Abstract
Sera (n = 781) from four African countries were used to determine the prevalence of herpes simplex virus type 2 (HSV-2) antibodies by using the HerpeSelect HSV-2 enzyme-linked immunosorbent assay (ELISA; Focus Technologies) and Western blotting (WB). Also, an HSV inhibition assay was developed to evaluate the discordant sample results between HerpesSelect and WB. The seroprevalence of HSV-2 ranged from 17% in the South African panel to nearly 70% in panels from Kenya, Uganda, and Zimbabwe. Overall, HerpeSelect was 100% sensitive and 88% specific compared to WB and 100% sensitive and 96% specific compared to the inhibition assay. There was 100% concordance among all three assays for samples from South Africa and Zimbabwe. The discordant results occurred in samples from Kenya and Uganda.
The detection and differentiation of type-specific antibodies to herpes simplex virus type 1 (HSV-1) and HSV-2 has been limited to reference laboratories due to the limited laboratory tools for HSV type-specific antibody testing. Tests such as the monoclonal inhibition assay (14) and Western blotting (WB) (4, 6, 8) are tedious and require a high level of expertise to perform. It is now possible to take advantage of the immunological distinction of the glycoproteins gG1 and gG2 of HSV-1 and HSV-2, respectively, to differentiate type-specific HSV antibodies in an ELISA and immunoblot format. Since both gG1 and gG2 proteins are highly conserved in HSV, gG-based serology tests allow for the detection of type-specific antibody in individuals infected with HSV (1, 2, 7, 10, 12, 13). The development of gG-based serologic tests allows for expanded seroprevalence and natural history studies relating to HSV and genital herpes by facilitating the detection of HSV type-specific antibodies in routine laboratory settings worldwide.
Seroprevalence studies have been initiated worldwide to support potential vaccine trials, aid in antiviral treatment, monitor sexually transmitted disease (STD) trends, and assess the risk of human immunodeficiency virus (HIV) transmission in the presence of STD. To date, various studies have shown a high degree of concordance in the United States and Europe between gG-based serologic assays and “gold standard” assays such as the HSV WB assay performed at the University of Washington (UW) (3, 5, 11). Recently, an HSV seroprevalence study conducted in Africa raised concern that the recombinant gG2 (rgG2) ELISA may give false-positive results in certain African sera (E. Van Dyck, A. Buvé, D. Brown, and M. Loga, Abstr. 14th ISSTDR/17th IUSTI, Berlin, Germany, abstr. T079, 2001). The current study is the largest to date to evaluate the presence of HSV antibody using a rgG2 ELISA and WB from various geographic locations in Africa. It appears that certain samples present interpretation difficulties regardless of the assay method used to detect HSV type-specific antibodies. In order to evaluate samples giving discordant results with ELISA and WB, an HSV-2 inhibition assay was developed. The assay is based on the ability of native gG2 present in cell culture lysates to inhibit the binding of gG2-specific antibodies to rgG2. The inhibition assay, based on differential absorption of type-specific antibodies, allows the identification of sera yielding false-positive results. Although a high degree of concordance was found between rgG2 ELISA and WB in certain geographic locations, the discordant samples were limited to two countries. The inhibition assay allowed for a further characterization of the discordant sera.
MATERIALS AND METHODS
Serum panels. (i) Kenya.
Two panels were obtained from Kenya for a total of 235 sera. Kenya-A samples were HIV negative (n = 150), and Kenya-B samples were HIV positive (n = 85). All samples were collected from randomized women attending an outpatient clinic in Mombasa, Kenya, as part of a vitamin A study. The median age of the women was 26 years (range, 18 to 45 years). The sera were collected, frozen, and shipped to the University of Washington for the vitamin studies. The samples were subsequently used in this study and, therefore, went through a second freeze-thaw cycle.
(ii) Uganda-A.
Fifty-one random sera were collected from a central blood bank (Nakasero) in Kampala in 1989 for an HIV seroprevalence study of blood donors. The sera were processed, frozen, and shipped to the University of California, Davis, for HIV serology studies. The samples were thawed for testing, aliquots were obtained, and the samples were refrozen until used in the present study.
(iii) Uganda-B.
A total of 176 serum samples were obtained from HIV-negative women between the ages of 18 to 35 who were recruited from urban family planning clinics. After the serum samples were obtained, they were immediately frozen and remained frozen during shipment. The samples were thawed only once prior to testing for this study.
