Abstract
Currently, diagnosis of Chagas' disease is based on serological methods, but due to the high occurrence of inconclusive results, more reliable methods are needed. The use of recombinant antigens for serodiagnosis of Chagas' disease is recommended in order to increase the sensitivity and specificity of the serological tests. The Trypanosoma cruzi complement regulatory protein (CRP) is a surface glycoprotein present on the trypomastigote forms of the parasite, and the recombinant CRP (rCRP) was cloned in a mammalian expression system and purified by affinity chromatography. The purified recombinant protein was used as an antigen in an enzyme-linked immunosorbent assay (rCRP ELISA) in order to verify its sensitivity and specificity compared with other established methods. In this evaluation, a panel of 184 serum samples distributed among chronic chagasic patients (n = 65), blood bank donors (n = 100), and patients infected with Leishmania spp. (n = 19) was used. The sensitivity and specificity of the rCRP ELISA were 100% when compared to conventional serology and complement-mediated lysis tests from these groups. When hemoculture and PCR tests were evaluated for diagnosis of chronic chagasic patients, using the rCRP ELISA as a reference test, the positivities were found to be 64.62 and 81.54%, respectively, showing a higher degree of sensitivity of the test. The data demonstrate that rCRP ELISA was able to discriminate between chronic chagasic patients and nonchagasic individuals, such as blood donors and patients with leishmaniasis. Thus, the rCRP is an excellent antigen for use in Chagas' disease diagnosis, due to the absence of false-negative or false-positive results.
Chagas' disease, first described by Carlos Chagas in 1909, remains a public health concern even after several efforts to reduce the incidence of cases, which affect 16 to 18 million people in Latin America (52). The etiological agent, Trypanosoma cruzi, is normally transmitted by infected triatomid bugs; however, cases associated with blood transfusion, organ transplant, and congenital infection have been described even in countries to which the disease is not endemic (7, 18, 45, 47).
The diagnosis of Chagas' disease can be made by the detection of the parasite by indirect parasitological methods (xenodiagnosis and hemoculture) or, more frequently, by detection of immunoglobulin G (IgG) antibodies against T. cruzi in sera from chronically chagasic patients. The parasitological methods show high specificity but are limited in terms of sensitivity due to the low parasitemia in the chronic phase. By such methods parasites are detectable in only 25 to 75% of individuals known to be infected by T. cruzi (15, 44). The use of conventional serologic tests (enzyme-linked immunosorbent assay (ELISA), indirect immunofluorescence, and indirect hemagglutination) is still the main method for the Chagas' disease diagnosis in the chronic phase. The epimastigote form of T. cruzi is generally used as a source of antigen because it is easily cultured in acellular medium and adequate amounts of antigen can be readily obtained (13). However, its antigenic fraction contains complex molecules, which favors the appearance of false-positive reactions and cross-reactivity with sera from patients bearing other parasites, especially Leishmania spp. and Trypanosoma rangeli (3, 16). In addition, this antigenic heterogeneity does not allow the differential diagnosis between the acute and chronic phases and also among the clinical manifestations (48). Indeed, discrepancies or inconclusive results, such as false-positive reactions caused by cross-reactivity with antibodies induced by other pathogens (mainly Leishmania) and false-negatives, may occur (35, 41, 42). For these reasons, the World Health Organization recommends using at least two of those serologic tests in parallel (52). The use of well-characterized antigens and their preparation under quality-control conditions have introduced a source variability in the final agent, and controversial results have been obtained with the reagents from whole or semipurified extracts of T. cruzi (12).
In recent years, development of recombinant DNA techniques permitted construction of different bacterial and eukaryotic gene expression systems which allow production of parasite antigens in large amounts and with a high level of purity. Several T. cruzi genes were isolated and cloned, and some recombinant antigens have been assayed and tested for diagnosis. The results, however, showed variable efficacy for the different tested antigens, and it was observed that some of them displayed better performance when used in combination than separately, producing results with sensitivities and specificities that reached up to 100% (1, 2, 17, 23, 24, 31, 32, 40, 49, 54).
More recently, use of the PCR was suggested as an alternative means of diagnosing Chagas' disease (6, 9, 51); however, variable levels of sensitivity in the detection of T. cruzi DNA have been reported by several laboratories. The initial data reported values from 96 to 100% compared with conventional serology (6, 51), but lower sensitivity levels were also observed (9, 22, 25) due to the extremely low parasitemia in the chronic phase of disease. The occurrence of these problems affecting sensitivity and specificity in the various tests cited above points to the necessity of developing more-reliable assays with well-defined antigen preparations for diagnosis (4).
The complement-mediated lysis (CoML) test detects the presence of protective antibodies during chronic infections with 100% sensitivity and specificity, but not in the uninfected, immunized host (26, 30). These antibodies, which induce lysis of T. cruzi in the presence of complement and are referred to as lytic antibodies, recognize antigens on the surfaces of living trypomastigotes and represent a class of antibodies distinct from those detected by conventional serologic tests (30). In addition, the CoML test may be the best indicator of an ongoing infection and an indication of treatment failure in chagasic patients (32). However, this method is not applicable for routine diagnosis in the clinical laboratory due to the manipulation of live infectious parasites, although the test is excellent for use as a reference (30). One of the antigenic targets of lytic antibodies was found to be a 160-kDa trypomastigote-specific surface glycoprotein (34, 36). This protein was later purified from the parasites and characterized as a complement regulatory protein (CRP) that functions to inhibit complement activation and lysis of the parasites (37). It was shown that antibodies to this protein could neutralize the complement inhibitory function, thus supporting complement-mediated lysis. Since the CRP is a target of complement lytic antibodies and the CoML test has been shown to be one of the best tests of ongoing infection, we sought to determine if a recombinant CRP-based ELISA could effectively replace the CoML and other diagnostic tests for detection of ongoing T. cruzi infection.
