Abstract
The autoimmune cascade that culminates in diabetes initiates within pancreatic lymph nodes (PLNs). Here, we show that developmentally controlled lymphogenesis establishes a preferential trafficking route from the gut to the PLN, where T cells can be activated by antigens drained from the peritoneum and the gastrointestinal tract. Furthermore, intestinal stress modifies the presentation of pancreatic self-antigens in PLNs. The convergence of endocrine and intestinal contents within PLNs has significant implications for type 1 diabetes and may help to explain the link between autoimmune pathogenesis and environmental provocation.
Keywords: autoimmunity, diabetes, peritoneal lymphatics, enteropathy
Type 1 diabetes (T1D) is an autoimmune disorder characterized by destruction of the insulin-producing β cells of the endocrine pancreas. The first stage of disease, known as insulitis, entails leukocyte invasion of the pancreatic islets; the second stage, overt diabetes, is marked by massive death of islet β cells and the subsequent loss of glucose homeostasis (1). The immune assault on β cells that preludes diabetes is orchestrated by T cells. Naïve, β cell-reactive T cells initially encounter their cognate antigen in pancreatic lymph nodes (PLNs) (2). Islet antigens are shuttled to these nodes by CD11b+ dendritic cells and subsequently presented to T lymphocytes (3). Thus, PLNs are key to diabetes pathogenesis (4, 5), the location where tolerance to pancreatic self-antigens is first broken.
Environmental factors impinge on an individual's genetic susceptibility to type 1 diabetes (6). Alimentary agents such as enteroviruses (e.g., coxsackie virus) and dietary antigens (e.g., gluten) are associated with T1D (7-13), and there is a frequent association between T1D and celiac disease (14). However, the precise mechanisms by which these factors might influence the autoimmune response to pancreatic β cells remain elusive. The common entry route of such environmental components certainly raises the question of how the gastrointestinal tract relates to the pancreatic axis.
Materials and Methods
Mice. All mice were bred and maintained under barrier conditions in the Joslin Diabetes Center animal facility in accordance with National Institutes of Health guidelines. BDC2.5/NOD, OT-I/Rag-10, and OT-II TCR transgenic (tg) mice are described in refs. 15-17. NOD, NOD/SCID, and B6.H2g7/g7 mice were bred at the Joslin Diabetes Center animal facility, and B6 mice were obtained from The Jackson Laboratory.
Antigens. Mice were injected i.p., intragastrically, or i.v. with chicken ovalbumin (OVA) (Sigma), BSA (Sigma), or saline (as controls), and OVA-coated beads, OVA-loaded apoptotic cells, or purified pancreatic islets. Soluble OVA was adsorbed onto 0.5-μm polystyrene microparticles (beads) (Polysciences) according to the manufacturer's protocol. Purified pancreatic islets were purified from 4- to 6-week-old NOD mice at the Islet Core of the Juvenile Diabetes Research Foundation Center at Harvard Medical School and were resuspended in PBS for injection.
Reagents. For Gαi inhibition, donor splenocytes were treated with 100 ng/ml Pertussis toxin (Sigma) for 30 min at 37°C, washed, and resuspended in PBS for i.p. injection. Large intestine injury was performed with dextran sulfate sodium (DSS) (MW 40,000; ICN) as described in ref. 18. DSS-containing drinking water (2% or 5% wt/vol) was administered to the mice for different lengths of time depending on the experiments. For adoptive transfers, mice were given DSS water for 2 days and then received normal drinking water for 1-3 days before T cells were injected. Small intestine injury was induced as described in ref. 19. Mice received a single s.c. injection of indomethacin (INDO) (85 mg/kg) 24 h before adoptive transfers. Control animals were fed a standard mouse diet with 20% protein, whereas the low-protein (LP) cohort was fed an 8%-protein diet (TestDiet) for 2-5 days before adoptive transfers and then switched to control diet for the duration of the experiment.
Assessment of Donor Cell Distribution. Spleens were harvested from adult and infant donors and processed into single-cell suspensions. After red blood cell lysis, B cells and CD4+ T cells were purified by negative depletion using streptavidin-labeled Abs and biotinylated MACS beads (Miltenyi Biotec). For B cell selection, Abs to CD3, CD11b, and CD11c were used; for CD4+ T cell selection, Abs to B220, CD8αα, CD11b, and CD11c were used. Purified lymphocyte populations or unfractionated splenocytes were then labeled with the cytoplasmic dye 5,6-carboxy-succinimidyl-fluorescein-ester (CFSE). Fluorescently labeled cells were then injected at different doses into the peritoneal cavity or the bloodstream (tail vein or retroorbital sinus). Recipients were killed no longer than 28 h after donor-cell injections, and the relevant lymphoid organs were harvested and processed into single-cell suspensions. After counting, the organ suspensions were subjected to cytofluorimetric analysis, and the fluorescently labeled cells were quantitated. Samples were acquired by using Coulter instrumentation and analyzed with expo32 software.