(iv) South Africa.
A total of 150 sera were collected from Cape Town, Port Elizabeth, George, and Bloemfontein, South Africa, and from Namibia. The random samples were collected from healthy, primarily middle-income individuals for HIV screening. The sera were processed, frozen, and shipped directly to the California testing laboratory for this study. The samples were not thawed until immediately prior to testing.
(v) Zimbabwe.
A total of 174 sera were collected from healthy, HIV-negative women aged 18 to 35 attending an STD clinic in Harare as part of an HIV seroprevalence study. The sera were collected, aliquoted, and frozen. The frozen samples were shipped to the California testing laboratory for this study.
rgG2 HSV-2 ELISA.
Focus Technologies' HSV-2 HerpeSelect ELISA was used to screen all sera for the presence of HSV-2 antibody. The rgG2 antigen used in the assay is a column purified, baculovirus-derived, truncated gG2 protein propagated in Sf9 cells. All samples were run per the package insert that calls for a 1:101 dilution of serum, a 1-h serum incubation step, and a 30-min conjugate incubation step. An index value was obtained for each sample based on the absorbance of the patient sample divided by the absorbance of cutoff calibrator supplied by the HerpeSelect kit. An index value of <0.9 was negative, values from 0.9 to 1.1 were considered equivocal, and a value of >1.1 was positive.
HSV UW-WB.
The UW-WB has been previously described (4). Briefly, the WB banding pattern of sera against separate HSV-1 and HSV-2 strips was compared and evaluated. If necessary, the WB was repeated for problematic samples after absorption with HSV-1 and HSV-2 soluble antigens. A sample was considered “atypical” if either a definitive gG band is not present but the overall pattern is consistent with a type-specific HSV antibody or a limited banding pattern is present and suggests either HSV-1 or HSV-2 antibody.
gG2/HSV-2 inhibition assay design.
HSV-1 (MacIntyre strain) and HSV-2 (MS strain) were used to infect Vero cells. When the Vero cell monolayer was nearly 100% infected but prior to significant cell death, the infected monolayers were harvested by mechanical disruption, washed once in phosphate-buffered saline, and suspended in 1% Triton X-100-1 mM phenylmethylsulfonyl fluoride-0.1% sodium dodecyl sulfate-50 mM Tris (pH 8.0). The HSV-1 and HSV-2 detergent lysates were used at a final concentration of ca. 1 mg/ml. The serum samples were first diluted 1:50 in the HerpeSelect ELISA dilution buffer and then diluted with an equal volume of either the HSV-1 or the HSV-2 lysate and incubated for 1 h at room temperature on an orbital shaker. The preincubated samples were then added to recombinant gG2 ELISA wells and incubated for 1 h at room temperature, and the conjugate and chromogen were subsequently added. The index value of the HSV-1 lysate well was determined, as were the index values of the HSV-2 lysate well. The percent inhibition due to the preincubation with HSV-2 lysate was determined by using the following formula: [1 − (index of HSV-2 lysate well/index of HSV-1 lysate well)] × 100. The HSV-1 lysate reaction controlled for any non-HSV reactivity present in any sample so that the percent inhibition detected with the HSV-2 lysate was due only to the absorption of gG2 reactive antibodies.
As noted below, a limited number of HSV-2 ELISA-positive samples were tested in the inhibition assay at a final dilution of 1:400 and tested with a 2× concentration of the lysates at both 1:100 and 1:400 final dilutions. All other parameters of the inhibition assay were unchanged.
RESULTS
HSV-2 seroprevalence as determined by ELISA and WB.
Table 1 summarizes, by country, the HSV-2 seroprevalence as determined by the two methods. Both the ELISA and WB methods detected the same positive samples in the Zimbabwe panel (females, HIV negative), in which 66% of the samples were positive for HSV-2. The South African panel (males and females, HIV status unknown) had the same HSV-2 seropositivity rate of 17% by both the ELISA and WB. Four South African samples that were HSV-2 positive by ELISA had “atypical” patterns for HSV-2 by the WB. The combined Kenyan panels (females: panel A, HIV negative; panel B, HIV positive) had 76 and 70% seroprevalence rates as determined by ELISA and WB, respectively, whereas the combined Uganda panels (males and females, panel A, HIV status unknown; panel B, females, HIV negative) had 78 versus 66% rates as determined by ELISA and WB. The HSV-1 seroprevalence rate as determined by WB was similar for the four countries: Kenya, 95%; South Africa, 89%; Uganda, 100%; and Zimbabwe, 95%.