A full-length cDNA encoding the T. cruzi CRP was previously isolated (38) and stably transfected in mammalian cells, allowing its large-scale production and purification (M. Beucher, W. S. F. Meira, V. Zegarra, L. M. C. Galvão, E. Chiari, and K. A. Norris, submitted for publication). In this study, we report the standardization of an ELISA test using the recombinant CRP (rCRP) of T. cruzi and the evaluation of its efficacy in Chagas' disease diagnosis compared with well-established methods, such as hemoculture, PCR, complement-mediated lysis, and conventional serology.
MATERIALS AND METHODS
Human sera.
A total of 184 sera were used in this study. Sixty-five serum samples from chronic chagasic patients as determined by positive results in conventional serological tests, such as indirect immunofluorescence, indirect hemagglutination, and enzyme-linked immunosorbent assay, were collected at the “Ambulatório de Doença de Chagas, Hospital das Clínicas, Universidade Federal de Minas Gerais” (Belo Horizonte, Brazil). One hundred serum samples found to be negative by serological testing of blood donors from the blood bank of the “Clínica Romeu Ibrahim de Carvalho, Hospital Felício Rocho” (Belo Horizonte, Brazil) were used as negative controls. Serum samples of 10 patients with visceral leishmaniasis and 9 patients with cutaneous leishmaniasis were selected by positive direct parasitological examination to be tested in the rCRP-ELISA. This study was carried out with the consent of the participants and was approved by the Ethics Committee of the “Hospital das Clínicas/087/99, Universidade Federal de Minas Gerais, Belo Horizonte” (Minas Gerais, Brazil).
Protein extraction and purification.
For T. cruzi rCRP extraction, we used the clone pcDNA3-CRP-his-stop transfected in CHO-K1 cells (Beucher et al., submitted). A total of 3 × 108 transfected cell lines were centrifuged, the pellet was solubilized at 107 cells/ml with 2% Triton X-114 (Pierce Chemical Co., Rockford, Ill.) in Tris-buffered saline (50 mM Tris [pH 7.4], 150 mM NaCl), and the aqueous phase was collected after centrifugation and incubation at 37°C for 3 min. Protease inhibitors (leupeptin, aprotinin, and E-64; Sigma Chemical Co., St. Louis, Mo.) were added to buffers at a final concentration of 1 μg/ml each. Nickel-nitrilotriacetic acid affinity chromatography purification was performed using 4 ml of a ProBond Ni2+ resin washed with 30 ml of ddH2O and with 50 ml of binding buffer (20 mM NaH2PO4 [pH 7.8], 500 mM NaCl). The total protein extracted from the cell lysate (∼27 mg) was added to the resin in binding buffer and incubated for 1 h at room temperature with shaking. The resin was washed with 50 ml of washing buffer (20 mM NaH2PO4 [pH 6.0], 500 mM NaCl) containing 25 mM of imidazole, and the fractions were eluted with the same washing buffer but with a crescent concentration gradient of imidazole from 50 to 300 mM. The fractions were collected in 2-ml aliquots, and the purified recombinant protein was eluted at an imidazole concentration of 100 to 150 mM of in elution buffer (20 mM NaH2PO4 [pH 6.0], 500 mM NaCl). Protein fraction concentrations were determined using Bio-Rad (Hercules, Calif.) protein assay dye reagent concentrate. The T. cruzi rCRP was also purified from culture media after 3 ml of the culture medium was incubated with 200 μl of the ProBond Ni2+ resin for 2 h at room temperature with shaking. This mixture was centrifuged, and the pellet was suspended in 200 μl of a 1× sodium dodecyl sulfate-polyacrylamide gel electrophoresis sample buffer for protein elution. The supernatant was analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (Beucher et al., submitted).
ELISA.
An ELISA for antibody detection in serum was standardized basically as described previously (50). The assay was performed using flat-bottom 96-well microtiter plates for enzyme immunoassay-radioimmunoassay (Hybond-polystyrene plates; Costar, Cambridge, Mass.) coated with 50 μl of solution containing 2.5 μg of purified T. cruzi rCRP/ml in carbonate buffer (0.05 M, pH 9.6), incubating for 24 h at 4°C. After incubation, the plates were washed two times with phosphate-buffered saline-0.05% Tween 20 and blocked for 2 h at 37°C with 200 μl of 5% nonfat milk (Nestlé Brasil Ltda., Araçatuba, São Paulo, Brasil) in phosphate-buffered saline (block solution) per well. Fifty microliters of sera diluted 1:200 in block solution was added per well. Plates were incubated for 16 h at 4°C and washed three times as previously mentioned. Then, 50 μl of peroxidase-conjugated rabbit anti-human IgG (DAKO Corp., Carpinteria, Calif.) diluted 1:7,500 in block solution was added, and the plates were incubated for 1 h at 37°C. After four washes as already described, the reaction was developed by addition of 100 μl of 3,3′,5,5′-tetramethylbenzidine chromogen (DAKO Corp.) and incubation for 20 min at 37°C. The reaction was stopped with 100 μl of 1 M H2SO4, and the optical density at 450 nm (OD450) was determined in an ELISA reader (Benchmark Microplate Reader; Bio-Rad). The cutoff (CO) values were calculated for each plate as follows: CO = m + 2.SD, in which m is the absorbance average of the negative controls (n = 8) and SD is the standard deviation.
