Abstract
Studies that detected an association between Streptococcus bovis endocarditis and colon carcinoma have not taken into account the recently identified genetic diversity among organisms historically classified as S. bovis. With near full-length 16S ribosomal DNA sequence analysis, organisms cultured from the blood of endocarditis patients at the Mayo Clinic from 1975 to 1985 and previously identified as S. bovis or streptococcus group D nonenterococci were shown to represent S. bovis biotypes I (11 isolates) and II/2 (1 isolate), S. salivarius (1 isolate), and S. macedonicus (1 isolate). Two of the S. bovis biotype I cases were associated with colon cancer. Whether S. bovis biotype II or other organisms closely related to and historically identified as S. bovis (e.g., S. macedonicus) are associated with malignant (or premalignant) colon lesions in humans remains to be definitively determined.
Streptococcus bovis is the causative agent of 5 to 14% of cases of endocarditis (4). An association between fecal carriage of S. bovis and carcinoma of the colon has been recognized for 2.5 decades (11), and an association between S. bovis endocarditis and carcinoma of the colon has been appreciated for a similar time period (1, 9). S. bovis NCTC 8133 and ATCC 41344 have been demonstrated to adhere to intestinal epithelial cells and stimulate the production of cytokines that promote vasodilation and increased capillary permeability, creating a potential portal of entry for microbes (5). S. bovis NCTC 8133 has been further shown to be a promoter of early preneoplastic lesions in the colons of rats (6). Importantly, since the early 1980s, genetic and biochemical diversity has been noted among organisms classically designated S. bovis; it is now understood that organisms historically categorized as S. bovis (7) may represent one of several biotypes of S. bovis or even non-S. bovis streptococci. Because of the relationship between S. bovis and carcinoma of the colon, as well as that between S. bovis and endocarditis, it may be important to accurately identify these organisms.
Recently published studies either have not addressed the diversity of S. bovis isolates in the context of S. bovis endocarditis (4, 12, 14) or have addressed this issue in a preliminary and often contradictory fashion (2, 3). Prior to the description of S. gallolyticus, Ruoff et al. reported that among S. bovis biotypes identified by the API Rapid Strep system (Analytab Products, Plainview, N.Y.) and cellular fatty acid content, biotype I was more likely than biotype II to be associated with both endocarditis and malignant or premalignant colonic lesions (15). Following the description of S. gallolyticus, Devriese et al. used whole-cell protein analysis to show that all six bacterial isolates studied, which were derived from patients with endocarditis and identified by conventional techniques as S. bovis, were in fact S. gallolyticus (3). Devriese et al. suggested that S. gallolyticus is more likely to be involved in human infections than is S. bovis (3). Schlegel et al. further suggested that most of the mannitol-positive group D streptococci isolated from blood, which had been reported to be responsible for human endocarditis associated with colonic cancer (15), might actually be S. gallolyticus (16). More recently, however, Clarridge et al. used partial 16S ribosomal DNA (rDNA) sequence analysis to show that none of five bacterial isolates associated with human endocarditis and identified by conventional techniques as S. bovis were S. gallolyticus (2). In their study, endocarditis isolates were all mannitol negative and were S. bovis biotype I (1 isolate), S. bovis biotype II/1 (1 isolate), or S. bovis biotype II/2 (3 isolates) (2).
The purpose of this study was to determine whether organisms cultured from the blood of endocarditis patients at the Mayo Clinic from 1975 to 1985 and previously identified as S. bovis represent S. bovis biotype I, S. bovis biotype II/1, S. bovis biotype II/2, S. gallolyticus, S. salivarius, or other recently described streptococci, as determined by near full-length 16S rDNA sequence analysis.
The records of the Mayo Clinic endocarditis database (1975 to 1985) were reviewed to identify S. bovis or streptococcus group D nonenterococcus endocarditis cases. We identified 14 cases (patient ages, 45 to 81; 9 males; 12 had native-valve involvement, and 2 had prosthetic-valve involvement) for which the infecting microorganism had been archived at −70°C (Table 1).
TABLE 1.