TABLE 1.
HSV-2 seroprevalence as determined by HerpeSelect ELISA and WB
| Country (n) | No. of positive samples (%) as determined by:
|
|
|---|---|---|
| HerpeSelect ELISA | WB | |
| Kenya (235) | 179 (76) | 166 (72) |
| South Africa (150) | 26 (17) | 26 (17) |
| Uganda (227) | 177 (78) | 153 (67) |
| Zimbabwe (173) | 115 (66) | 115 (66) |
| All countries (785) | 497 (63) | 460 (59) |
The detailed comparison of ELISA and WB results for the six serum panels is given in Table 2. All 451 WB-positive samples were also ELISA positive; therefore, the rgG2 ELISA was 100% sensitive versus the WB. A total of 11 atypical WB samples (9 ELISA positive and 2 ELISA negative) were detected. The atypical WB samples are discussed in greater detail below. Five samples were equivocal with the ELISA, and all were WB negative. Of the 497 ELISA-positive samples, 451 (91%) were WB positive, 9 (2%) were WB atypical, and 37 (7%) were WB negative, with all of the 37 WB-negative, ELISA-positive samples coming from the Kenyan and Ugandan panels. No HSV-2 ELISA-WB discordant results were found with the samples from the Zimbabwe and South African panels; therefore, 100% concordance was found between the rgG2 ELISA and the WB for the 323 samples from these two countries.
TABLE 2.
Comparison of HSV-2 results determined by HerpeSelect ELISA and WB for each serum panela
| Serum panel | WB result | HerpeSelect ELISA result (no. of samples)
|
|||
|---|---|---|---|---|---|
| POS | NEG | EQ | Total | ||
| Kenya-A | POS | 85 | 85 | ||
| NEG | 12 | 51 | 1 | 64 | |
| ATYP | 1 | 1 | |||
| Total | 98 | 51 | 1 | 150 | |
| Kenya-B | POS | 80 | 80 | ||
| NEG | 1 | 1 | |||
| Total | 81 | 81 | |||
| South Africa | POS | 22 | 22 | ||
| NEG | 124 | 124 | |||
| ATYP | 4 | 4 | |||
| Total | 26 | 124 | 0 | 150 | |
| Uganda-A | POS | 40 | 40 | ||
| NEG | 6 | 2 | 1 | 9 | |
| ATYP | 2 | 2 | |||
| Total | 48 | 2 | 1 | 51 | |
| Uganda-B | POS | 109 | 109 | ||
| NEG | 18 | 42 | 3 | 63 | |
| ATYP | 2 | 2 | 4 | ||
| Total | 129 | 44 | 3 | 176 | |
| Zimbabwe | POS | 115 | 115 | ||
| NEG | 58 | 58 | |||
| Total | 115 | 58 | 0 | 173 | |
| Total | 497 | 279 | 5 | 781 | |
ATYP, atypical WB profile for HSV-2 antibody; POS, positive; NEG, negative; EQ, equivocal.
HSV-2 inhibition assay.
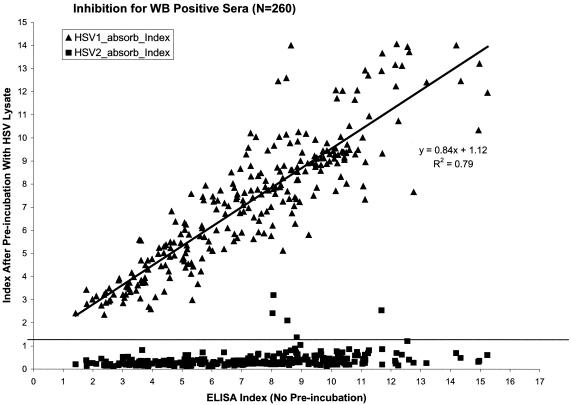
The inhibition assay was developed as a tool to investigate the cause of the ELISA and WB discordant samples from the Uganda and Kenya panels. Since the rgG2 ELISA uses a purified baculovirus-derived recombinant protein, the use of native HSV antigens to inhibit the binding of gG2 antibodies to the recombinant protein was investigated as a way to discriminate gG2-specific from gG2-nonspecific antibody binding. To determine the effectiveness of the differential blocking abilities of HSV-1 lysate and HSV-2 lysate, 260 HSV-2-positive samples that were concordant between the HerpeSelect and WB for their HSV-2 results were evaluated. The 260 samples were from the Kenya-A, South Africa, Uganda-A, and Zimbabwe panels. Figure 1 compares the HSV-2 ELISA index value obtained for each sample by using the standard HSV-2 ELISA assay with the index value obtained after each sample was absorbed with either HSV-1 lysate or HSV-2 lysate. Each serum sample has two datum points in Fig. 1: one datum point for the HSV-1 lysate preincubation and a second datum point for the HSV-2 lysate preincubation. As shown, all 260 samples had significant inhibition when preincubated with HSV-2 lysate, with 253 of the 260 samples having post-HSV-2 preincubation index values of <1.0. However, none of the 260 samples had a significant decrease in the index value when preincubated with HSV-1 lysate, and no samples had a post-HSV-1 preincubation index value of <2.3. Therefore, good discrimination was found between the blocking of HSV-2 antibody binding to rGg2 by HSV-2 lysate, whereas the HSV-1 lysate did not block HSV-2 antibody binding to rgG2.
