Abstract
A survey was conducted among Spanish children with gastroenteritis treated in an emergency room. Reverse transcription-PCR with specimens negative for other enteric pathogens was used. The minimum incidence of human calicivirus infection was 7.7%, with Lordsdale as the predominant genotype. The clinical features and severity of calicivirus and rotavirus were similar.
Norwalk-like virus (NLV) and Sapporo-like virus (SLV) are members of the family Caliciviridae (7), which has been considered one of the most common causes of nonbacterial gastroenteritis outbreaks and sporadic cases in adults and children (3, 6, 11, 25).
Routine detection is not yet possible due to the lack of a simple diagnostic test. Thus, the most-used human calicivirus (HuCV) detection assay is generic reverse transcription-PCR (RT-PCR) with the RNA polymerase gene (pol) as the target. Thus far, the RT-PCR assay has not been routinely used for the detection of childhood gastroenteritis etiologic agents. Although HuCV is emerging as a cause of sporadic gastroenteritis as a sole pathogen in young children, there are not many data about the incidence of this viral infection. During previous studies in other countries, this infection was detected in 3.5 to 20% of sporadic cases among young children (1, 2, 5, 9, 10, 12, 14, 15, 16, 22, 24). To date, there is to our knowledge a lack of data from Spain. In order to clarify the epidemiologic role and clinical significance of calicivirus-associated gastroenteritis in our country, we have conducted a surveillance study among children with gastroenteritis treated in an emergency room in Madrid, Spain. Our objectives were to determine the incidence of HuCV infection and the clinical characteristics of the disease and to establish the genetic diversity of HuCV strains. A total of 822 fecal specimens were collected from children of less than 4 years of age with gastroenteritis who visited the emergency room of Severo Ochoa Hospital in Madrid between October 1996 and September 1997. A gastroenteritis episode was defined as at least three looser-than-normal stools within a 24-h period or an episode of forceful vomiting with any loose stools. Clinical information was collected for all patients, including age, sex, presenting symptoms, and duration of illness prior to admission. Each episode was graded by using a 20-point severity score scale as previously described (17). Fecal specimens were screened for etiologic agents of diarrhea. Bacteria (Salmonella, Shigella, Yersinia, and Campylobacter spp. and Vibrionaceae) were detected by routine cultivation methods, and viruses (group A rotavirus, adenovirus, and astrovirus) were detected by commercial enzyme immunoassays.
No pathogens were detected in fecal specimens from 292 (35.5%) children. A subset of 201 of these samples with an adequate volume of fecal specimen was tested for the presence of HuCVs (NLV and SLV) by using RT-PCR with the RNA polymerase gene (pol) as the target. For this, RNA was extracted from 20% of the stool suspensions in phosphate-buffered saline by using the guanidine thiocyanate method (23) and the RNaid Spin kit (Q-BIOgene; Bio 101). Samples were first tested with the JV12-JV13 primer pair, which detects only NLV agents (21), and the samples that tested negative with this test were then analyzed with the p289-290 primer pair, which detects NLV and SLV agents (8). PCR products were analyzed by electrophoresis in 2% (wt/vol) agarose-Tris-borate-EDTA gels and detected by UV illumination after staining with ethidium bromide.
A subset of HuCV-positive samples was genetically characterized by either reverse line blot hybridization (RLB) (20) or sequence analysis. The sequences were analyzed with CLUSTALX, version 1.8, methods.
Statistical analysis was performed with the Mann-Whitney U test method, which was used to compare the medians of clinical symptom scores between rotavirus- and HuCV-associated cases. For comparison of proportions, chi-square or Fisher's exact test was used. All tests were two-tailed and considered significant when the P value was ≤0.05.
HuCV was detected by RT-PCR in 63 (31%) of 201 diarrhea samples that were negative for other common enteric pathogens. Of these 63 samples, 54 were positive with the JV12-JV13 (21) primer pair, which detects only the NLV genus, and 9 samples were positive with the p289-290 primer pair, which is able to detect the NLV and SLV genera (8).
Twenty-nine samples were genotyped either by RLB or by sequence analysis (the RLB method was used for 24 samples testing positive with the JV12-JV13 primer pair).
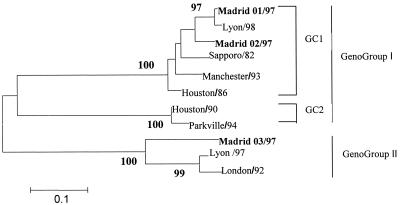
This genotype analysis showed that 26 samples belonged to the NLV genus and 3 samples belonged to the SLV genus. Of the 26 NLV samples, 23 were related to the Lordsdale virus, 2 were related to the Mexico virus, and 1 was related to the Queen Arms virus. Of the three SLV samples, two were related to genogroup I genetic cluster 1 (Sapporo/82) and one was related to genogroup II genetic cluster 1 (London/92) (Fig. 1) (18).