Characteristics of the 14 patients with endocarditis caused by bacteria previously characterized as S. bovis or streptococcus group D nonenterococcia
| Patient | Age | Gender | Date of diagnosis | Valve involved | N or P valve | Gastrointestinal pathology | Organism identification (initial) | Organism identification by 16S rDNA sequence analysis |
|---|---|---|---|---|---|---|---|---|
| 1 | 50 | M | 09/04/75 | A/MI | N | Not assessed | SDNE | S. bovis biotype I |
| 2 | 57 | F | 12/28/75 | A/MI | N | Not assessed | SDNE | S. bovis biotype I |
| 3 | 70 | M | 07/27/76 | A | N | Not assessedb | SDNE | S. bovis biotype II/2 |
| 4 | 60 | M | 11/01/76 | A | N | Gastric adenomatous polyps | SDNE | S. bovis biotype I |
| 5 | 59 | F | 02/08/77 | A/MI | N | Normal colon X ray, primary biliary cirrhosis | SDNE | S. bovis biotype I |
| 6 | 45 | M | 08/30/77 | A | N | Ulcerative colitis | SDNE | S. bovis biotype I |
| 7 | 62 | M | 11/07/77 | MI | N | Sigmoid colon adenocarcinoma | SDNE | S. bovis biotype I |
| 8 | 78 | F | 12/01/77 | A | N | Normal colonoscopy | SDNE | S. bovis biotype I |
| 9 | 70 | M | 06/29/78 | MI | N | Cecal polyp | SDNE | S. macedonicus |
| 10 | 58 | F | 01/03/80 | A | N | Ulcerative colitis | S. bovis | S. bovis biotype I |
| 11 | 74 | M | 07/08/80 | MI/T | N | Sigmoid colon villous adenoma | S. bovis | S. bovis biotype I |
| 12 | 72 | F | 12/06/80 | A | P | Not assessed | S. bovis | S. salivarius |
| 13 | 81 | M | 09/09/83 | A | P | Sigmoid colon diverticulosis | S. bovis | S. bovis biotype I |
| 14 | 73 | M | 02/10/85 | A/Pul | N | Ascending colon adenocarcinoma | S. bovis | S. bovis biotype I |
M, male; F, female; N, native valve; P, prosthetic valve; MI, mitral valve; T, tricuspid valve; A, aortic valve; Pul, pulmonary valve; SDNE, streptococcus group D nonenterococcus.
This patient had iron deficiency anemia and occult blood in the stool.
16S rDNA PCR amplification and bidirectional sequencing of 1,430 nucleotides were performed using previously described cycling conditions and primers (10) and previously described PCR mixtures (13). Sequence data were analyzed using Sequencher 3.0 (Gene Codes Corporation, Ann Arbor, Mich.).
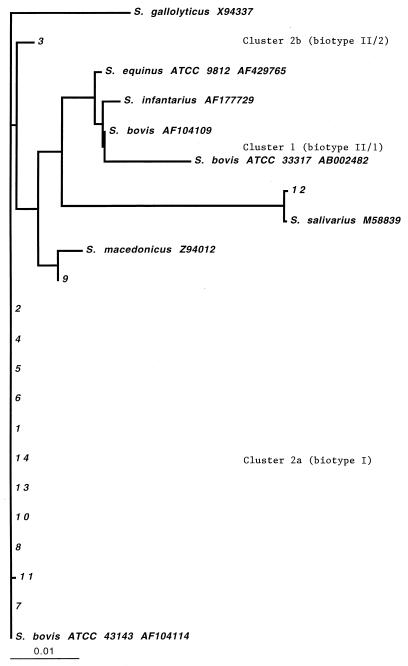
The 16S rDNA sequences of 11 isolates were identical to (n = 10) or different in one base pair from (n = 1) that of the type strain S. bovis ATCC 43143 (Fig. 1). These 11 isolates belonged to cluster 2a, which is S. bovis biotype I as defined by Clarridge et al. (2). One isolate (isolate 12) was most closely related to S. salivarius, and another isolate (isolate 9) was most closely related to S. macedonicus (17). The sequence of one isolate (isolate 3) was identical to the available sequence of VAMC blood3395. This places the isolate in cluster 2b, which is S. bovis biotype II/2 as defined by Clarridge et al. (2). None of the isolates studied clustered with S. bovis biotype II/1 as defined by Clarridge et al. (2), and none clustered with S. gallolyticus. All isolates produced acid from trehalose; all except two (isolates 3 and 12) produced acid from mannitol; all except one (isolate 12) were urease negative and bile esculin positive.
FIG. 1.
Dendrogram of phylogenetic relationships among study isolates, American Type Culture Collection type strains, and GenBank entries. Distances were calculated by the neighbor-joining method. The bar indicates a 1% difference measured horizontally. Isolate numbers correspond to case numbers cited in the text. The sequence of isolate 12 had one base different from that of S. salivarius M58839. Isolate 9 had five bases different from S. macedonius ATCC 43143. Clusters are indicated as defined by Clarridge et al. (2).