Complement-mediated lysis.
Lytic antibodies were detected by the CoML test (21, 26, 29) as follows: 6 × 106 to 7 × 106 trypomastigotes per ml were incubated with human serum as the complement source (HuC) at 37°C for 30 min and counted to assure total resistance to complement lysis in the absence of immune serum. Fifty microliters of a suspension of such trypomastigotes and 50 μl of the test serum, diluted two- and fourfold, were mixed in 5-ml plastic tubes and incubated at 37°C for 30 min and then placed on ice. Fifty microliters of HuC were added to 50 μl of each sample, and the parasites were counted in a hemocytometer. The tubes were incubated at 37°C for 45 min and replaced on ice, and the parasites were recounted. The lysis percentage was calculated as 100 − (number of parasites in sample at 45 min) × 100 ÷ (initial number of parasites in sample).
Hemoculture.
The technique of Chiari et al. (15) was used as follows: 30 ml of venous blood was collected into vacuum tubes containing sodium heparin and centrifuged for 10 min at 300 × g, at 4°C, to separate red blood cells from plasma. The plasma supernatant was centrifuged at 900 × g (4°C) for 20 min, and 3 ml of liver infusion tryptose medium (LIT) (10) was added to the pellet. The packed red blood cells were washed by the same centrifugation and resuspended in 6 ml of LIT, homogenized, and distributed into six plastic tubes (Falcon) each containing 3 ml of LIT. All tubes were maintained at 27 to 28°C, mixed gently twice weekly, and examined monthly for up to 120 days. Ten microliters of each preparation was examined microscopically under 22-mm2 coverslips at magnification ×150.
Preparation of DNA for PCR.
In order to obtain DNA for PCR, 10 ml of blood samples was collected simultaneously with hemoculture and added to 50-ml plastic tubes (Falcon) containing 10 ml of a mixture of 6 M guanidine-HCl and 0.2 M EDTA (Sigma), pH 8.0 (5). The samples were stored for 5 days at room temperature, boiled in water at 100°C for 15 min to shear the DNA molecules, and stored at 4°C until the time of use, when a 200-μl aliquot was collected from each sample and DNA extraction was performed (8, 22).
PCR conditions.
PCR amplification was performed in a total volume of 20 μl containing 0.1% Triton X-100, 10 mM Tris-HCl (pH 9.0), 75 mM KCl, 5 mM Cl2Mg3, 0.2 mM (each) dATP, dTTP, dGTP, and dCTP (Sigma), 1 μl of Taq DNA polymerase (Promega Corp.), 20 pmol of 121 (5′-AAATAATGTACGG(T/G)GAGATGCATGA-3′) and 122 (5′-GGTTCGATTGGGGTTGGTGTAATATA-3′) primers (Operon Technologies Inc.), and 2 μl of DNA of each sample (20, 22). The reaction mixture was overlaid with 30 μl of mineral oil (Sigma) to prevent evaporation and subjected to 35 cycles of amplification in an automatic thermocycler (MJ Research programmable thermal controller PTC-100) using plastic 0.5-ml microtubes. The temperature profile was as follows: 95°C for 1 min for denaturation (with a longer initial time of 5 min at 95°C), 65°C for 1 min for primer annealing, and 72°C for 1 min for extension, with a final incubation at 72°C for 10 min to extend the annealed primers. The PCR products were visualized by 6% polyacrylamide gel electrophoresis and silver stained (43). All DNA extraction steps and reaction mixtures used for PCR were monitored and compared with positive and negative controls. To avoid contamination, the reaction steps were performed in separate environments, using equipment and reagents destined exclusively to each stage. To test whether inhibition of the reaction was occurring, DNA from T. cruzi culture was obtained and used as a positive control. The sizes of the amplified bands were monitored using a 100-bp ladder molecular size marker (Promega).
Slot blot hybridization.
All samples were submitted to the hybridization technique in a slot blot (Hoefer Scientific Instruments) with a specific probe that hybridized internally to the 330-bp fragment amplified by PCR. This probe consisted of the oligonucleotide 5′-TGGTTTTGGGAGGGGCGTTCAAATTT-3′ labeled with alkaline phosphatase (46) and was synthesized by Life Technologies (Rockville, Md.). This technique confirms the specificity of the amplified product and/or increases the sensitivity of the protocol. When PCR alone is employed, 10 fg of parasite DNA may be detected in polyacrylamide gel, while hybridization permits detection of as little as 0.1 fg (22).
Data analysis.
The OD450s from different samples were plotted using computer graphics software (Prizm GraphPad, Version 2.01, San Diego, Calif.). The values of sensitivity and specificity were calculated according to the method of Camargo (11), and the degree of concordance between results was estimated by the Youden index (J) (53). The differences among positivity percentages were tested using the chi-square test with 1 degree of freedom by the statistics software package EPI-INFO/EPITABLE with the confidence interval at the level of 95%.