Assessment of T Cell Activation. T cell proliferation was assessed by using an adoptive transfer system with CFSE-labeled lymphocytes as described in ref. 2. Various lymphoid organs, including PLNs, mesenteric lymph nodes (MLNs), various s.c. lymph nodes (SLNs), and Peyer's patches, were harvested 2-3 days after i.v. transfer of CFSE-labeled donor T cells, and single-cell suspensions were prepared by using glass-slide disruption. For cytofluorimetric analysis of T cell activation, lymph node (LN) cells were then stained with fluorescently labeled monoclonal Abs specific for Vβ4 and CD4 (BDC2.5), Vα2 and CD8 (OT-I), and Vα2 and CD4 (OT-II) T cells. In assessments of T cell activation, Vβ4 was replaced with antibodies to CD44 or CD69. The extent of T cell proliferation was determined simultaneously by CFSE-dilution analysis.
Immunizations. Two days after treatment with intestinal perturbants was terminated, NOD mice were anaesthetized, and a single hind footpad was injected with 10 μg of the BDC2.5 mimic peptide.
Insulitis Scoring. For insulitis studies, BDC2.5/NOD mice were put on DSS-containing drinking water at weaning (21 days of age). After 7 days, experimental and control littermates were killed, and pancreata were promptly excised, formalin-fixed, and embedded in paraffin. Thin sections were stained with hematoxylin/eosin and examined by light microscopy. Multiple non-consecutive sections per animal were scored for insulitis (at least 50 islets per individual). The same fixation and staining procedures were used for gut histology.
Results
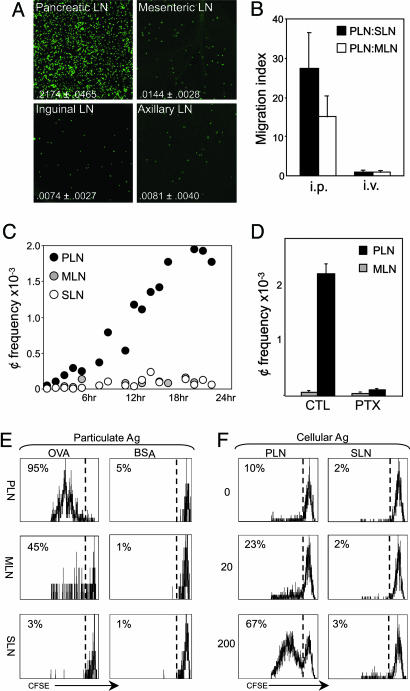
The experiments reported here may provide a cellular and molecular substratum to understand how environmental factors that enter the body via the gastrointestinal route influence pancreas-directed autoimmunity. The first observations were made through pure serendipity, however. For purposes of in vivo imaging, we were attempting to transfer fluorescently labeled splenocytes to SLNs of young NOD mice by i.p. injection. When SLNs were harvested from recipient mice 24 h later and examined by confocal microscopy, we observed fewer cells than expected. This was also true in MLNs and in several SLNs; the inguinal, axillary, and brachial SLNs were examined but yielded identical results (and will be referred to generically as SLN throughout this study). Unexpectedly, a far larger density of donor cells was found in PLNs, compared with SLNs or even MLNs, which one might have expected to be a primary draining site (Fig. 1A). Quantitation by cytofluorimetry over several such transfers confirmed the preferential localization to PLNs of cells infused in the peritoneum, because the density of fluorescent cells in these nodes was 17- to 37-fold higher than in SLNs and 10- to 20-fold higher than in MLNs (Fig. 1 A). The reduced frequency of donor cells in the MLNs or SLNs after peritoneal injection did not reflect dilution of the label provoked by some activation event at those sites as the cells did not proliferate at such short times after transfer (data not shown). The selective accumulation in PLNs was not an artifact of organ size and saturation, because PLN and SLNs are of roughly similar sizes in normal mice, and the PLN preference was observed over a wide range of donor-cell numbers (ranging from 2.5 to 60 × 106 cells; data not shown).
Fig. 1.