Of the 14 patients studied, 2 had colon cancer; 1 (case 7) of these was diagnosed simultaneously with endocarditis, and the other (case 14) was diagnosed 1 month prior to the onset of endocarditis symptoms. Six patients had other gastrointestinal pathologies (ulcerative colitis [cases 6 and 10], sigmoid colon villous adenoma [case 11], a cecal polyp [case 9], gastric adenomatous polyps [case 4], and sigmoid colon diverticulosis [case 13]). Two further patients had negative colonoscopic or double-contrast barium enema studies; of these patients, one had primary biliary cirrhosis (case 5). Four patients (cases 1, 2, 3, and 12) did not undergo colonic evaluation; however, one of these four (case 3) had findings indicative of a gastrointestinal-tract abnormality (iron deficiency anemia and occult blood detected in the stool) at the time of endocarditis diagnosis.
This is the largest series of S. bovis endocarditis isolates subjected to near full-length 16S rDNA sequence analysis. The organisms involved in these endocarditis cases were shown to be predominantly S. bovis biotype I (11 isolates), followed by S. bovis biotype II/2 (1 isolate), S. salivarius (1 isolate), and S. macedonicus (1 isolate). Our findings support earlier findings based on the API Rapid Strep system and cellular fatty acid studies that S. bovis biotype I is most frequently associated with endocarditis in humans (15) and refute the suggestion of Devriese et al. that S. gallolyticus is a more common pathogen than S. bovis in human endocarditis (3).
It has previously been observed that S. salivarius can be misidentified as S. bovis (15). Since S. salivarius has been shown to be less likely to be associated with colonic lesions or endocarditis than S. bovis (15), differentiating S. salivarius from S. bovis, which can be accomplished by the methods described herein, can provide clinically useful information. Our S. salivarius endocarditis patient did not have known colonic pathology.
This is the first description of human infection caused by S. macedonicus, an organism originally isolated from naturally fermented Greek Kasseri cheese (8, 17). It is unknown whether S. macedonicus endocarditis is associated with colonic carcinoma; our single case was associated with a cecal polyp.
16S rDNA sequencing and sequence analysis are being increasingly used in clinical microbiology laboratories for bacterial identification. This tool enables accurate species and subspecies level identification of S. bovis and closely related bacteria such as S. salivarius, S. macedonicus, and S. gallolyticus. The association or lack thereof of endocarditis with colon pathology needs to be considered when patients with endocarditis caused by these organisms are evaluated. The isolate determined to be a promoter of early preneoplastic lesions in the colons of rats is biotype II/1 (Marie Scholler-Guinard, personal communication) (6). Whether other biotypes of S. bovis and/or other organisms closely related to and historically identified as S. bovis (e.g., S. macedonicus) would yield similar results in this rat model remains to be determined. The isolates determined to adhere to intestinal epithelial cells and stimulate the production of cytokines that promote vasodilation and increased capillary permeability (5) include the aforementioned biotype II/1 isolate, as well as ATCC 31344, which, on the basis of 16S rDNA sequence analysis (GenBank accession number AF104115), was determined to be biotype II/2 as defined by Clarridge et al. (2). Based on our results for humans, S. bovis biotype I is associated with both endocarditis and malignant and premalignant colon lesions. Whether S. bovis biotype II or other organisms closely related to and historically identified as S. bovis (e.g., S. macedonicus) are associated with malignant and premalignant colon lesions in humans remains to be definitively determined.
Nucleotide sequence accession numbers.
16S rDNA sequences have been deposited in GenBank under accession numbers AF459434 and AF459433 for isolates 9 and 12, respectively.
Acknowledgments
We acknowledge Marlene K. Hopkins for her assistance with biochemical identification of the study isolates.