FIG. 1.
HSV-2-positive sera (n = 260) as determined by both WB and ELISA after preincubation with either HSV-1 lysate (▴) or HSV-2 lysate (▪).
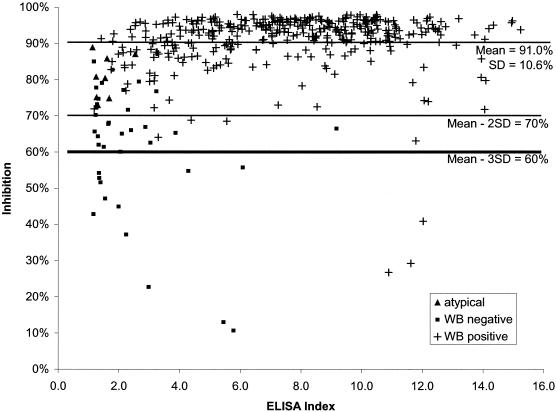
The differential reactivity obtained with HSV-1 and HSV-2 lysates was used to establish cutoff criteria for the inhibition assay. The percent inhibition due to preincubation with HSV-2 lysate was determined by using the formula given previously. The use of the HSV-1 lysate controlled for the loss of antibody binding to gG2 due to factors other than native gG2 present in the HSV-2 lysate. Therefore, the calculated percent inhibition was only due to the specific removal of gG2-reactive antibodies by the HSV-2 lysate. The mean percent inhibition obtained by using the 260 HSV-2-positive concordant samples was 91% with a standard deviation (SD) of 10.6%; therefore, the cutoff values at 2 SD and at 3 SD were 70 and 60%, respectively. Figure 2 demonstrates the percent inhibition obtained with 417 ELISA-positive samples. The 80 HSV-2 concordant samples from the Kenya-B panel were not submitted for the inhibition assay. At the 60% (3 SD) inhibition level, 402 of 417 (96.4%) ELISA-positive samples were confirmed to be positive. Of the 37 WB-negative, ELISA-positive samples, 25 (68%) were confirmed to be HSV-2 positive in the inhibition assay. The ELISA-WB-discrepant samples tended to have lower antibody levels; 21 of 37 (57%) had index values of between 1.1 and 2.0, 9 of 37 (24%) had index values of between 2.1 and 3.0, and 7 of 37samples (19%) had index values of >3.0. The 25 WB-negative samples that were determined to be HSV-2 positive by the inhibition assay had a mean inhibition of 70% compared to the ELISA-WB-concordant positive samples that had a mean inhibition of 92%. The two samples with the lowest percent inhibition were found to be highly reactive for HSV immunoglobulin M (IgM) as determined by a native-antigen ELISA (data not shown).
FIG. 2.
The HSV inhibition assay with the 497 samples determined to be positive by the gG2 ELISA. An inhibition result of ≥60% was considered positive for HSV-2 antibodies. WB positive (+), negative (▪), and atypical (▴) results are indicated.