Preferential migration to PLNs. (A) Distribution of transferred cells was examined in PLNs, MLNs, inguinal LNs, and axillary LNs of 4-week-old NOD mice 24 h after i.p. injection with 10 × 106 CFSE-labeled splenocytes from 4-week-old NOD donors. Shown is representative fluorescence in recipient LNs as captured by confocal microscopy (magnification ×250). Data are representative of 10-20 mice. The donor cell proportion among total LN cells is indicated. (B) Cytofluorimetric enumeration of donor cells in recipient LNs after i.p. or i.v. transfer. The migration index is calculated as the ratio of donor cell density in PLNs to the donor cell density in SLNs (filled columns) or MLNs (open columns). Shown is data from 4-10 mice. (C) Kinetics of donor cell trafficking to PLNs, MLNs, and SLNs was determined by cytofluorimetry. Data are shown as the number of CFSE+ donor cells (¢) × 10-3 per 1 × 106 recipient LN cells. Three or four mice were examined at each time point. (D) Donor cells were pretreated with 100 ng/ml Pertussis toxin, washed, transferred i.p. into age- and strain-matched recipients, and analyzed in recipient LNs 24 h later by flow cytometry. Data are shown as the number of CFSE+ donor cells (¢) × 10-3 per 1 × 106 recipient LN cells in PLNs (filled column) and MLNs (open column). Three to four mice were used per condition. (E) Proliferation of transferred naive OT-I T cells (gated on CD8+Vα2+ cells) in PLNs, MLNs, and SLNs after i.p. injection of BSA-coated beads (total of 20 μg of BSA per recipient) or OVA-coated beads (total of 20 μg of OVA per recipient) into adult B6 mice. Proliferation of OT-I T cells was assessed 48 h after transfer by CFSE dilution in donor T cells. Values depict the frequency of divided OT-I T cells among the donor CD8+ T cells. Data are representative of four to five mice per condition. (F) Proliferation of transferred, naive BDC2.5 T cells (gated on CD4+Vβ4+ cells) in PLNs and SLNs after i.p. injection of graded doses of pancreatic islets or PBS into juvenile NOD mice. Proliferation of BDC2.5 T cells was assessed in all experiments 70 h after transfer by CFSE dilution in CD4+ T cells. Values depict the frequency of divided BDC2.5 T cells among the donor CD4+ T cells.
In marked contrast, fluorescently labeled splenocytes transferred i.v. were uniformly distributed among the LNs (Fig. 1B). Thus, there seemed to be no selective advantage in homing of cells to PLNs or retention within them via blood vessel high endothelial venules (HEVs). The preferential homing after i.p. administration appeared to denote a favored lymphatic drainage from the peritoneum to PLNs, rather than a selective retention, which would have also been observed after homing from blood.
To define the kinetics of this unforeseen trafficking route, we enumerated the donor cells within LNs at various time points after i.p. infusion. Small numbers of donor cells could be detected uniformly in LNs in the first hours, but their density began to increase specifically in PLNs after 8 h and continued to increase (Fig. 1C). The frequency of donor cells in MLNs and SLNs, on the other hand, remained flat throughout the time course.
To define the specificity of this trafficking route, purified populations of B and T lymphocytes were used as donor cells to test whether preferential homing to PLNs is restricted to a particular lymphocyte subset. Both populations behaved like the unfractionated splenocytes in their selective migration to PLNs (Fig. 5A, which is published as supporting information on the PNAS web site).
We sought to determine whether cellular traffic from the peritoneum to PLNs was an active process, as might be suggested by these kinetics. Splenocytes were pretreated with Pertussis toxin, an inhibitor of Gαi signaling that impedes signal processing, and in particular the response to chemotactic cues (20), before transfer into the peritoneum. Treated donor cells were unable to selectively access PLNs (Fig. 1D), confirming that the selective migration to the pancreas is an active process.
To ascertain whether this trafficking pattern was unique to NOD and/or resulted from ongoing pancreatic inflammation, identical transfer experiments were carried out with diabetes-resistant mouse strains. As with NOD, donor cells introduced into the peritoneum of B6 and B6.H2g7 animals also settled preferentially in the PLNs (Fig. 5B). To determine whether recipient lymphocytes played any role in establishing this polarized distribution, we transferred wild-type splenocytes into alymphoid SCID mice. Donor cell distribution in immunodeficient recipients was as skewed as in wild-type recipients, indicating that recipient lymphocytes do not instruct this process (Fig. 5C). Thus, the selectivity of cellular migration from the peritoneum to PLNs is a general phenomenon not particular to any mouse strain or cell type, or to being prone to T1D.
