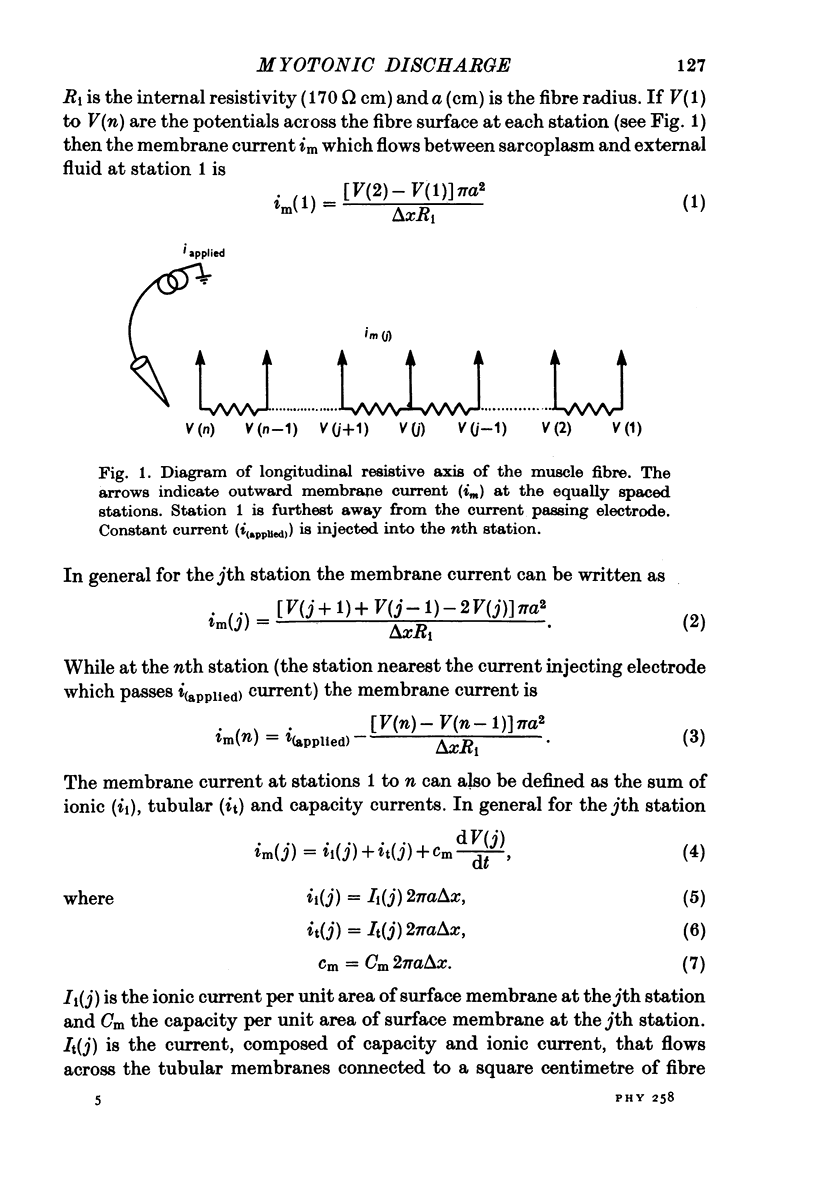
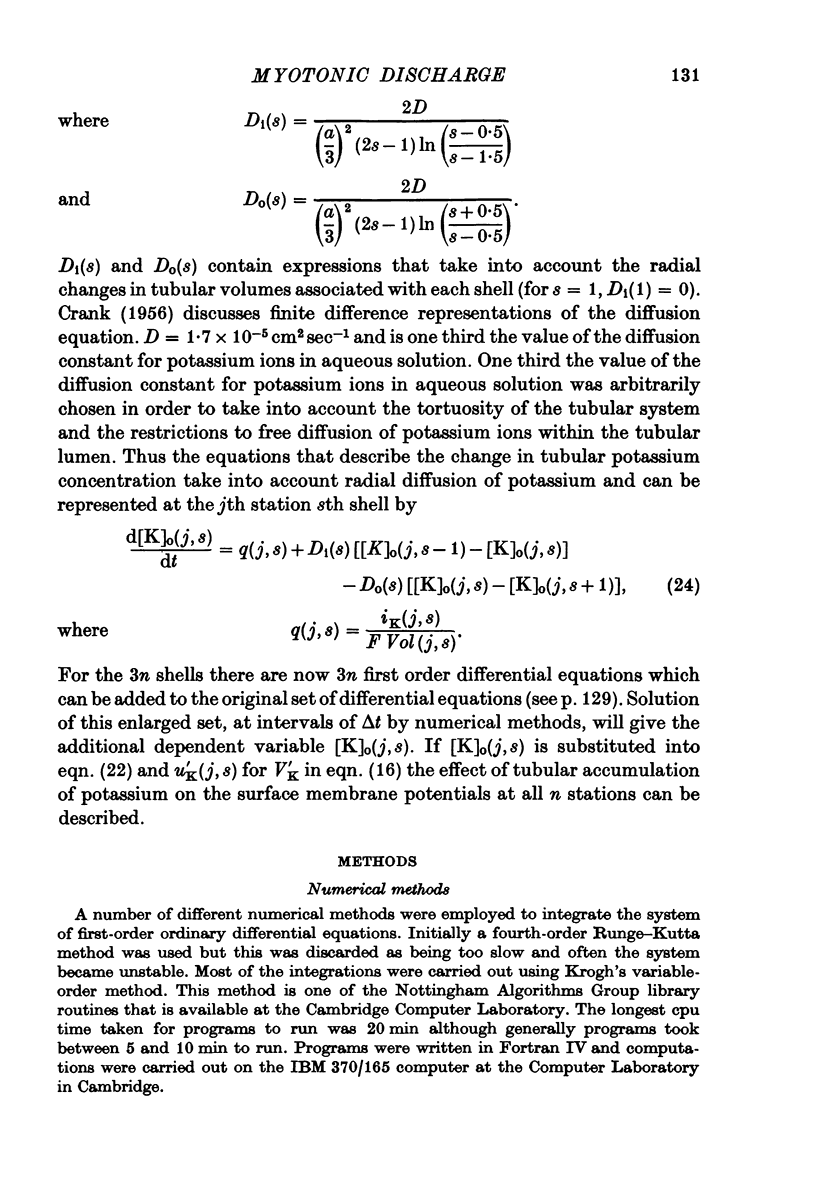
Abstract
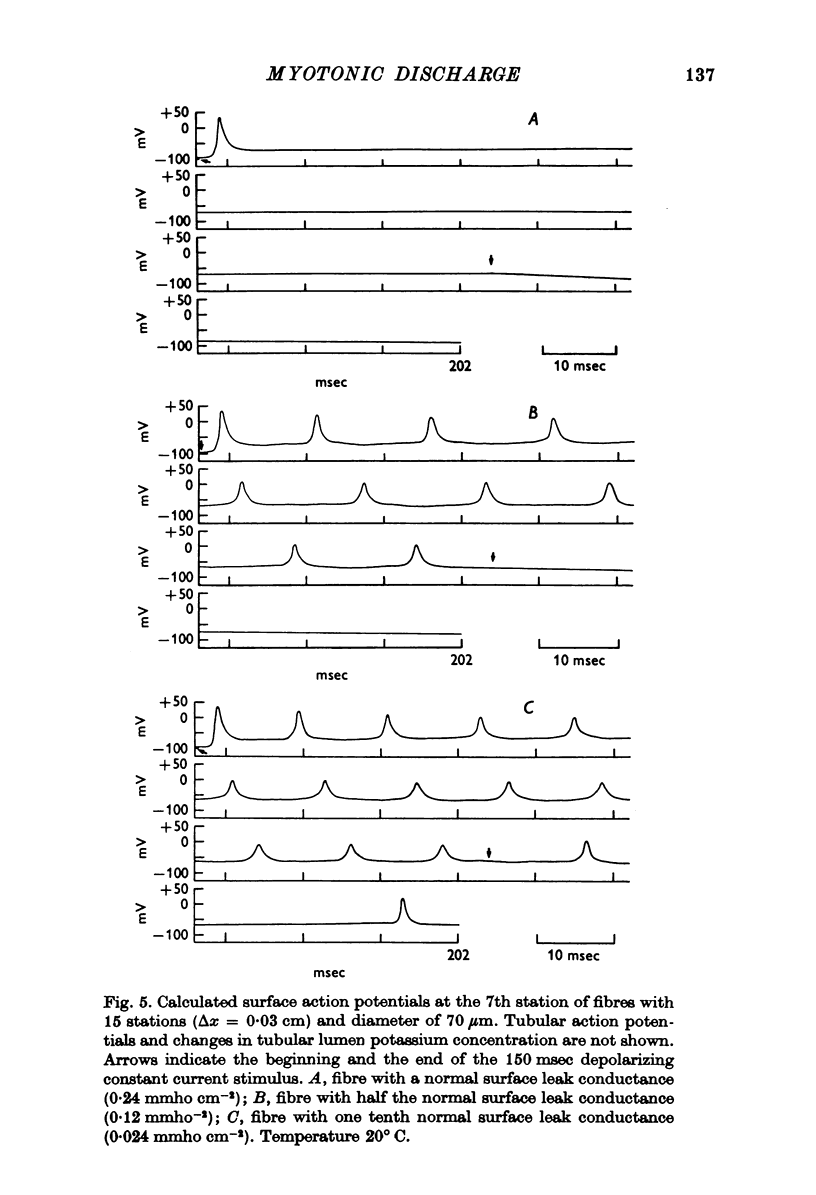
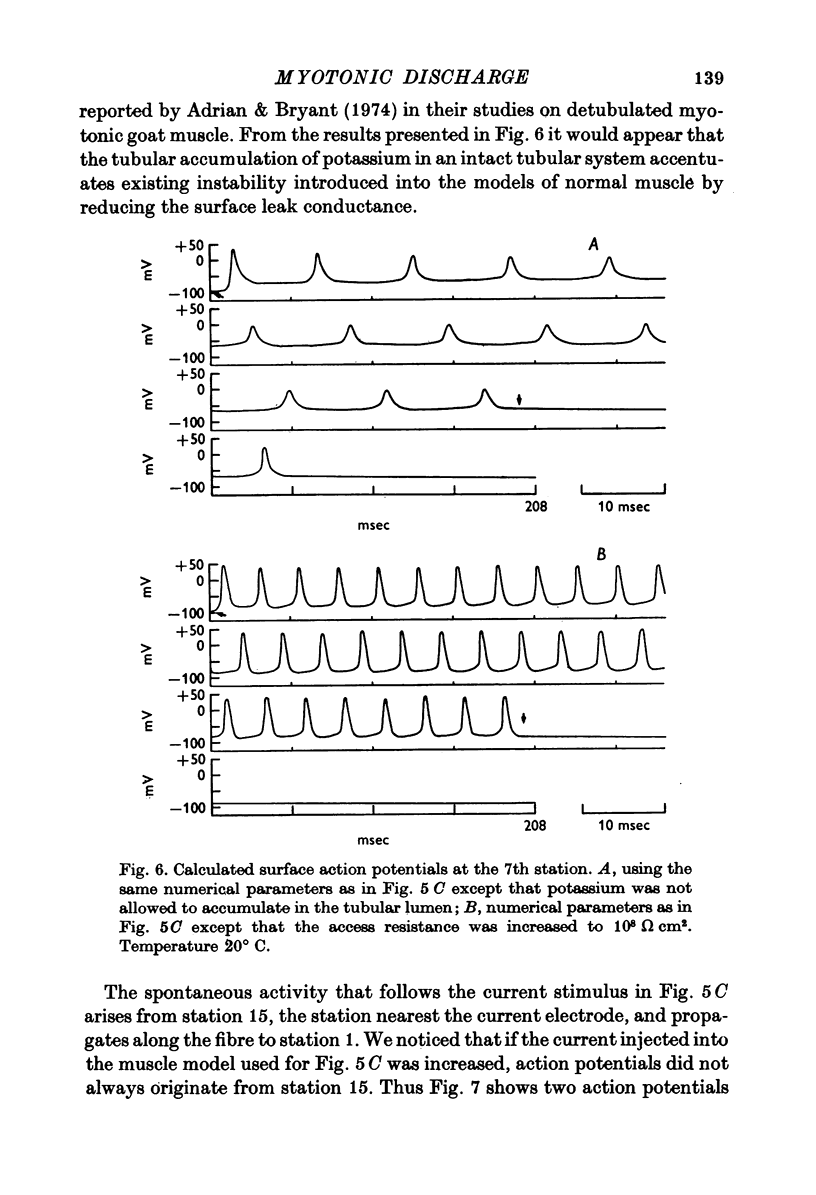
1. Muscle fibres from goats with myotonia congenita show characteristic responses to stimulation with intracellular currents (Adrian & Bryant, 1974). To test whether the reduced surface chloride conductance can account for these myotonic discharges, we have calculated responses of a model 'muscle fibre' to intracellular current of long duration (greater than 100 msec), assuming that the current is applied at the end of the fibre, that the fibre is of finite length, that a regenerative action potential occurs in the transverse tubular system as well as the surface, and that the potassium current in the wall of the transverse tubular system raises the potassium in the tubular lumen. In the absence of information about the kinetic parameters of the ionic currents in mammalian muscle we have used numerical values from frog muscle (Adrian, Chandler & Hodgkin, 1970). 2. In calculations with a normal surface chloride conductance a long maintained current gives only one action potential. Reduction of the chloride conductance to a half produces repetitive firing during the current; reduction to a tenth produces repetitive firing during and a small number of action potentials after the end of the current. Elimination of the tubular potassium accumulation from the calculation reduces the number but does not eliminate action potentials arising after the end of the applied current. 3. With a tenth of the normal chloride conductance calculated responses show maintained firing following a constant current if the deactivating rate of the sodium channels (betam) is reduced by 25%. As before, eliminating potassium accumulation reduces the number of post-stimulus action potentials, but it does not eliminate them altogether. 4. We conclude that in the absence of a surface chloride conductance tubular potassium accumulation could certainly contribute to the instability of the membrane, but it is clear that potassium accumulation is not the only reason for the instability of myotonic muscle fibres. The kinetics of the sodium channels are important and we do not know that they are the same in normal and myotonic fibres. Nevertheless the presence of a surface chloride conductance does stabilize the response of a fibre to constant current or to repetitive stimulation, and its absence could be a sufficient condition for myotonic behaviour.