FIG. 1.
Phylogenic tree based on analyses of a 236-bp region of the RNA polymerase gene of three Spanish strains of SLV obtained in this study. Distances were calculated by the Kimura 2p method, available in the MEGA, version 2.1, analytical package. The tree was drawn by using the neighbor-joining method. Bootstrap values for each node are given if they are >80%. Genogroups and genetic clusters (GC) were named according to the Schuffenecker study of genetic classifications of SLVs (18). GenBank accession numbers for the SLV sequences used in this study are as follows: Lyon/98, AJ251991; Sapporo/82, U65427; Manchester/93, X86560; Houston/86, U95643; Houston/90, U95644; Parkville/94, U73124; Lyon/97, AJ271056; London/92, U95645. Spanish strains are marked in bold.
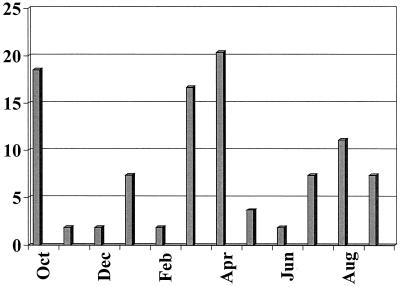
The median age of children with HuCV was 15.12 months (range, 1 to 47 months). The seasonal distribution of gastroenteritis episodes caused by HuCV is shown in Fig. 2. HuCV was detected year-round, with two peak seasons in October and in March and April.
FIG. 2.
Monthly distribution of HuCV-associated cases as percentages of HuCV-positive samples.
From the 63 cases positive for HuCV, 52 (82.5%) were associated with vomiting, 20 (31.7%) were associated with fever, 15 (24%) were associated with mild dehydration, and 1 (1.6%) was associated with severe dehydration. Hospitalization was required in 8 (12.6%) cases.
To further assess the medical importance of HuCV infection, the clinical characteristics of children infected with HuCV were compared with those found in patients infected with rotavirus (205 of 822) (Table 1). The clinical features and the severity of HuCV and rotavirus gastroenteritis episodes were similar, although children with HuCV infection were less likely to be dehydrated (P < 0.05), despite the longer duration of diarrhea observed among these patients.
TABLE 1.
Clinical characteristics of acute gastroenteritis associated with HuCV or rotavirus
| Variable | Median (range) for patients with:
|
Statistical significance (P)a | |
|---|---|---|---|
| HuCV (n = 63) | Rotavirus (n = 205) | ||
| Diarrhea (days) | 2 (1-13) | 1 (1-8) | <0.05 |
| Diarrhea (maximum no. of times/day) | 4.5 (1-15) | 5 (1-20) | NS |
| Vomiting (days) | 1 (0-7) | 1 (0-5) | NS |
| Vomiting (maximum no. of times/day) | 3 (0-15) | 3 (0-20) | NS |
| Maximum fever (°C) | 37 (37-40) | 37 (36-40) | NS |
| Severity score (points)b | 10 (2-16) | 10 (1-16) | NS |
| Dehydration (%) | 26 | 41 | <0.05 |
| Hospitalization (%) | 12.6 | 19.5 | NS |
NS, not significant.
The maximum possible severity score was 20 points.
This is the first report, to our knowledge, of HuCV detection among Spanish children with sporadic gastroenteritis. Our results demonstrate that HuCV might be an important and under-appreciated cause of diarrhea in Spanish children. This study provides the lowest possible estimate of the magnitude of the problem (63 of 822 cases, 7.7%) because we screened only those specimens which had no other pathogens. In addition, some positive samples could escape detection with the primer pairs used.
Our results confirm those of studies done in The Netherlands (10), South Africa (24), France (Rouen) (12), and China (16). However, this incidence is lower than that found in studies from Australia (9), France (Dijon) (1), Finland (14, 15), Mexico (4), and Chile (13). The reasons for this discrepancy could be attributable to the different methods used to diagnose HuCV as well as the diversity of the studies.
As described in previous studies, most of the HuCVs identified worldwide belonged to the NLV genus (89.6%) and the majority of the strains were related to the Lordsdale virus (4, 5, 9, 19). SLV appears to be an uncommon cause of severe gastroenteritis in young Spanish children, although further studies are needed to confirm this data.