RESULTS
Development of the rCRP ELISA.
T. cruzi rCRP was produced in transfected cell lines and purified by affinity chromatography on a nickel-nitrilotriacetic acid resin. A total of 783 μg of purified recombinant antigen was obtained after the three immunoreactive eluted fractions were combined. The recombinant antigen was specifically recognized by a Western blotting assay against chicken anti-CRP IgY antibody (data not shown). After rCRP ELISA standardization, optimal results were obtained when the assay was performed under the following conditions: microtiter plates were coated with 50 μl of the antigen at a concentration of 2.5 μg/ml per well, the primary antibody was used in a dilution of 1:200, and the second antibody was diluted at 1:7.500.
Sensitivity and specificity of rCRP ELISA.
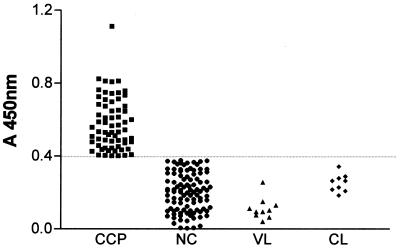
The sensitivity and specificity of rCRP ELISA in relation to the conventional serology tests were calculated using serum samples from individuals in the chronic phase of Chagas' disease and from blood bank donors as positive and negative samples, respectively. The analysis of sensitivity, specificity, and concordance (Youden) showed a result of 100%. A negative percentage of 100% was also observed when sera from patients with visceral and cutaneous leishmaniasis were tested (Table 1). The rCRP ELISA results in all sera groups are also demonstrated in a scatter plot (Fig. 1). The cutoff value was calculated as described previously (50) and is indicated by the dashed line in the graphic. The comparison among the CoML and rCRP ELISA results demonstrated a sensitivity of 98.46%, a specificity of 95.0%, and concordance of 92% with a confidence interval from 0.85 to 0.98 (Table 1), confirming the high concordance between the methods. The difference of these two methods was not considered statistically significant in the sample tested according to the chi-square values calculated. These data showed clearly that the recombinant antigen was positive for all samples, without any false-positive or false-negative reactions. No cross-reaction was observed with leishmaniasis sera samples tested.
TABLE 1.
Sensitivity, specificity, and positive percentage comparison between rCRP ELISA, conventional serology, CoML, hemoculture, and PCR tests with sera obtained from individuals infected with T. cruzi or Leishmania spp. and blood donorsa
| Sera group (n)b | Result (%)
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| rCRP ELISA
|
Conv. serology
|
CoML
|
Hemoculture
|
PCR
|
||||||
| Pos. | Neg. | Pos. | Neg. | Pos. | Neg. | Pos. | Neg. | Pos. | Neg. | |
| CCP (65) | 100 | 0 | 100 | 0 | 98.46 | 1.54 | 64.62 | 33.38 | 81.54 | 18.46 |
| NC (100) | 0 | 100 | 0 | 100 | 5.0 | 95.0 | ND | ND | ND | ND |
| Leish (19) | 0 | 100 | ND | ND | ND | ND | ND | ND | ND | ND |
CCP, chronic chagasic patients; NC, nonchagasic individuals (blood donors); Leish, patients infected with Leishamania spp.; ND, not done; Conv., conventional; Pos., positive; Neg., negative.
n, no. of serum samples.
FIG. 1.
Distribution of OD450s for different groups of individuals. CCP, chronic chagasic patients; NC, nonchagasic; VL, visceral leishmaniasis patients; CL, cutaneous leishmaniasis patients. The horizontal dashed line indicates the cutoff value calculated.
Comparison between rCRP ELISA and other methods.
The hemoculture test and the PCR followed by hybridization were performed with the sera samples from chronic chagasic patients for comparison with the rCRP ELISA results. This analysis was done using the difference between positive percentage values obtained by rCRP ELISA (100%) and by hemoculture (64,62%) (χ2 = 27.94; P = 0.00001) (Table 1). The PCR-hybridization results also presented a significantly lower positivity of 81.54% (χ2 = 13.22; P = 0.000277) compared with the rCRP ELISA. The statistical analysis chi-square and P values demonstrated that the difference between the results of the different methods has high significance with a confidence interval of 95%. These data showed that the rCRP ELISA gives greater sensitivity than hemoculture and PCR in the diagnosis of Chagas' disease.
DISCUSSION
Serological methods, based on the detection of antibodies against T. cruzi, are widely used for diagnosis of Chagas' disease. During the infection, the patients produce several antibodies against different T. cruzi antigens, and these antibodies show qualitative and quantitative differences. Among them, a small subset is considered specific for antigens from different developmental stages of the parasite. The majority of these antibodies usually show different degrees of nonspecific reaction with antigens from other closely related organisms, resulting in cross-reactions and consequently false-positive diagnosis (16, 33, 35). To overcome this problem, several antigens are being produced in different manners and evaluated for diagnosis of chagasic patients.