Next, we sought to determine whether preferential trafficking of cells to PLNs also applies to antigens. We traced the drainage of several particulate antigens, each administered i.p., through their ability to elicit the proliferation of antigen-specific T cells. Mice received chicken OVA- or control-coated microspheres, and presentation was evaluated by assessing dilution of CFSE label in OVA-specific T cells from OT-I tg mice (16) transferred i.v. In animals that received OVA microspheres, OT-I T cells divided extensively in PLNs after 48 h, to a lesser degree in MLNs, and not at all in SLNs (Fig. 1E). Infusion of OVA-containing splenocytes gave similar results, with elevated OT-I activation in PLNs as compared with other LNs (data not shown). Pancreatic islets were used as a third form of particulate antigen, probably the form most directly relevant to diabetes. Here, presentation was measured as proliferation of naïve β cell-reactive CD4+ T cells derived from BDC2.5 TCR tg mice (15) [using the time window between initiation of traffic from the peritoneum to PLNs, described below, and the onset of presentation of pancreatic self-antigen at 15 days of age (2, 3)]. Graded numbers of pancreatic islets were infused into 12-day-old animals. Within 3 days of islet infusion, transferred T cells proliferated extensively in PLNs but not in other LNs (Fig. 1F), indicating that antigen-presenting cells transported islet antigens-derived MHC class II molecule/peptide complexes to PLNs. These data demonstrate that particulate as well as cell-associated antigens from the i.p. cavity are presented to CD4+ and CD8+ T cells within the PLNs of adult mice in a preferential manner.
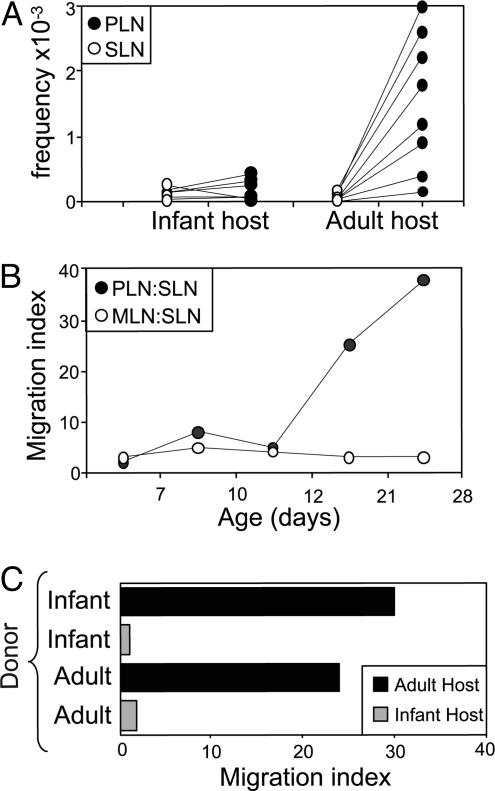
The presentation of islet-β cell autoantigens in PLNs is a developmentally regulated process, beginning between 10 and 20 days of age in mice (2, 3). This observation prompted us to address the question of when preferential lymphatic access to the PLNs is established, in particular whether there is any temporal relationship with the appearance of pancreatic antigens in PLNs. To that end, labeled splenocytes from adult mice were transferred into the peritoneal cavities of infant (≈1 week old) or adult (4 weeks old) animals, and donor cells were counted in LNs 24 h later. The pattern of donor-cell trafficking in the 1-week-old recipients was strikingly different from that in adults, with no enhanced traffic to PLNs in the former case (Fig. 2A). This pattern did not change when the dose of donor cells was varied (data not shown). To establish more precisely when PLNs develop into a site of convergence for lymphatic traffic, we transferred labeled splenocytes into the peritoneum of recipients ranging in age from 4 to 28 days. The distribution of donor cells changed markedly between 11 and 18 days of age, showing the polarization toward PLNs observed in adult recipients (Fig. 2B). Reciprocal transfers of splenocytes between infant and adult donors and recipients clearly indicated that trafficking from the peritoneum to the PLNs is not dictated by the migrating lymphocytes but by the developmental stage of the host recipient tissue (Fig. 2C). These data indicate that nonvascular access to PLNs develops ≈12 days after birth. This time frame is strikingly reminiscent of that when stimulatory dendritic cells presenting pancreatic autoantigens make their debut.
Fig. 2.