At high HSV-2 index values (index of >8.0) nine samples were found to be both HSV-2 ELISA positive and WB positive but had inhibition values of <65% (range, 22 to 65%); five of the nine samples had inhibition levels below the 60% threshold. It was possible that the inability to reach maximum inhibition with these sera was due to antibody excess. To test for antibody excess, eight of the samples were retested by incubating with a 2× concentration of HSV lysate and by diluting the samples to 1:400 in addition to the standard 1:100 dilution. Both doubling the HSV lysate concentration and increasing the sample dilution to 1:400 had a similar effect on all eight samples tested. Five of the eight samples had increased inhibition values at the 1:400 dilution as with the 2× lysate concentration. Two of the five samples with increased inhibition had initial inhibition values of <60% that became >60% after the incubation parameters were changed. These two samples then matched the results obtained with WB and HerpeSelect. However, no enhanced inhibition was exhibited by three of the samples, and the inhibition values remained <60% for all of the inhibition assay parameters tested. Although all three samples were WB and ELISA positive for HSV-2, they were considered HSV-2 negative by the inhibition assay. These three samples were the only discordant samples between the WB and the inhibition assay at high (>6) index values of the HerpeSelect ELISA.
Eleven samples gave atypical HSV-2 WB profiles. Nine of the eleven samples were ELISA positive, and two were ELISA negative (Table 3). All nine ELISA positive samples gave high inhibition values ranging from 73 to 95% (mean, 79%). It appears that most of the atypical HSV-2 WB samples are positive for HSV-2 antibodies. The samples giving atypical WB profiles were similar to the WB-ELISA-discordant samples in that the majority were found to be only slightly positive in the ELISA and tended to have lower inhibition values than the concordant samples.
TABLE 3.
Index values of samples with atypical profiles as determined by WB
| Sample | ELISA index | % Inhibition |
|---|---|---|
| South Africa | 5.6 | 95 |
| South Africa | 2.5 | 87 |
| Uganda-B | 1.7 | 75 |
| South Africa | 1.6 | 86 |
| Uganda-B | 1.6 | 80 |
| Uganda-A | 1.3 | 73 |
| Uganda-B | 1.3 | 75 |
| Kenya-A | 1.2 | 81 |
| South Africa | 1.1 | 89 |
| Uganda-B | 0.8 | |
| Uganda-B | 0.8 |
Assay concordance.
The calculations to determine concordance, sensitivity, and specificity of the ELISA results were based on 765 samples with definitive test results. Eleven sera with atypical WB profiles were considered to be HSV-2 positive for these calculations; however, the five sera with equivocal results as determined by ELISA were excluded from the calculations. As shown in Table 2, there was a 95.2% overall concordance between the ELISA and WB results. The concordance, by country, was 88% for Uganda, 96% for Kenya, and 100% for both South Africa and Zimbabwe. Table 4 summarizes the sensitivity and specificity of the ELISA versus both WB and the inhibition assay. Using an ELISA index value of ≥1.1 as an indication of a positive result, the sensitivity was 99.6% versus both WB and the inhibition assay and the specificities were 88 and 95% versus the WB and the inhibition assay, respectively. Since the majority of the ELISA-WB-discordant samples from Uganda and Kenya had low ELISA index values, the sensitivities and specificities of the ELISA for all 765 samples were recalculated by using higher index values as the cutoffs for a positive result. Table 4 shows that incrementally increasing the index cutoff value increased the specificity with only a modest impact on the sensitivity. For example, with a 2.1 index value the sensitivity decreased to 96%, whereas the specificity increased to 95% for the entire study population. However, with an index of 2.1 for only the Kenya and Uganda samples, the sensitivity decreased to 97% but the specificity remained at 88%.
TABLE 4.
Sensitivity and specificity of the HerpeSelect ELISA versus WB and the inhibition assay
| Parameter | % Sensitivity or specificity versus:
|
||||||
|---|---|---|---|---|---|---|---|
| WB at indexa:
|
Inhibition assay at index
|
||||||
| 1.1 | 1.5 | 2.1 | 2.5 | 3.1 | 1.1 | 2.1 | |
| Sensitivity | 99.6 | 98.0 | 95.9 | 93.7 | 90.5 | 100 | 92.8 |
| Specificity | 88.0 | 93.0 | 94.9 | 96.5 | 97.8 | 94.9 | 96.9 |
Index refers to the ELISA index value above which the sample is considered positive.