In the present report, we evaluated T cruzi rCRP, a trypomastigote-specific antigen, in the development of a sensitive and specific ELISA system for the diagnosis of Chagas' disease. The method was standardized to establish the optimal antigen concentration and dilution of antibodies. The assay was performed with 184 individual sera, distributed between chronic chagasic patients (n = 65), nonchagasic individuals (n = 100), and patients with leishmaniasis (n = 19). For the evaluation in the first two groups, we considered conventional serology as the reference test and compared these results with those obtained by the rCRP ELISA. This comparison showed a total concordance between the methods and allowed us to affirm that the new ELISA test shows sensitivity and specificity of 100%. When patients infected with Leishmania spp., considered negative samples, were tested by rCRP ELISA, no positive reaction was observed, confirming the high specificity of the method. The complement-mediated lysis was tested in the same group of patients and in the group of blood donors, resulting in a lower sensitivity and specificity than rCRP ELISA. This difference was not statistically significant for the number of samples analyzed. These data reinforce the strength of the rCRP ELISA as a Chagas' disease diagnostic tool.
The rCRP ELISA was considered a reference test to be compared with hemoculture and PCR methods. The hemoculture, which is a highly specific test, was positive for 64.62% of the patients compared with ELISA results, confirming a low sensitivity of this method as reported previously. The positivity of 81.54% verified for the PCR also confirmed preceding data. Our results reinforce that both methods, although reliable, cannot be applied in clinical laboratory testing or in blood bank screenings due to the high level of variable results. The sensitivity of these methods seems to reflect the different genetic constitution of circulating T. cruzi strains, which vary according to geographic origin (19). The presence of the parasite in the blood at a given time depends on the parasite's life cycle as well as on the immunological equilibrium between the parasite and the host (14). The analysis of these results leads us to conclude that this recombinant antigen is a strong candidate for use in diagnosis of Chagas' disease.
In addition to the inadequacies of the present methods for diagnosing chronic Chagas' disease, there is also a lack of reliable or applicable means to verify cure of patients after drug treatment, particularly in the chronic phase of the disease. The effectiveness of treatment with nitroimidazole derivatives and other drugs has been difficult to assess, since conventional serologic tests may remain positive for several years in some patients despite repeated negative direct parasite detection tests, such as hemoculture or xenodiagnosis (15, 21, 26, 27, 28, 39). This lack of a means of detection of ongoing infection has hampered the development of drugs that may be effective in treating the chronic phase of the disease. The CoML test has been proposed by Kettli and Brener as a means to detect parasite clearance posttreatment (30). This method has opened new perspectives regarding the evaluation of cure in chagasic patients and shows clear discordance among conventional serology and lytic antibodies in both human patients and experimental animals, the former being classified as “dissociated” (35). It was previously shown that the T. cruzi CRP is a principal effector molecule restricting complement activation and lysis of the parasites and also elicits the production of lytic antibodies in humans (37). The results presented here suggest that the T. cruzi rCRP may be a candidate antigen for monitoring cure in chagasic patients after treatment.
Acknowledgments
This work was supported by CNPq (Conselho Nacional de Pesquisa e Desenvolvimento Tecnológico), CAPES (Coordenação de Aperfeiçoamento de Pessoal do Ensino Superior), and FAPEMIG (Fundação de Amparo à Pesquisa do Estado de Minas Gerais) and the National Institutes of Health, Public Health Service Grant AI-32719.
We thank Orlando Carlos Magno and Afonso Da Costa Viana for technical assistance, Renato Saphler Avelar for the blood harvest of the patients, Ana Lúcia Teles Rabelo and Zélia Maria Profeta Da Luz, who kindly provided the serum samples from patients with leishmaniasis, and Patrícia Carvalho Carneiro de Mendonça for the serum samples from blood bank donors.
REFERENCES
- 1.Affranchino, J. L., C. F. Ilbanez, A. O. Luquetti, A. Rassi, M. B. Reyes, R. A. Macina, L. Aslund, U. Pettersson, and A. C. Frash. 1989. Identification of Trypanosoma cruzi antigen that is shed during the acute phase of Chagas' disease. Mol. Biochem. Parasitol. 34:221-228. [DOI] [PubMed] [Google Scholar]
- 2.Almeida, E., M. A. Krieger, M. R. Carvalho, W. Oelemann, and S. Goldenberg. 1990. Use of recombinant antigens for the diagnosis of Chagas' disease and blood bank screening. Mem. Inst. Oswaldo Cruz 85:513-517. [DOI] [PubMed] [Google Scholar]
- 3.Araújo, F. G. 1986. Analysis of Trypanosoma cruzi antigens bound by specific antibodies and by antibodies to related tripanosomatids. Infect. Immun. 53:179-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Araújo, F. G. 1992. Perspectives for confirmatory tests, p. 219-223. In S. Wendel, Z. Brener, M. E. Camargo, and A. Rassi (ed.), Chagas' disease (American trypanosomiasis): its impact on transfusion and clinical medicine. Sociedade Brasileira de Hematologia e Hemoterapia, São Paulo, Brazil.
