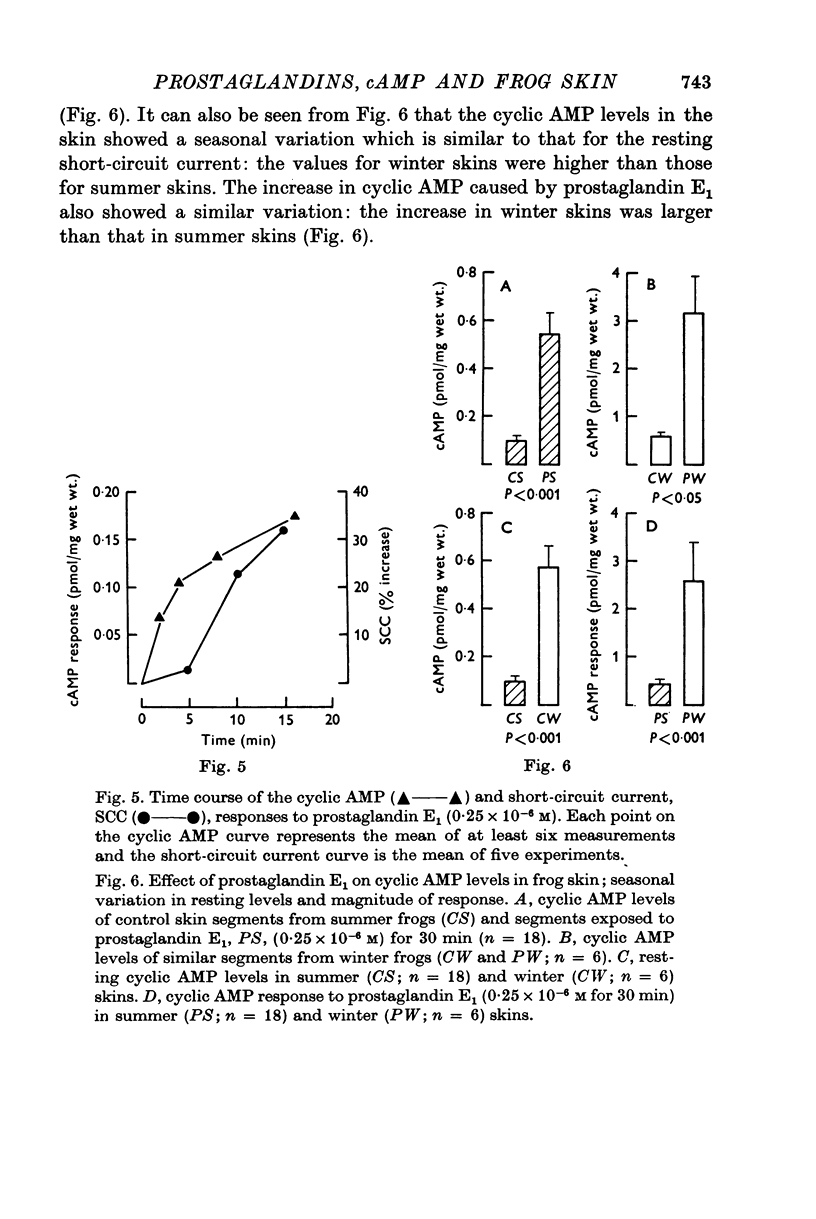
Abstract
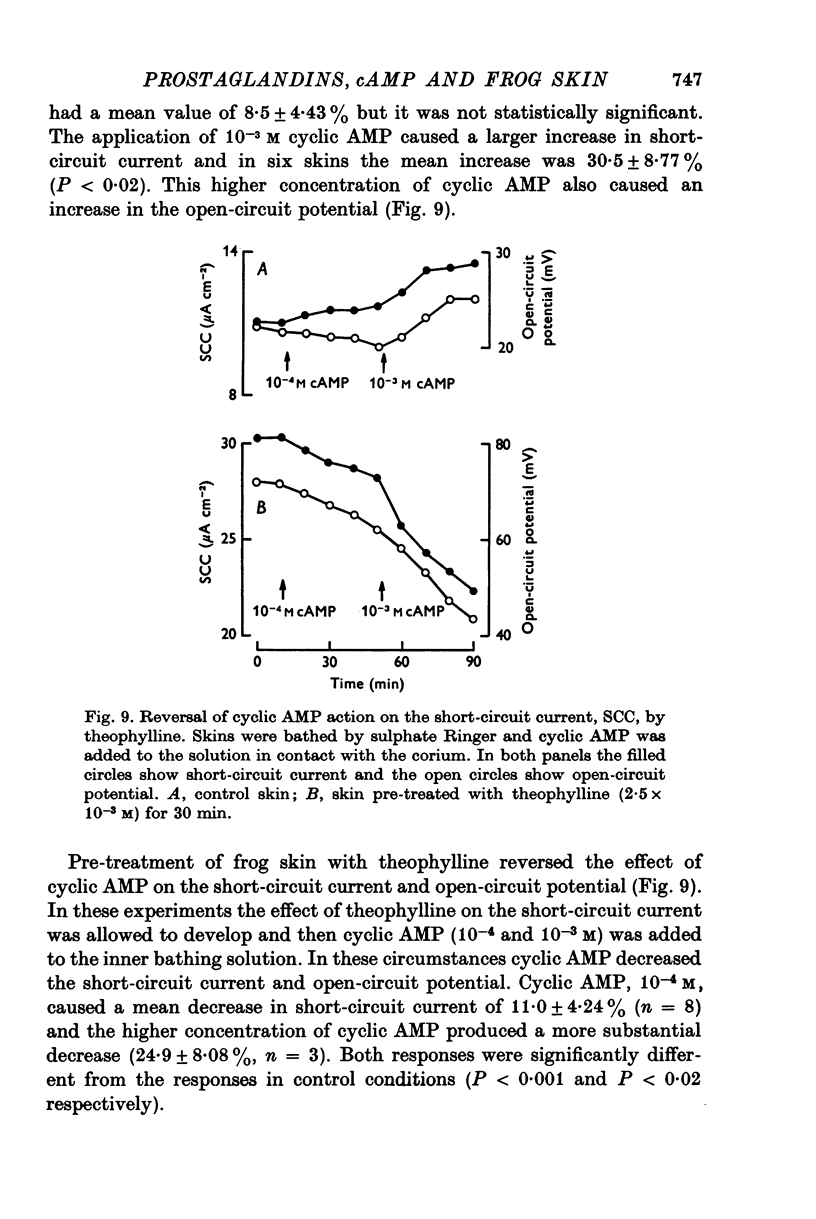
1. Sodium transport across isolated frog skin, as measured by the short-circuit current, was decreased by acetylsalicylic acid, mefenamic acid, paracetamol and phenylbutazone. Indomethacin (6 X 10(-6) M) had a biphasic effect on the short-circuit current: a transient increase followed by a sustained decrease. 2. The release of prostaglandin-like material from the skin was reduced by acetylsalicylic acid and indomethacin. Paracetamol caused a significant reduction in the short-circuit current response of the skin to low doses of arachidonic acid, but the response to the highest dose tested was not significantly altered. 3. Indomethacin (6 X 10(-6) M) increased the sensitivity of the skin to applied prostaglandin E1. The other prostaglandin synthetase inhibitors did not have this effect. Indomethacin (6 X 10(-6) M) also enhanced the effect of antidiuretic hormone on the short-circuit current. 4. Indomethacin (30 X 10(-6) M) increased the short-circuit current and diminished the response to applied prostaglandin E1. 5. In sulphate Ringer, theophylline increased the short-circuit current and diminished the response to prostaglandin E1. 6. Prostaglandin E1 increased the levels of cyclic AMP in frog skin and these increases preceded the increases in short-circuit current. There was a seasonal variation in the level of cyclic AMP in the skin: the levels in winter exceeded those in summer. There was also a seasonal variation in the cyclic AMP response to prostaglandin E1: the winter response was greater than that in summer. 