively, TRAP 11-mers could bind along the same three-fold axes of AT, but from the opposite sides of the AT 12-mer (not shown). In both cases, the interactions would involve zinc-binding domains of AT, but, in the latter case, each 11-mer of TRAP would interact with three subunits of AT belonging to three different trimers. AT/TRAP interactions could involve either main-chain or exposed side-chain atoms of zinc-binding domains of AT. Continuing studies must be directed toward understanding the details of these interactions.
The phenomenon, where two macromolecular components with mismatching rotational symmetries interact with each other, has been observed in several macromolecular systems. In the F1-ATPase (50), a circular array of αβ-subunits interacts with another component (γ-subunit) possessing no circular symmetry. DNA translocating molecular motors of several double-stranded DNA bacteriophages contain a twelvefold symmetrical portal protein embedded in a fivefold symmetrical vertex of the capsid (51). Available evidence suggests that, in these systems, the symmetry mismatch generates rotation of one component relative to the other. Unlike transient complexes observed in molecular motors, we expect AT to form a more stable complex with TRAP. Here, we discussed several possibilities for the functional complex that are consistent with structural observations, but it remains for the future to solve the exact structure that forms in the AT/TRAP complex.
Supplementary Material
Acknowledgments
We thank Charles Yanofsky for critically reading the manuscript and useful discussions, and Andrey Lebedev for help during structure determination. We also thank the Wellcome Trust for the allocation of synchrotron beam time at the Synchrotron Radiation Source and at the European Synchrotron Radiation Facility, and Miroslav Papiz and Martin Walsh for help during the data collection. This work was supported by Wellcome Trust Fellowship 067416 (to A.A.A.) and by National Institutes of Health Grant GM62750 and National Science Foundation funds (to P.G.).
Author contributions: M.B.S. and Y.C. performed research; M.B.S., Y.C., P.G., and A.A.A. analyzed data; P.G. and A.A.A. designed research; and M.B.S., P.G., and A.A.A. wrote the paper.
Conflict of interest statement: No conflicts declared.
Abbreviations: TRAP, trp RNA-binding attenuation protein; AT, anti-TRAP protein.
Data deposition: The atomic coordinates and structure factor amplitudes have been deposited in the Protein Data Bank, www.pdb.org (PDB ID code 2bx9).
References
- 1.Babitzke, P. & Gollnick, P. (2001) J. Bacteriol. 183, 5795–5802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Babitzke, P. (2004) Curr. Opin. Microbiol. 7, 132–139. [DOI] [PubMed] [Google Scholar]
- 3.Yanofsky, C. (2004) Trends Genet. 20, 367–374. [DOI] [PubMed] [Google Scholar]
- 4.Gollnick, P., Babitzke, P., Antson, A. & Yanofsky, C. (2005) Annu. Rev. Genet. 39, 47–68. [DOI] [PubMed] [Google Scholar]
- 5.Antson, A. A., Otridge, J., Brzozowski, A. M., Dodson, E. J., Dodson, G. G., Wilson, K. S., Smith, T. M., Yang, M., Kurecki, T. & Gollnick, P. (1995) Nature 374, 693–700. [DOI] [PubMed] [Google Scholar]
- 6.Chen, X., Antson, A. A., Yang, M., Li, P., Baumann, C., Dodson, E. J., Dodson, G. G. & Gollnick, P. (1999) J. Mol. Biol. 289, 1003–1016. [DOI] [PubMed] [Google Scholar]
- 7.Baumann, C., Otridge, J. & Gollnick, P. (1996) J. Biol. Chem. 271, 12269–12274. [DOI] [PubMed] [Google Scholar]
- 8.Babitzke, P., Stults, J. T., Shire, S. J. & Yanofsky, C. (1994) J. Biol. Chem. 269, 16597–16604. [PubMed] [Google Scholar]
- 9.Babitzke, P., Bear, D. G. & Yanofsky, C. (1995) Proc. Natl. Acad. Sci. USA 92, 7916–7920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Merino, E., Babitzke, P. & Yanofsky, C. (1995) J. Bacteriol. 177, 6362–6370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Du, H. & Babitzke, P. (1998) J. Biol. Chem. 273, 20494–20503. [DOI] [PubMed] [Google Scholar]
- 12.Yang, M., de Saizieu, A., van Loon, A. P. & Gollnick, P. (1995) J. Bacteriol. 177, 4272–4278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Du, H., Tarpey, R. & Babitzke, P. (1997) J. Bacteriol. 179, 2582–2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sarsero, J. P., Merino, E. & Yanofsky, C. (2000) J. Bacteriol. 182, 2329–2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yakhnin, H., Zhang, H., Yakhnin, A. V. & Babitzke, P. (2004) J. Bacteriol. 186, 278–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sarsero, J. P., Merino, E. & Yanofsky, C. (2000) Proc. Natl. Acad. Sci. USA 97, 2656–2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen, G. & Yanofsky, C. (2003) Science 301, 211–213. [DOI] [PubMed] [Google Scholar]
- 18.Henkin, T. M. & Yanofsky, C. (2002) BioEssays 24, 700–707. [DOI] [PubMed] [Google Scholar]
- 19.Valbuzzi, A., Gollnick, P., Babitzke, P. & Yanofsky, C. (2002) J. Biol. Chem. 277, 10608–10613. [DOI] [PubMed] [Google Scholar]
- 20.Chen, G. & Yanofsky, C. (2004) Mol. Cell 13, 703–711. [DOI] [PubMed] [Google Scholar]
- 21.Valbuzzi, A. & Yanofsky, C. (2001) Science 293, 2057–2059. [DOI] [PubMed] [Google Scholar]
- 22.Valbuzzi, A. & Yanofsky, C. (2002) J. Biol. Chem. 277, 48574–48578. [DOI] [PubMed] [Google Scholar]
- 23.Antson, A. A., Dodson, E. J., Dodson, G., Greaves, R. B., Chen, X. & Gollnick, P. (1999) Nature 401, 235–242. [DOI] [PubMed] [Google Scholar]
- 24.Hopcroft, N. H., Wendt, A. L., Gollnick, P. & Antson, A. A. (2002) Acta Crystallogr. D 58, 615–621. [DOI] [PubMed] [Google Scholar]
- 25.Hopcroft, N. H., Manfredo, A., Wendt, A. L., Brzozowski, A. M., Gollnick, P. & Antson, A. A. (2004) J. Mol. Biol. 338, 43–53. [DOI] [PubMed] [Google Scholar]
- 26.Snyder, D., Lary, J., Chen, Y., Gollnick, P. & Cole, J. L. (2004) J. Mol. Biol. 338, 669–682. [DOI] [PubMed] [Google Scholar]
- 27.Shevtsov, M. B., Chen, Y., Gollnick, P. & Antson, A. A. (2004) Acta Crystallogr. D 60, 1311–1314. [DOI] [PubMed] [Google Scholar]
- 28.Leslie, A. G. W. (1993) in Proceedings of CCP4 Study Weekend (CLRC Daresbury Laboratory, Warrington, U.K.), pp. 44–51.