The clinical severity of HuCV infection has been reported only in Finnish (14) and Japanese (26) studies up until now. Our findings showed that the mean HuCV clinical severity was equal to that in rotavirus gastroenteritis, as in the Japanese study (26). However, our data are at variance with those of the Finnish study (15).
Undoubtedly, future studies should be applied to determine the true epidemiologic role of HuCV diarrhea among Spanish children.
Nucleotide sequence accession numbers.
GenBank accession numbers for the SLV sequences analyzed in this study are as follows: AY102703 (Madrid01/97), AY102704 (Madrid02/97), and AY102705 (Madrid03/97).
Acknowledgments
This study was supported in part by FIS grant no. 200/2000 and EU grant no. QLK1-199-00594 for the study of food-borne viruses in Europe.
We thank E. Cubero, R. Ramiro, and V. Montero for technical assistance. We are also grateful to H. Vennema and M. Koopmans (RIVM, Bilthoven, The Netherlands) for providing an RLB membrane and to J. Colomina and M. Koopmans for critical readings of the manuscript. We are grateful to Roger Glass for fruitful discussion.
REFERENCES
- 1.Bon, F., P. Fascia, M. Dauvergne, D. Tenenbaum, H. Planson, A. M. Petion, P. Pothier, and E. Kohli. 1999. Prevalence of group A rotavirus, human calicivirus, astrovirus, and adenovirus type 40 and 41 infections among children with acute gastroenteritis in Dijon, France. J. Clin. Microbiol. 37:3055-3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.deWit, M. A., M. Koopmans, L. Kortbeek, W. Wannet, J. Vinje, F. van Leusden, A. Bartelds, and Y. van Duynjoven. 2001. Sensor, a population-based cohort study on gastroenteritis in The Netherlands: incidence and etiology. Am. J. Epidemiol. 154:666-674. [DOI] [PubMed] [Google Scholar]
- 3.Fankhauser, R. L., J. S. Noel, S. S. Monroe, T. Ando, and R. I. Glass. 1998. Molecular epidemiology of “Norwalk-like viruses” in outbreaks of gastroenteritis in the United States. J. Infect. Dis. 178:1571-1578. [DOI] [PubMed] [Google Scholar]
- 4.Farkas, T., X. Jiang, M. L. Guerrero, W. Zhong, N. Wilton, T. Berke, D. Matson, L. K. Pickering, and G. Ruiz-Palacios. 2000. Prevalence and genetic diversity of human caliciviruses (HuCVs) in Mexican children. J. Med. Virol. 62:217-223. [PubMed] [Google Scholar]
- 5.Foley, B., J. O'Mahony, C. Hill, and J. G. Morgan. 2001. Molecular detection and sequencing of “Norwalk-like viruses” in outbreaks and sporadic cases of gastroenteritis in Ireland. J. Med. Virol. 65:388-394. [DOI] [PubMed] [Google Scholar]
- 6.Glass, R. I., J. Noel, T. Ando, R. Frankhauser, G. Belliot, A. Mounts, U. D. Parashar, J. S. Bresee, and S. S. Monroe. 2000. The epidemiology of enteric caliciviruses from humans: a reassessment using new diagnostics. J. Infect. Dis. 181(Suppl. 2):S254-S261. [DOI] [PubMed] [Google Scholar]
- 7.Green, K. Y., T. Ando, M. S. Balayan, et al. 2000. Taxonomy of caliciviruses. J. Infect. Dis. 181(Suppl. 2):S322-S330. [DOI] [PubMed] [Google Scholar]
- 8.Jiang, X., P. W. Huang, W. M. Zhong, T. Farkas, D. W. Cubitt, and D. O. Matson. 1999. Design and evaluation of a primer pair that detects both Norwalk- and Sapporo-like caliciviruses by RT-PCR. J. Virol. Methods 83:145-154. [DOI] [PubMed] [Google Scholar]
- 9.Kirkwood, C. D., and R. F. Bishop. 2001. Molecular detection of human calicivirus in young children hospitalized with acute gastroenteritis in Melbourne, Australia, during 1999. J. Clin. Microbiol. 39:2722-2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koopmans, M., J. Vinje, M. de Wit, I. Leenen, W. Poel, and Y. Duynhoven. 2000. Molecular epidemiology of human enteric caliciviruses in The Netherlands. J. Infect. Dis. 181(Suppl. 2):S262-S269. [DOI] [PubMed] [Google Scholar]