Lymphatic access to PLNs is developmentally regulated. (A) Fluorescently labeled splenocytes from adult NOD donors were transferred i.p. into infant and adult NOD recipients and then enumerated 24 h later in PLNs and SLNs by cytofluorimetry. Data are shown as the number of CFSE+ donor cells (¢) × 10-3 per 1 × 106 recipient LN cells in PLNs and SLNs of both recipients. Shown are data from seven to eight mice per age group. (B) Donor cell trafficking to PLNs, MLNs, and SLNs in recipients of different ages was determined by flow cytometry. The migration index is calculated as the ratio of donor cell density in PLNs to the donor cell density in MLNs (filled circles) or the ratio of donor cell density in SLNs to the donor cell density in MLNs (open circles). Data are representative of 3-15 mice. (C) Fluorescently labeled splenocytes from adult and infant NOD donors were transferred i.p. into adult and infant recipients and then enumerated 24 h later in recipient LNs by cytofluorimetry. The migration index is calculated as the ratio of donor cell density in PLNs to the donor cell density in SLNs.
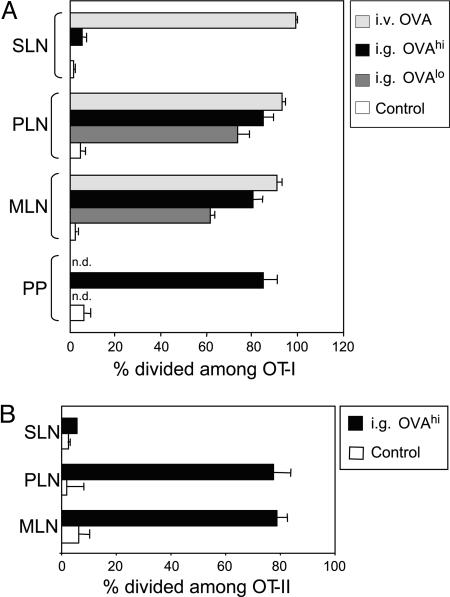
The peritoneum is the site of developmentally primitive lymphoid organs, like the omentum, and a niche for innate-like immune system populations such as B-1 B cells. Although normally sterile, the peritoneum may be the first site under attack by enteric pathogens, commensals, and dietary contents once intestinal barrier function is breached. Given the close connection between the peritoneum and PLNs demonstrated above, we asked whether luminal contents of the gastrointestinal tract might also preferentially enter PLNs. To test for this possibility, we fed B6 mice with OVA protein and tested whether the antigen had been ferried to LNs by assessing the response of OVA-specific T cells (again measuring proliferation as dilution of fluorescent label in OT-I tg T cells). OT-I T cells divided in response to oral OVA in MLNs and Peyer's patches, as expected from previous reports (10), but was also evident to a similar extent in PLNs (Fig. 3A). In contrast, it was essentially absent in all other LNs tested (SLNs). With 10-fold lower doses of fed antigen, the extent of OVA presentation in PLNs was consistently equivalent to or slightly higher than in MLNs (Fig. 3A). Similar results were obtained with MHC class II-restricted, OVA-specific, OT-II tg T cells (17) (Fig. 3B). Therefore, PLNs can be considered a gut-associated lymphoid tissue (GALT) in their ability to present antigens from the gastrointestinal contents.
Fig. 3.
Gut antigens are presented to CD8+ and CD4+ T cells in PLNs. (A) Proliferation of transferred naive OT-I T cells (gated on CD8+Vα2+ cells) in SLNs, Peyer's patches (PPs), MLNs, and PLNs after intragastric administration of 60 mg of OVA (IG OVAhi) or 2 mg of OVA (IG OVAlo) or i.v. injection of 2 mg of OVA (IV OVA) into adult B6 mice as compared with controls. Proliferation of OT-I T cells was assessed 48 h after transfer by CFSE dilution in donor T cells. Each symbol represents an individual mouse. (B) Proliferation of transferred naive OT-II T cells (gated on CD4+Vα2+ cells) in MLNs and PLNs after intragastric administration of 60 mg of OVA (IG OVAhi) into adult B6 mice. Proliferation of OT-II T cells was assessed 48 h after transfer by CFSE dilution in donor T cells. Each symbol represents an individual mouse.