- 5.Ávila, H. A., A. M. Gonçalves, N. S. Nehme, C. M. Morel, and L. Simpson. 1990. Schizodeme analysis of Trypanosoma cruzi stocks from South and Central America by analysis of PCR amplified minicircle variable region sequences. Mol. Biochem. Parasitol. 42:175-188. [DOI] [PubMed] [Google Scholar]
- 6.Ávila, H., J. Borges-Pereira, O. Thiemann, E. de Paiva, W. Degrave, C. M. Morel, and L. Simpson. 1993. Detection of Trypanosoma cruzi in blood specimens of chronic chagasic patients by polymerase chain reaction amplification of kinetoplast minicircle DNA: comparison with serology and xenodiagnosis. J. Clin. Microbiol. 31:2421-2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bittencourt, A. L. 1976. Congenital Chagas disease. Am. J. Dis. Child. 130:97-103. [DOI] [PubMed] [Google Scholar]
- 8.Britto, C., M. A. Cardoso, P. Wincker, and C. M. Morel. 1993. A simple protocol for the physical cleavage of Trypanosoma cruzi kinetoplast DNA present in blood samples and its use in polymerase chain reaction (PCR)-based diagnosis of chronic Chagas' disease. Mem. Inst. Oswaldo Cruz 88:171-172. [DOI] [PubMed] [Google Scholar]
- 9.Britto, C., M. A. Cardoso, C. M. M. Vanni, A. Hasslocher-Moreno, S. Xavier, W. Oeleman, A. Santoro, C. Pimimez, C. M. Morel, and P. Wincker. 1995. Polymerase chain reaction detection of Trypanosoma cruzi in blood samples as a tool for diagnosis and treatment evolution. Parasitology 110:241-247. [DOI] [PubMed] [Google Scholar]
- 10.Camargo, E. P. 1964. Growth and differentiation in Trypanosoma cruzi. Origin of metacyclic trypanosomes in liquid media. Rev. Inst. Med. Trop. São Paulo 6:93-100. [PubMed] [Google Scholar]
- 11.Camargo, M. E. 1992. An appraisal of Chagas' disease serodiagnosis, p. 165-168. In S. Wendel, Z. Brener, M. E. Camargo, and A. Rassi. (ed.), Chagas' disease (American Trypanosomiasis): its impact on transfusion and clinical medicine. Sociedade Brasileira de Hematologia e Hemoterapia, São Paulo, Brazil.
- 12.Camargo, M. E., E. L. Segura, I. G. Kagan, J. M. Souza, J. R. Carvalheiro, J. F. Yanovsky, and M. C. Guimaraes. 1986. Three years of collaboration on the standardization of Chagas' disease serodiagnosis in the Americas: an appraisal. Bull. Pan. Am. Health Organ. 20:233-244. [PubMed] [Google Scholar]
- 13.Camargo, M. E., and V. Amato-Neto. 1974. Anti-Trypanosoma cruzi IgM antibodies as serological evidence in recent of recent infection. Rev. Inst. Med. Trop. São Paulo 16:200-202. [PubMed] [Google Scholar]
- 14.Castro, C., and A. Prata. 2000. Absence of both circadian rhythm and Trypanosoma cruzi periodicity with xenodiagnosis in chronic chagasic individuals. Rev. Soc. Bras. Med. Trop. 33:427-430. [DOI] [PubMed] [Google Scholar]
- 15.Chiari, E., J. C. Dias, M. Lana, and C. A. Cifiari. 1989. Hemocultures for the parasitological diagnosis of human chronic Chagas' disease. Rev. Soc. Bras. Med. Trop. 22:19-23. [DOI] [PubMed] [Google Scholar]
- 16.Chiller, T. M. M. A., and Z. Z. Samudio. 1990. IgG antibody reactivity with Trypanosoma cruzi and Leishmania antigens in sera of patients with Chagas' disease and leishmaniasis. Am. J. Trop. Med. Hyg. 43:650-656. [DOI] [PubMed] [Google Scholar]
- 17.Cotrim, P. C., G. P. Paranhos, R. A. Mortara, J. Wanderley, A. Rassi, M. E. Camargo, and J. Franco da Silveira. 1990. Expression in Escherichia coli of a dominant immunogen of Trypanosoma cruzi recognized by human chagasic sera. J. Clin. Microbiol. 28:519-524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coura, J. R., E. S. Nogueira, and J. R. Silva. 1966. Índices de transmissão da doença de Chagas por transfusão de sangue de doadores na fase crônica da doença. O Hosp. 69:115-122. [PubMed] [Google Scholar]
- 19.Coura, J. R., L. L. De Abreu, L. E. Dubois, F. D. Lima, E. De Arruda Junior, H. P. Willcox, N. Anunziato, and W. Petana. 1984. Morbidity of Chagas' disease. II. Sectional studies in 4 field areas in Brazil. Mem. Inst. Oswaldo Cruz 79:101-124. [DOI] [PubMed] [Google Scholar]
- 20.Degrave, W., S. P. Fragoso, C. Britto, H. Van-Heuverswyn, G. Z. Kidane, M. A. Cardoso, R. U. Mueller, L. Simpson, and C. M. Morel. 1988. Peculiar sequence organization of kinetoplast DNA minicircles from Trypanosoma cruzi. Mol. Biochem. Parasitol. 27:63-70. [DOI] [PubMed] [Google Scholar]