DISCUSSION
The HSV-2 inhibition assay was developed to investigate the cause of the discordant results between the ELISA and WB methods. The HSV-2-infected cell culture lysate inhibited binding of ELISA-WB-concordant HSV-2-positive sera to the rgG2 in all 260 samples initially tested, whereas none of the 260 samples showed significant inhibition of antibody binding to rgG2 when HSV-1 lysate alone was added to the assay. The use of HSV-1 lysate in the inhibition assay controlled for any inhibitory effect that was contributed by Vero cell antigens, denaturing agents, ionic strength, HSV type-common antigens, and detergents present in either the HSV-1 or HSV-2 lysates. Therefore, the only antigenic differences between the HSV-1 and HSV-2 lysates are due to the presence of HSV type-specific antigens. The mean percent inhibition of the 260 concordant samples was 92% (SD = 10.6%); therefore, >60% inhibition (mean, 3 SD) was considered significant. Any ELISA-positive sample with an inhibition value of <60% was considered a false-positive ELISA result. Based on the inhibition assay, 25 of 37 (67%) discrepant samples were positive for HSV-2 antibodies (false negative by WB), and 12 (33%) were negative for HSV-2 antibodies (false positive by ELISA). Only 3 of the 451 (0.7%) samples positive for HSV-2 by both the HerpeSelect and WB assays had inhibition values of <60%.
There was a high level of overall concordance between the ELISA and inhibition assay (98%), whereas the concordance between the ELISA and WB assays was slightly lower at 95%. All 37 discordant samples were ELISA positive and WB negative and were limited geographically to Kenya and Uganda; the majority of the discordant samples were weakly positive as determined by the ELISA. The factors causing discordant results between the ELISA and WB methods were beyond the scope of the present study; however, there are several avenues for future studies to determine the source of the discordance. The ability of the two methods to detect early HSV-2 acquisition may account for some of the discordant results. Recently, Ashley et al. found that the HerpeSelect HSV-2 ELISA and the atypical HSV WB profile detected seroconversion in HSV-2 primary infection sooner than did the classic WB profile (R. L. Ashley, E. Krantz, and A. Wald, unpublished data). In the present study, 9 of 11 WB samples with atypical profiles were both HerpeSelect ELISA and inhibition ELISA positive for HSV-2. Also, two samples from Kenya with high ELISA index values and negative WB had high levels of HSV IgM, indicating possible recent exposure to HSV. It is possible that at least a portion of the ELISA- and WB-discordant samples could be due the recent acquisition of HSV-2 and to low levels of IgG being present, especially since the present study's population included young adult women from areas of high HSV-2 acquisition.
Another possible explanation may be the presence of antibodies to a non-HSV-2 virus that react to the rgG2. It is unlikely that this reactivity would be due to HSV-1 since all of the serum panels in the present study had a high incidence of HSV-1 antibody (88 to 100%). Also, the use of HSV-1 lysate in the inhibition assay as a baseline control for each sample tested would indicate that the antibody reactivity is probably limited to the reactivity to the gG2 protein. Few HSV-2 isolates are available from Africa, and none were collected in the present study to determine whether HSV-2 antigenic difference exist in this region. Although Liljeqvist et al. (9) found a gG2 epitope, as determined by using a monoclonal antibody, to be highly conserved among 2,400 HSV-2 isolates, these isolates were from northern Europe, and no studies have evaluated the relatedness of HSV-2 isolates in Africa. Lastly, the positive predictive value of HerpeSelect is high in the population studied; however, the high HSV-2 seroprevelance in most of the populations studied would favor a high positive predictive value for the ELISA. However, the South African panel (n = 150) had a seroprevalance rate of only 17% though the positive predictive value remained at 100% for the ELISA.
The performance of new diagnostic tools is determined by comparison to the gold standard assays available at that point in time. For the serodiagnosis of HSV, WB has been the gold standard against which gG-based HSV serology tests have been measured for U.S. Food and Drug Administration approval (2, 4). Other tests, such as the monoclonal inhibition assay, have also been shown to be useful in detecting and discriminating HSV-1 and HSV-2 antibodies (14). However, even these two gold standard assays have different performance characteristics. For example, Slomka et al. demonstrated a high degree of concordance between WB and the monoclonal inhibition assay for HSV-1; however, the monoclonal inhibition assay detected 20% fewer positive samples than did WB in the first episodes of primary HSV-2 genital herpes (14). The inhibition assay described in the present study shows a very high concordance with WB and provides a sound approach for evaluating unclear HSV results. The HerpeSelect ELISA has a very high degree of concordance with both the inhibition assay and WB. Discordant samples are geographically limited and may be due to strain differences, assay sensitivity, or restricted antibody cross-reactivity.