- 11.Lopman, B. A., D. W. Brown, and M. Koopmans. 2002. Human caliciviruses in Europe. J. Clin. Virol. 24:137-160. [DOI] [PubMed] [Google Scholar]
- 12.Marie-Cardine, A., K. Gourlain, O. Mouterde, N. Castignolles, M. F. Hellot, E. Mallet, and C. Buffet-Janvresse. 2002. Epidemiology of acute viral gastroenteritis in children hospitalized in Rouen, France. Clin. Infect. Dis. 34:1170-1178. [DOI] [PubMed] [Google Scholar]
- 13.O'Ryan, M. L., N. Mamani, A. Gaggero, L. F. Avendano, S. Prieto, A. Pena, X. Jiang, and D. O. Matson. 2000. Human caliciviruses are a significant pathogen of acute sporadic diarrhea in children of Santiago, Chile. J. Infect. Dis. 182:1519-1522. [DOI] [PubMed] [Google Scholar]
- 14.Pang, X.-L., S. Honma, S. Nakata, and T. Vesikari. 2000. Human caliciviruses in acute gastroenteritis of young children in the community. J. Infect. Dis. 181(Suppl. 2):S288-S294. [DOI] [PubMed] [Google Scholar]
- 15.Pang, X.-L., S.-Q. Zeng, S. Honma, S. Nakata, and T. Vesikari. 2001. Effect of rotavirus vaccine on Sapporo virus gastroenteritis in Finnish infants. Pediatr. Infect. Dis. J. 20:295-300. [DOI] [PubMed] [Google Scholar]
- 16.Qiao, H., M. Nilsson, E. R. Abreu, K.-O. Hedland, K. Johansen, G. Zaoru, and L. Svensson. 1999. Viral diarrhea in children in Beijing, China. J. Med. Virol. 57:390-396. [PubMed] [Google Scholar]
- 17.Ruuska, T., and T. Vesikari. 1990. Rotavirus disease in Finnish children: use of numerical scores for clinical severity of diarrhoeal episodes. Scand. J. Infect. Dis. 22:259-267. [DOI] [PubMed] [Google Scholar]
- 18.Schuffenecker, I., T. Ando, D. Thouvenot, B. Lina, and M. Aymard. 2001. Genetic classification of “Sapporo-like viruses.” Arch. Virol. 146:2115-2132. [DOI] [PubMed] [Google Scholar]
- 19.Vainio, K., K. Stene-Johansen, T. Oystein-Jonassen, A. L. Bruu, and B. Grinde. 2001. Molecular epidemiology of calicivirus infections in Norway. J. Med. Virol. 65:309-314. [DOI] [PubMed] [Google Scholar]
- 20.Vinjé, J., and M. P. G. Koopmans. 2000. Simultaneous detection and genotyping of “Norwalk-like viruses” by oligonucleotide array in a reverse line blot hybridization format. J. Clin. Microbiol. 38:2595-2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vinje, J., and M. P. Koopmans. 1996. Molecular detection and epidemiology of small round-structured viruses in outbreaks of gastroenteritis in the Netherlands. J. Infect. Dis. 174:610-615. [DOI] [PubMed] [Google Scholar]
- 22.Wheeler, J., D. Sethi, J. Cowden, P. Wall, L. Rodrigues, D. Tompkins, M. Hudson, P. Roderik, et al. 1999. Study of the infectious intestinal disease in England: rates in the community, presenting to general practice, and reported to general surveillance. Br. Med. J. 318:1046-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilhelmi, I., C. Mier, E. Román, J. Colomina, J. Prat, and A. Sánchez-Fauquier. 1999. Epidemiología molecular de rotavirus en niños españoles. Enferm. Infecc. Microbiol. Clin. 17:509-514. [PubMed] [Google Scholar]
- 24.Wolfaardt, M., M. Taylor, H. Booysen, L. Engelbrecht, W. Grabow, and X. Jiang. 1997. Incidence of human calicivirus and rotavirus infection in patients with gastroenteritis in South Africa. J. Med. Virol. 51:290-296. [DOI] [PubMed] [Google Scholar]
- 25.Wright, P. J., I. C. Gunesekere, J. C. Doultree, and J. A. Marshall. 1998. Small round-structured (Norwalk-like) viruses and classical human caliciviruses in southeastern Australia, 1980-1996. J. Med. Virol. 55:312-320. [PubMed] [Google Scholar]
- 26.Yoshiyuki, S., N. Shuyi, H. Shinjiro, T. Masatoshi, N. Kazuko, and C. Shunzo. 2001. Clinical severity of Norwalk and Sapporo virus gastroenteritis in children in Hokkaido, Japan. Pediatr. Infect. Dis. J. 20:849-853. [DOI] [PubMed] [Google Scholar]