- 29.Evans, P. R. (1997) in Proceedings of CCP4 Study Weekend (CLRC Daresbury Laboratory, Warrington, U.K.), pp. 97–102.
- 30.Uson, I. & Sheldrick, G. M. (1999) Curr. Opin. Struct. Biol. 9, 643–648. [DOI] [PubMed] [Google Scholar]
- 31.Bricogne, G., Vonrhein, C., Flensburg, C., Schiltz, M. & Paciorek, W. (2003) Acta Crystallogr. D 59, 2023–2030. [DOI] [PubMed] [Google Scholar]
- 32.Terwilliger, T. C. (2002) Acta Crystallogr. D 58, 1937–1940. [DOI] [PubMed] [Google Scholar]
- 33.Cowtan, K. D. & Main, P. (1996) Acta Crystallogr. D 52, 43–48. [DOI] [PubMed] [Google Scholar]
- 34.Murshudov, G. N., Vagin, A. A. & Dodson, E. J. (1997) Acta Crystallogr. D 53, 240–255. [DOI] [PubMed] [Google Scholar]
- 35.Winn, M. D., Isupov, M. N. & Murshudov, G. N. (2001) Acta Crystallogr. D 57, 122–133. [DOI] [PubMed] [Google Scholar]
- 36.DeLano, W. L. (2002) pymol Molecular Graphics System (DeLano Scientific, San Carlos, CA).
- 37.Thompson, J. D., Higgins, D. G. & Gibson, T. J. (1994) Nucleic Acids Res. 22, 4673–4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Holm, L. & Sander, C. (1993) J. Mol. Biol. 233, 123–138. [DOI] [PubMed] [Google Scholar]
- 39.Martinez-Yamout, M., Legge, G. B., Zhang, O., Wright, P. E. & Dyson, H. J. (2000) J. Mol. Biol. 300, 805–818. [DOI] [PubMed] [Google Scholar]
- 40.Li, J., Qian, X. & Sha, B. (2003) Structure 11, 1475–1483. [DOI] [PubMed] [Google Scholar]
- 41.Szabo, A., Korszun, R., Hartl, F. U. & Flanagan, J. (1996) EMBO J. 15, 408–417. [PMC free article] [PubMed] [Google Scholar]
- 42.McCabe, B. C. & Gollnick, P. (2004) J. Bacteriol. 186, 5157–5159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang, W. J. & Yanofsky, C. (2005) J. Bacteriol. 187, 1884–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Linke, K., Wolfram, T., Bussemer, J. & Jakob, U. (2003) J. Biol. Chem. 278, 44457–44466. [DOI] [PubMed] [Google Scholar]
- 45.Banecki, B., Liberek, K., Wall, D., Wawrzynow, A., Georgopoulos, C., Bertoli, E., Tanfani, F. & Zylicz, M. (1996) J. Biol. Chem. 271, 14840–14848. [DOI] [PubMed] [Google Scholar]
- 46.Lu, Z. & Cyr, D. M. (1998) J. Biol. Chem. 273, 5970–5978. [DOI] [PubMed] [Google Scholar]
- 47.Li, J. & Sha, B. (2005) Biochem. J. 386, 453–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McElroy, C., Manfredo, A., Wendt, A., Gollnick, P. & Foster, M. (2002) J. Mol. Biol. 323, 463–473. [DOI] [PubMed] [Google Scholar]
- 49.McCammon, M. G., Hernandez, H., Sobott, F. & Robinson, C. V. (2004) J. Am. Chem. Soc. 126, 5950–5951. [DOI] [PubMed] [Google Scholar]
- 50.Abrahams, J. P., Leslie, A. G. W., Lutter, R. & Walker, J. E. (1994) Nature 370, 621–628. [DOI] [PubMed] [Google Scholar]
- 51.Hendrix, R. W. (1978) Proc. Natl. Acad. Sci. USA 75, 4779–4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.