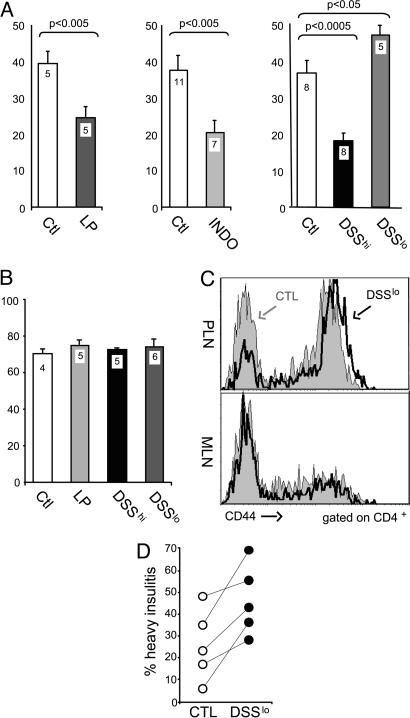
Given that enteric proteins are presented by antigen-presenting cells within PLNs, we hypothesized that perturbations occurring in the gut may also reach the PLNs and affect their ability to present endocrine self-antigens. For instance, strong T cell responses to gut antigens in the PLN might enhance bystander responses to pancreatic autoantigens; alternatively, enhanced activation of gut antigen-presenting cells by microbial stimuli might alter the phenotype of PLN antigen-presenting cells, eliciting more aggressive responses. To test this hypothesis, we examined the priming of diabetogenic T cells in NOD mice treated with several agents that induce diverse forms of intestinal stress or injury. INDO is a nonsteroidal antiinflammatory drug that reproducibly causes ulceration in the small intestine of mice (and humans) within 24 h of administration (19). LP diet also perturbs the small intestine by modifying the composition of the gut flora, and adaptation to the altered flora elicits histologically detectable changes in the ileal lining (21). DSS is a sulfated polysaccharide that disturbs permeability of the colonic mucosa, leading to epithelial injury and erosion in a dose- and time-dependent manner (18, 22). CFSE-labeled T cells from BDC2.5 mice were transferred into NOD mice that had been pretreated with LP diet, INDO, or DSS (treatments were stopped at or before transfer to avoid indirect effects on responder T cells). BDC2.5 T cells proliferated to a lesser extent in PLNs of mice on LP (47% inhibition on average) or INDO (40% inhibition) regimens, compared with littermate controls (Fig. 4A). The effect of DSS varied with the dose. At high doses, it reduced BDC2.5 T cell activation (54% inhibition); at low doses, DSS actually accentuated the proliferation (22% increase). This enhancement was specific to PLNs: there was no broadening of the response to pancreatic autoantigen in other locations, ruling out a systemic mitogenic impact on BDC2.5 T cells by DSS treatment (data not shown). Furthermore, treatment with these agents had no effect on the response elicited by mimotope peptide in SLNs (Fig. 4B), establishing that the impact of gut-perturbing agents on the PLN is highly specific. The effect of low doses of DSS also affected the activation of endogenous T cells in treated BDC2.5 mice: higher levels of the CD44 activation marker were observed in the PLN, but not in the MLN (Fig. 4C). Finally, we asked whether similar perturbations would also influence insulitis. Consistent with the results above, DSS enhanced the severity of insulitis in 4-week-old BDC2.5 mice (Fig. 4D).
Fig. 4.
Intestinal alterations influence the autoimmune response in PLNs. (A) Proliferation of transferred naive BDC2.5 T cells (gated on CD4+Vβ4+ cells) in PLNs of NOD mice on a LP or control (Ctl) diet or pretreated with 85 mg/kg INDO, 5% DSS (DSShi), or 2% DSS (DSSlo) as compared with control littermates (Ctl). T cell proliferation, assessed by CFSE dilution in BDC2.5 T cells, was reduced 47% by LP (P = 0.002), 40% by INDO (P = 0.004), and 54% by 5% DSS (P = 0.001) and was enhanced 22% by 2% DSS (P = 02). Data are shown as the percentage of divided BDC2.5 T cells among the donor CD4+ T cells. Values in columns depict number of mice per condition. (B) Proliferation of transferred naive BDC2.5 T cells (gated on CD4+Vβ4+ cells) in popliteal LNs of NOD mice on a LP diet or pretreated with 5% DSS (DSShi) or 2% DSS (DSSlo) after footpad immunization with BDC2.5 peptide mimic. Data are shown as the percentage of divided BDC2.5 T cells among the donor CD4+ T cells. Values in columns depict number of mice per condition. (C) Representative histograms of CD44 staining on donor BDC2.5 T cells in PLNs and MLNs of NOD mice after transfer into mice on 2% DSS or control (CTL) littermates. (D) Frequency of pancreatic islets with heavy leukocyte infiltration in BDC2.5/NOD mice treated with low-dose DSS. Each symbol represents an individual mouse. Data shown as the percentage of islets with heavy leukocyte infiltration among total islets. Each symbol represents an individual mouse.