Acknowledgments
We thank the following sources for providing the serum panels used in this study: for the Zimbabwe and Uganda B panels, we thank the collaborators of the Hormonal Contraception and the Risk of HIV Acquisition Study at the University of Zimbabwe, University of California at San Francisco, Makere University, Case Western Reserve University, Family Health International, and the Statistical Center for HIV/AIDS Research and Prevention; for the Kenya A and B panels, we thank Jared Baeton, University of Washington, Seattle; for the South Africa panel, we thank Pierre Schoeman; and for the Uganda A panel, we thank JoAnn Yee, University of California, Davis. We thank Anne Cent for assistance with WB testing, Harry Prince for critical review of the manuscript, and Manya Cerna for assistance in preparing the manuscript.
REFERENCES
- 1.Ashley, R. L. 1998. Type-specific antibodies to HSV-1 and 2: review of methodology. Herpes 5:33-38. [Google Scholar]
- 2.Ashley, R. L. 2001. Sorting out the new HSV type specific antibody tests. Sex. Transm. Infect. 77:232-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ashley, R. L., and M. Eagleton. 1998. Evaluation of a novel point of care test for antibodies to herpes simplex virus type 2. Sex. Transm. Infect. 74:229-231. [PubMed] [Google Scholar]
- 4.Ashley, R. L., J. Militoni, F. Lee, A. Nahmias, and L. Corey. 1988. Comparison of Western blot (immunoblot) and G-specific immunoblot enzyme assays for detecting antibodies to herpes simplex virus types 1 and 2 in human sera. J. Clin. Microbiol. 26:662-667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ashley, R. L., L. Wa, and J. W. Pickering. 1998. Premarket evaluation of a commercial glycoprotein G-based enzyme immunoassay for herpes simples virus type-specific antibodies. J. Clin. Microbiol. 36:294-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bernstein, D. I., M. A. Lovett, and Y. J. Bryson. 1984. Serologic analysis of first-episode nonprimary genital herpes simples virus infection. Am. J. Med. 77:1055-1060. [DOI] [PubMed] [Google Scholar]
- 7.Eis-Hübinger, A. M., M. Dämer, B. Matz, and K. E. Schneweis. 1999. Evaluation of glycoprotein G2-based enzyme immunoassays for detection of antibodies to herpes simplex virus type 2 in human serum. J. Clin. Microbiol. 37:1242-1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ho, D. W. T., P. R. Field, W. L. Irving, D. R. Packham, and A. L. Cunningham. 1993. Detection of immunoglobulin M antibodies to glycoprotein G2 by Western blot (immunoblot) for diagnosis of initial herpes simplex virus type 2 genital infections. J. Clin. Microbiol. 31:3157-3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liljeqvist, J. A., B. Svennerholm, and T. Bergström. 1999. Typing of clinical herpes simplex virus type 1 and type 2 isolates with monoclonal antibodies. J. Clin. Microbiol. 37:2717-2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martins, T. B., R. D. Woolstenhulme, T. D. Jaskowski, H. R. Hill, and C. M. Litwin. 2001. Comparison of four enzyme immunoassays with a Western blot for the determination of type-specific antibodies to herpes simplex virus. Am. J. Clin. Pathol. 115:272-277. [DOI] [PubMed] [Google Scholar]
- 11.Prince, H. E., C. E. Ernst, and W. R. Hogrefe. 2000. Evaluation of an enzyme immunoassay for measuring herpes simplex virus (HSV) type 1- and HSV type 2-specific IgG antibodies. J. Clin. Lab. Anal. 14:14-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ribes, J. A., M. Hayes, A. Smith, J. L. Winters, and D. J. Baker. 2001. Comparitive performance of herpes simplex virus type 2-specific serologic assays from Meridian Diagnostics and MRL Diagnostics. J. Clin. Microbiol. 39:3740-3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Safrin, S., A. Arvin, J. Mills, and R. Ashley. 1992. Comparison of the Western immunoblot assay and a glycoprotein G enzyme immunoassay for detection of serum antibodies to herpes simplex virus type2 in patients with AIDS. J. Clin. Microbiol. 30:1312-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Slomka, M. J., R. L. Ashley, F. M. Cowan, A. Cross, and D. W. G. Brown. 1995. Monoclonal blocking antibody tests for the detection of HSV-1- and HSV-2-specific humoral responses: comparison with Western blot assay. J. Virol. Methods 55:27-35. [DOI] [PubMed] [Google Scholar]