REFERENCES
- 1.Ballet, M., G. Gevigney, J. P. Gare, F. Delahaye, J. Etienne, and J. P. Delahaye. 1975. Infective endocarditis due to Streptococcus bovis. A report of 53 cases. Eur. Heart J. 16:1975-1980. [DOI] [PubMed] [Google Scholar]
- 2.Clarridge, J. E., III, S. M. Attorri, Q. Zhang, and J. Bartell. 2001. 16S ribosomal DNA sequence analysis distinguishes biotypes of Streptococcus bovis: Streptococcus bovis biotype II/2 is a separate genospecies and the predominant clinical isolate in adult males. J. Clin. Microbiol. 39:1549-1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Devriese, L. A., P. Vandamme, B. Pot, M. Vanrobaeys, K. Kersters, and F. Haesebrouck. 1998. Differentiation between Streptococcus gallolyticus strains of human clinical and veterinary origins and Streptococcus bovis strains from the intestinal tracts of ruminants. J. Clin. Microbiol. 36:3520-3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duval, X., V. Papastamopoulos, P. Longuet, C. Benoit, C. Perronne, C. Leport, and J. L. Vilde. 2001. Definite Streptococcus bovis endocarditis: characteristics in 20 patients. Clin. Microbiol. Infect. 7:3-10. [DOI] [PubMed] [Google Scholar]
- 5.Ellmerich, S., N. Djouder, M. Scholler, and J. P. Klein. 2000. Production of cytokines by monocytes, epithelial and endothelial cells activated by Streptococcus bovis. Cytokine 12:26-31. [DOI] [PubMed] [Google Scholar]
- 6.Ellmerich, S., M. Scholler, B. Duranton, F. Gosse, M. Galluser, J. P. Klein, and F. Raul. 2000. Promotion of intestinal carcinogenesis by Streptococcus bovis. Carcinogenesis 21:753-756. [DOI] [PubMed] [Google Scholar]
- 7.Facklam, R. R. 1972. Recognition of group D streptococcal species of human origin by biochemical and physiological tests. Appl. Microbiol. 23:1131-1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Georgalaki, M. D., P. Sarantinopoulos, E. S. Ferreira, L. De Vuyst, G. Kalantzopoulos, and E. Tsakalidou. 2000. Biochemical properties of Streptococcus macedonicus strains isolated from Greek Kasseri cheese. J. Appl. Microbiol. 88:817-825. [DOI] [PubMed] [Google Scholar]
- 9.Grinberg, M., A. J. Mansur, D. O. Ferreira, G. Bellotti, and F. Pileggi. 1990. Endocarditis caused by Streptococcus bovis and colorectal neoplasms. Arq. Bras. Cardiol. 54:265-269. [PubMed] [Google Scholar]
- 10.Johnson, J. L. 1994. Similarity analysis of rRNAs, p. 683-700. In P. Gerhardt, R. G. E. Murray, W. A. Wood, and N. R. Krieg (ed.), Methods for general and molecular bacteriology. American Society for Microbiology, Washington, D.C.
- 11.Klein, R. S., R. A. Recco, M. T. Catalano, S. C. Edberg, J. I. Casey, and N. H. Steigbigel. 1977. Association of Streptococcus bovis with carcinoma of the colon. N. Engl. J. Med. 297:800-802. [DOI] [PubMed] [Google Scholar]
- 12.Kupferwasser, I., H. Darius, A. M. Muller, S. Mohr-Kahaly, T. Westermeier, H. Oelert, R. Erbel, and J. Meyer. 1998. Clinical and morphological characteristics in Streptococcus bovis endocarditis: a comparison with other causative microorganisms in 177 cases. Heart 80:276-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patel, R., K. E. Piper, M. S. Rouse, J. M. Steckelberg, J. R. Uhl, P. Kohner, M. K. Hopkins, F. R. Cockerill III, and B. C. Kline. 1998. Determination of 16S rRNA sequences of enterococci and application to species identification of nonmotile Enterococcus gallinarum isolates. J. Clin. Microbiol. 36:3399-3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pergola, V., G. Di Salvo, G. Habib, J. F. Avierinos, E. Philip, J. M. Vailloud, F. Thuny, J. P. Casalta, P. Ambrosi, M. Lambert, A. Riberi, A. Ferracci, T. Mesana, D. Metras, J. R. Harle, P. J. Weiller, D. Raoult, and R. Luccioni. 2001. Comparison of clinical and echocardiographic characteristics of Streptococcus bovis endocarditis with that caused by other pathogens. Am. J. Cardiol. 88:871-875. [DOI] [PubMed] [Google Scholar]
- 15.Ruoff, K. L., S. I. Miller, C. V. Garner, M. J. Ferraro, and S. B. Calderwood. 1989. Bacteremia with Streptococcus bovis and Streptococcus salivarius: clinical correlates of more accurate identification of isolates. J. Clin. Microbiol. 27:305-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schlegel, L., F. Grimont, M. D. Collins, B. Regnault, P. A. Grimont, and A. Bouvet. 2000. Streptococcus infantarius sp. nov., Streptococcus infantarius subsp. infantarius subsp. nov. and Streptococcus infantarius subsp. coli subsp. nov., isolated from humans and food. Int. J. Syst. E vol. Microbiol. 50:1425-1434. [DOI] [PubMed] [Google Scholar]
- 17.Tsakalidou, E., E. Zoidou, B. Pot, L. Wassill, W. Ludwig, L. A. Devriese, G. Kalantzopoulos, K. H. Schleifer, and K. Kersters. 1998. Identification of streptococci from Greek Kasseri cheese and description of Streptococcus macedonicus sp. nov. Int. J. Syst. Bacteriol. 48:519-527. [DOI] [PubMed] [Google Scholar]