Discussion
Thus, PLNs are at a very peculiar confluence. They sample self-antigens from the pancreas but also foreign antigens from the gastrointestinal tract and the peritoneum, and nonspecific perturbations of gut physiology have a direct and specific impact on the response of β cell-reactive T cells. This connection has strong conceptual implications for our understanding of pancreas-directed autoimmunity and its connection to environmental factors such as gut microbes and food antigens (8, 9, 11). In particular, the enhancement in diabetogenic T cell priming and insulitis severity by increased intestinal permeability and mild enteropathy provides a potential explanation for the special relationship between T1D and celiac disease. This inflammatory disorder of the small intestine is provoked by immune reactivity to gluten-associated proteins, which also have adjuvant activity (23). CD is very frequent in T1D patients, detectable in 30% of patients with HLA-DQ2 and 3-10% of diabetic children and adolescents (14, 24, 25). CD, but also T1D, can be ameliorated in human patients and in NOD mice by removing gluten-containing foods from the diet (9, 26). From the present results it follows that enteropathy, induced by gluten or other dietary factors, may provide inflammatory stimuli that make their way to and activate dendritic cells within PLNs. This interaction might increase an otherwise sluggish response to pancreatic autoantigens, by promoting the maturation or mobilization of CD11b+ dendritic cells.
Preferential homing to PLNs via peritoneal lymphatics was developmentally regulated and became fully established only after 10 days of age. Intriguingly, this is also the time frame during which pancreatic antigens are first presented to islet-reactive T cells in PLNs. Of course, this parallel might simply be a coincidence, but it could also be that lymphatic connectivity of PLNs may not occur before this time. We have shown that the initiation of pancreatic antigen presentation in PLNs is linked to a programmed increase in β cell apoptosis around this time (27); it was delayed by apoptosis blockers and accelerated by provoking β cell death (3). Thus, antigen availability and lymphatic connectivity might both contribute to the delayed presentation of pancreatic self in the neonatal period. Profound alterations take place in the gut around this transitional period, with marked changes in food intake and bacterial flora, and the epithelial barrier (28, 29). One might hypothesize that these events, relayed via immunological or metabolic cues, drive the establishment of lymphatic connections to PLNs as well as the increased supply of self-antigen.
In conclusion, the present study defines a fundamental link between the gastrointestinal tract and PLNs, providing a conduit for environmental agents to directly modify the immune response to pancreatic autoantigens.
Supplementary Material
Acknowledgments
We thank E. Hyatt and T. Lipatova for assistance with animal husbandry, M. Petruzzelli for expert instruction in gastric gavage, A. Pinkhasov and R. Bronson for help with the histology, and members of the Diabetes Group for their constructive input on this study. This work was supported by funds from the National Institutes of Health (Grant R01 DK59658) and by the Joslin Diabetes and Endocrinology Research Center core facilities (Grant P30 DK36836) and the cores of the Juvenile Diabetes Research Foundation Centers for Islet Transplantation and on Immunological Tolerance in Type-1 Diabetes at Harvard Medical School. S.J.T. received fellowship support from the Cancer Research Institute.
Author contributions: S.J.T. designed research; S.J.T., J.-W.L., and N.D.-S. performed research; S.J.T., D.M., and C.B. analyzed data; and S.J.T., D.M., and C.B. wrote the paper.
Conflict of interest statement: No conflicts declared.
Abbreviations: CFSE, 5,6-carboxy-succinimidyl-fluorescein-ester; DSS, dextran sulfate sodium; INDO, indomethacin; LN, lymph node; LP, low-protein; MLN, mesenteric LN; OVA, ovalbumin; PLN, pancreatic LN; SLN, s.c. LN; T1D, type 1 diabetes; tg, transgenic.