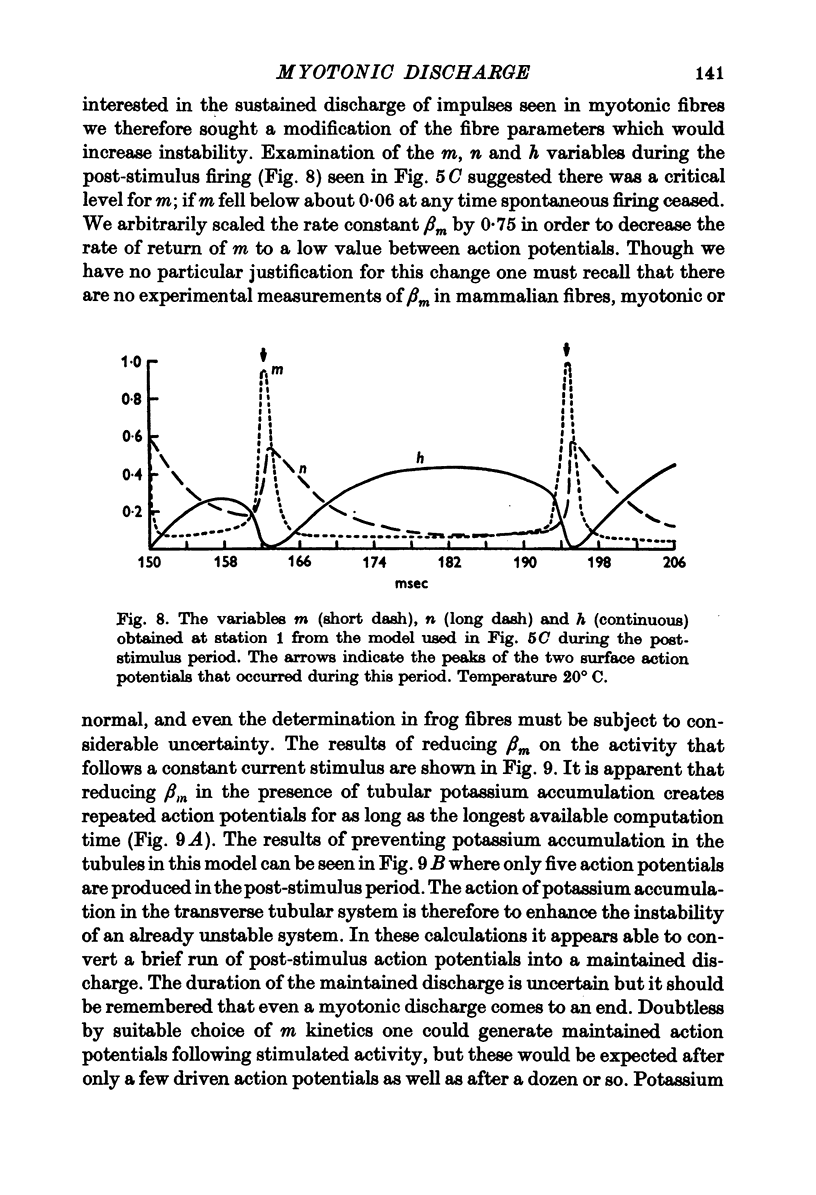
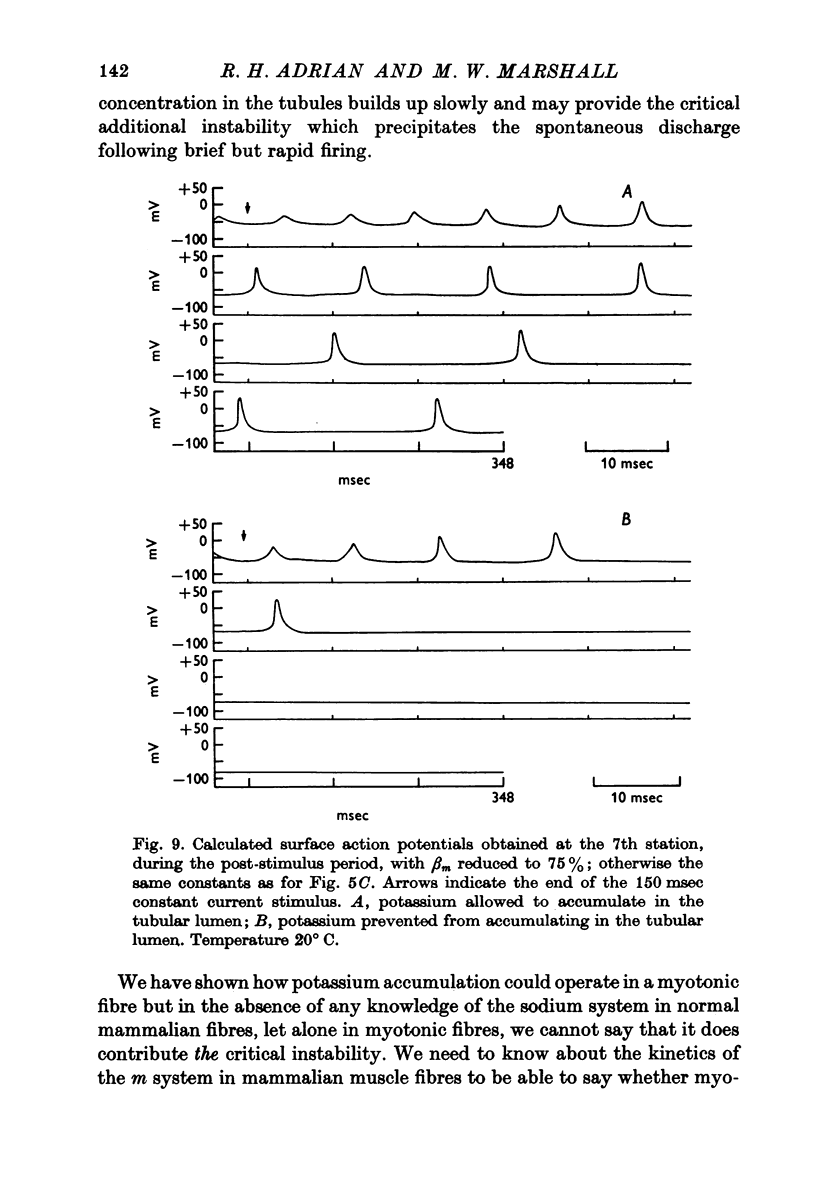
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adrian R. H., Bryant S. H. On the repetitive discharge in myotonic muscle fibres. J Physiol. 1974 Jul;240(2):505–515. doi: 10.1113/jphysiol.1974.sp010620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrian R. H., Chandler W. K., Hodgkin A. L. The kinetics of mechanical activation in frog muscle. J Physiol. 1969 Sep;204(1):207–230. doi: 10.1113/jphysiol.1969.sp008909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrian R. H., Chandler W. K., Hodgkin A. L. Voltage clamp experiments in striated muscle fibres. J Physiol. 1970 Jul;208(3):607–644. doi: 10.1113/jphysiol.1970.sp009139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrian R. H., Peachey L. D. Reconstruction of the action potential of frog sartorius muscle. J Physiol. 1973 Nov;235(1):103–131. doi: 10.1113/jphysiol.1973.sp010380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant S. H. Cable properties of external intercostal muscle fibres from myotonic and nonmyotonic goats. J Physiol. 1969 Oct;204(3):539–550. doi: 10.1113/jphysiol.1969.sp008930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein S. S., Rall W. Changes of action potential shape and velocity for changing core conductor geometry. Biophys J. 1974 Oct;14(10):731–757. doi: 10.1016/S0006-3495(74)85947-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipicky R. J., Bryant S. H., Salmon J. H. Cable parameters, sodium, potassium, chloride, and water content, and potassium efflux in isolated external intercostal muscle of normal volunteers and patients with myotonia congenita. J Clin Invest. 1971 Oct;50(10):2091–2103. doi: 10.1172/JCI106703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipicky R. J., Bryant S. H. Sodium, potassium, and chloride fluxes in intercostal muscle from normal goats and goats with hereditary myotonia. J Gen Physiol. 1966 Sep;50(1):89–111. doi: 10.1085/jgp.50.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]