- 21.Galvão, L. M. C., R. M. B. Nunes, J. R. Cançado, Z. Brener, and A. U. Krettli. 1993. Lytic antibody titre as a means of assessing cure after treatment of Chagas disease: a 10 years follow-up study. Trans. R. Soc. Trop. Med. Hyg. 87:220-223. [DOI] [PubMed] [Google Scholar]
- 22.Gomes, M. L., A. M. Macedo, A. R. Vago, S. D. J. Pena, L. M. C. Galvão, and E. Chiari. 1998. Trypanosoma cruzi: optimization of polymerase chain reaction for detection in human blood. Exp. Parasitol. 88:28-33. [DOI] [PubMed] [Google Scholar]
- 23.Gomes, Y. M., V. R. A. Pereira, M. Nakazawa, D. S. Rosa, M. N. D. S. Barros, A. G. P. Ferreira, E. D. Silva, S. F. Y. Ogatta, M. A. Krieger, and S. Goldenberg. 2001. Serodiagnosis of chronic Chagas infection by using EIE-Recombinant-Chagas-Biomanguinhos kit. Mem. Inst. Oswaldo Cruz 96:497-501. [DOI] [PubMed] [Google Scholar]
- 24.Guevara, A. G., A. Taibi, J. Alava, R. H. Guderian, and A. Ouaissi. 1995. Use of a recombinant Trypanosoma cruzi protein antigen to monitor cure of Chagas' disease. Trans. R. Soc. Med. Hyg. 89:447-448. [DOI] [PubMed] [Google Scholar]
- 25.Junqueira, A. C. V., E. Chiari, and P. Wincker. 1996. Comparison of the polymerase chain reaction with two classical parasitological methods for the diagnosis of Chagas' disease in an endemic region of north-eastern Brazil. Trans. R. Soc. Trop. Med. Hyg. 90:129-132. [DOI] [PubMed] [Google Scholar]
- 26.Krettli, A. U. 1984. Protective antibodies in Trypanosoma cruzi infections: detection, functional activity and possible mechanisms of trypomastigote killing in vivo and in vitro. Mem. Inst. Oswaldo Cruz 79:59-65.6748942 [Google Scholar]
- 27.Krettli, A. U., J. R. Cançado, and Z. Brener. 1982. Effect of specific chemotherapy on levels of lytic antibodies in Chagas' disease. Trans. R. Soc. Trop. Med. Hyg. 76:334-340. [DOI] [PubMed] [Google Scholar]
- 28.Krettli, A. U., J. R. Cançado, and Z. Brener. 1984. Criterion of cure of human Chagas' disease after specific chemotherapy: recent advances. Mem. Inst. Oswaldo Cruz. 79:157-164. [Google Scholar]
- 29.Krettli, A. U., P. Weisz-Carrington, and Z. Brener. 1979. Membrane-bound antibodies to bloodstream Trypanosoma cruzi in mice: strain differences in susceptibility to complement-mediated lysis. Clin. Exp. Immunol. 37:416-423. [PMC free article] [PubMed] [Google Scholar]
- 30.Krettli, A. U., and Z. Brener. 1982. Resistance against Trypanosoma cruzi associated to anti-living trypomastigotes antibodies. J. Immunol. 128:2009-2012. [PubMed] [Google Scholar]
- 31.Krieger, M. A., E. Almeida, W. Oelemann, J. J. Lafaile, J. B. Pereira, H. Krieger, M. R. Carvalho, and S. Goldenberg. 1992. Use of recombinant antigens for the accurate immunodiagnosis of Chagas' disease. Am. J. Trop. Med. Hyg. 46:427-434. [DOI] [PubMed] [Google Scholar]
- 32.Levin, M. J., E. A. Mesti, R. Benarous, G. Levitus, A. Schijman, P. Levy-Yeyati, A. Ruiz, A. Kahan, M. B. Rosembaum, H. N. Torres, and E. L. Segura. 1989. Identification of major Trypanosoma cruzi antigenic determinants in chronic Chagas' heart disease. Am. J. Trop. Med. Hyg. 41:530-539. [DOI] [PubMed] [Google Scholar]
- 33.Malchiodi, E. L., M. G. Chiaramonte, N. J. Taranto, N. W. Zwirner, and R. A. Margni. 1994. Cross-reactivity studies and differential serodiagnosis of human infections caused by Trypanosoma cruzi and Leishmania spp.; use of immunoblotting and ELISA with a purified antigen (Ag163B6). Clin. Exp. Immunol. 97:417-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martins, M. S., L. Hudsin, A. U. Krettli, J. R. Cançado, and Z. Brener. 1985. Human and mouse sera recognize the same polypeptide associated with immunological resistance to Trypanosoma cruzi infection. Clin. Exp. Immunol. 61:343-345. [PMC free article] [PubMed] [Google Scholar]
- 35.Mendes, R. P., S. Hoshino-Shimizu, A. M. M. Silva, I. Mota, R. A. G. Hereida, A. O. Luquetti, and P. G. Leser. 1997. Serological diagnosis of Chagas' disease: a potential confirmatory assay using preserved protein antigens of Trypanosoma cruzi. J. Clin. Microbiol. 35:1829-1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Norris, K. A., B. Bradt, N. Cooper, and M. So. 1991. Characterization of a Trypanosoma cruzi C3 binding protein with functional and genetic similarities to the complement regulatory protein, decay accelerating factor. J. Immunol. 147:2240-2247. [PubMed] [Google Scholar]