References
- 1.Atkinson, M. A. & Maclaren, N. K. (1994) N. Engl. J. Med. 331, 1428-1434. [DOI] [PubMed] [Google Scholar]
- 2.Hoglund, P., Mintern, J., Waltzinger, C., Heath, W., Benoist, C. & Mathis, D. (1999) J. Exp. Med. 189, 331-339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Turley, S., Poirot, L., Hattori, M., Benoist, C. & Mathis, D. (2003) J. Exp. Med. 198, 1527-1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gagnerault, M. C., Luan, J. J., Lotton, C. & Lepault, F. (2002) J. Exp. Med. 196, 369-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levisetti, M. G., Suri, A., Frederick, K. & Unanue, E. R. (2004) Diabetes 53, 3115-3119. [DOI] [PubMed] [Google Scholar]
- 6.Redondo, M. J., Rewers, M., Yu, L., Garg, S., Pilcher, C. C., Elliott, R. B. & Eisenbarth, G. S. (1999) Br. Med. J. 318, 698-702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herrath, M. G., Holz, A., Homann, D. & Oldstone, M. B. A. (1998) Semin. Immunol. 10, 87-100. [DOI] [PubMed] [Google Scholar]
- 8.Horwitz, M. S., Bradley, L. M., Harbertson, J., Krahl, T., Lee, J. & Sarvetnick, N. (1998) Nat. Med. 4, 781-785. [DOI] [PubMed] [Google Scholar]
- 9.Schmid, S., Koczwara, K., Schwinghammer, S., Lampasona, V., Ziegler, A. G. & Bonifacio, E. (2004) Clin. Immunol. 111, 108-118. [DOI] [PubMed] [Google Scholar]
- 10.Blanas, E., Carbone, F. R., Allison, J., Miller, J. F. & Heath, W. R. (1996) Science 274, 1707-1709. [DOI] [PubMed] [Google Scholar]
- 11.Norris, J. M., Barriga, K., Klingensmith, G., Hoffman, M., Eisenbarth, G. S., Erlich, H. A. & Rewers, M. (2003) J. Am. Med. Assoc. 290, 1713-1720. [DOI] [PubMed] [Google Scholar]
- 12.Ziegler, A. G., Schmid, S., Huber, D., Hummel, M. & Bonifacio, E. (2003) J. Am. Med. Assoc. 290, 1721-1728. [DOI] [PubMed] [Google Scholar]
- 13.Cronin, C. C. & Shanahan, F. (1997) Lancet 349, 1096-1097. [DOI] [PubMed] [Google Scholar]
- 14.Bao, F., Yu, L., Babu, S., Wang, T., Hoffenberg, E. J., Rewers, M. & Eisenbarth, G. S. (1999) J. Autoimmun. 13, 143-148. [DOI] [PubMed] [Google Scholar]
- 15.Katz, J. D., Wang, B., Haskins, K., Benoist, C. & Mathis, D. (1993) Cell 74, 1089-1100. [DOI] [PubMed] [Google Scholar]
- 16.Hogquist, K. A., Jameson, S. C., Heath, W. R., Howard, J. L., Bevan, M. J. & Carbone, F. R. (1994) Cell 76, 17-27. [DOI] [PubMed] [Google Scholar]
- 17.Barnden, M. J., Allison, J., Heath, W. R. & Carbone, F. R. (1998) Immunol. Cell Biol. 76, 34-40. [DOI] [PubMed] [Google Scholar]
- 18.Kitajima, S., Takuma, S. & Morimoto, M. (1999) Exp. Anim. 48, 137-143. [DOI] [PubMed] [Google Scholar]
- 19.Ettarh, R. R. & Carr, K. E. (1993) Scand. J. Gastroenterol. 28, 795-802. [DOI] [PubMed] [Google Scholar]
- 20.Luther, S. A. & Cyster, J. G. (2001) Nat. Immunol. 2, 102-107. [DOI] [PubMed] [Google Scholar]
- 21.Li, M., Specian, R. D., Berg, R. D. & Deitch, E. A. (1989) J. Parenter. Enteral. Nutr. 13, 572-578. [DOI] [PubMed] [Google Scholar]
- 22.Okayasu, I., Hatakeyama, S., Yamada, M., Ohkusa, T., Inagaki, Y. & Nakaya, R. (1990) Gastroenterology 98, 694-702. [DOI] [PubMed] [Google Scholar]
- 23.Sollid, L. M. (2002) Nat. Rev. Immunol. 2, 647-655. [DOI] [PubMed] [Google Scholar]
- 24.Jaeger, C., Hatziagelaki, E., Petzoldt, R. & Bretzel, R. G. (2001) Diabetes Care 24, 27-32. [DOI] [PubMed] [Google Scholar]
- 25.Barker, J. M., Yu, J., Yu, L., Wang, J., Miao, D., Bao, F., Hoffenberg, E., Nelson, J. C., Gottlieb, P. A., Rewers, M., et al. (2005) Diabetes Care 28, 850-855. [DOI] [PubMed] [Google Scholar]
- 26.Ventura, A., Neri, E., Ughi, C., Leopaldi, A., Citta, A. & Not, T. (2000) J. Pediatr. 137, 263-265. [DOI] [PubMed] [Google Scholar]
- 27.Scaglia, L., Cahill, C. J., Finegood, D. T. & Bonner-Weir, S. (1997) Endocrinology 138, 1736-1741. [DOI] [PubMed] [Google Scholar]
- 28.Meddings, J. B., Jarand, J., Urbanski, S. J., Hardin, J. & Gall, D. G. (1999) Am. J. Physiol. 276, G951-G957. [DOI] [PubMed] [Google Scholar]
- 29.Graham, S., Courtois, P., Malaisse, W. J., Rozing, J., Scott, F. W. & Mowat, A. M. (2004) Gut 53, 1437-1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.