- 37.Norris, K. A., G. Harth, and M. So. 1989. Purification of a Trypanosoma cruzi membrane glycoprotein which elicits lytic antibodies. Infect. Immun. 57:2372-2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Norris, K. A., J. E. Schrimpf, and M. J. Szabo. 1997. Identification of the gene family encoding the 160-kilodalton Trypanosoma cruzi complement regulatory protein. Infect. Immun. 65:349-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Norris, K. A., L. M. C. Galvão, J. E. Schrimpf, J. R. Cançado, and A. U. Krettli. 1994. Humoral immune response to the Trypanosoma cruzi complement regulatory protein as an indicator of parasitologic clearance in human Chagas' disease. Infect. Immun. 62:4072-4074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Paranhos, G. S., P. C. Cotrim, R. A. Mortara, A. Rassi, R. Corral, H. L. Freilij, S. Grinstein, J. Wanderley, M. E. Camargo, and J. Franco da Silveira. 1990. Trypanosoma cruzi: cloning and expression of an antigen recognized by acute and chronic human chagasic sera. Exp. Parasitol. 71:284-293. [DOI] [PubMed] [Google Scholar]
- 41.Passos, V. M. A.; A. C. Volpini, E. M. Braga, P. A. F. Lacerda, A. Ouaissi, M. V. C. Lima-Martins, and A. U. Krettli. 1997. Differential serodiagnosis of human infections caused by Trypanosoma cruzi and Leishmania spp. Using ELISA with a recombinant antigen (rTc 24). Mem. Inst. Oswaldo Cruz 92:791-793. [DOI] [PubMed] [Google Scholar]
- 42.Salles, N. A., E. C. Sabino, M. G. Cliquet, J. Eluf-Neto, A. Mayer, C. Almeida-Neto, M. C. Mendonça, P. Dorliach-Llacer, D. F. Chamone, and A. Saez-Alquézar. 1996. Risk of exposure to Chagas' disease among seroreactive Brazilian blood donors. Transfusion 36:969-973. [DOI] [PubMed] [Google Scholar]
- 43.Santos, F. R., S. D. J. Pena, and J. T. Epplen. 1993. Genetic and population study of a Y-linked tetranucleotide repeat DNA polymorphism with a simple non-isotopic technique. Hum. Genet. 90:655-656. [DOI] [PubMed] [Google Scholar]
- 44.Schenome, H., E. Alfaro, H. Reyes, and E. Taucher. 1968. Valor de xenodiagnóstico en la infección chagásica crónica. Bol. Chil. Parasitol. 23:149-154. [PubMed] [Google Scholar]
- 45.Shmunis, G. A. 1985. Chagas' disease and blood transfusion, p. 127-145. In R. V. Dodd and L. F. Barker (ed.), Infection, immunity and blood transfusion. Alan R. Liss, Inc., New York, N.Y.
- 46.Sturm, N. R., W. Degrave, C. M. Morel, and L. Simpson. 1989. Sensitive detection and schizodeme classification of Trypanosoma cruzi cells by amplification of kinetoplast minicircle DNA sequences: use in diagnosis of Chagas' disease. Mol. Biochem. Parasitol. 33:205-214. [DOI] [PubMed] [Google Scholar]
- 47.Szarfman, A., J. F. Yanovsky, and M. Traverso. 1968. Primer caso de Chagas congénito diagnosticado en vida en el Hospital de Niños de la Municipalidad de Buenos Aires. Arch. Argent. Pediatr. 66:164-166. [PubMed] [Google Scholar]
- 48.Umezawa, E. S., and J. Franco da Silveira. 1999. Serological diagnosis of Chagas disease with purified and defined Trypanosoma cruzi antigens. Mem. Inst. Oswaldo Cruz 94:285-288. [DOI] [PubMed] [Google Scholar]
- 49.Umezawa, E. S., S. F. Bastos, M. E. Camargo, L. M. Yamauchi, M. R. Santos, A. Gonzalez, B. Zingales, M. J. Levin, O. Sousa, R. Rangel-Aldao, and J. Franco da Silveira. 1999. Evaluation of recombinant antigens for serodiagnosis of Chagas' disease in South and Central America. J. Clin. Microbiol. 37:1554-1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Voller, A., C. Draper, D. E. Bidwell, and A. Bartlett. 1975. A microplate enzyme-linked immunosorbent assay (ELISA) for Chagas' disease. Lancet i:426-429. [DOI] [PubMed]
- 51.Wincker, P., C. Britto, J. Borges-Pereira, M. A. Cardoso, W. Oeleman, and C. M. Morel. 1994. Use of a simplified polymerase chain reaction procedure to detect Trypanosoma cruzi in blood samples from chronic chagasic patients in a rural endemic area. Am. J. Trop. Med. Hyg. 51:771-777. [DOI] [PubMed] [Google Scholar]
- 52.World Health Organization. 1991. Control of Chagas' disease. Report of a W. H. O. expert committee. WHO Tech. Rep. Ser. 811:1-95. [PubMed] [Google Scholar]
- 53.Youden, W. J. 1950. Index for rating diagnostic tests. Cancer 3:32.. [DOI] [PubMed] [Google Scholar]
- 54.Zingales, B., A. Gruber, C. B. Ramalho, E. S. Umezawa, and W. Colli. 1990. Use of two recombinant proteins of Trypanosoma cruzi in the serological diagnosis of Chagas' disease. Mem. Inst. Oswaldo Cruz 85:519-522. [DOI] [PubMed] [Google Scholar]