7. Indomethacin (6 X 10(-6) M) had a biphasic effect on cyclic AMP levels in the skin, an initial increase followed by a decrease. Indomethacin also potentiated prostaglandin E1 stimulated cyclic AMP accumulation. 8. Theophylline increased cyclic AMP levels in the skin and potentiated prostaglandin E1 stimulated cyclic AMP accumulation. 9. Pre-treatment of the skin with theophylline reversed the effects of cyclic AMP on the short-circuit current and open-circuit potential. 10. It is concluded that endogenous prostaglandins help to maintain sodium transport across isolated frog skin and that the effects of E-type prostaglandins on the short-circuit current are mediated by increased cyclic AMP levels. The transient increase in short-circuit current and the increased skin sensitivity caused by indomethacin (6 X 10(-6) M) are attributed to inhibition of phosphodiesterase activity. The failure of theophylline to potentiate the short-circuit current response of the skin to prostaglandin E1 is attributed to alteration of cyclic AMP action on the skin by theophylline.
Full text
PDF




















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe K., Butcher R. W., Nicholson W. E., Baird C. E., Liddle R. A., Liddle G. W. Adenosine 3',5'-monophosphate (cyclic AMP) as the mediator of the actions of melanocyte stimulating hormone (MSH) and norepinephrine on the frog skin. Endocrinology. 1969 Feb;84(2):362–368. doi: 10.1210/endo-84-2-362. [DOI] [PubMed] [Google Scholar]
- Albano J. D., Barnes G. D., Maudsley D. V., Brown B. L., Etkins R. P. Factors affecting the saturation assay of cyclic AMP in biological systems. Anal Biochem. 1974 Jul;60(1):130–141. doi: 10.1016/0003-2697(74)90137-7. [DOI] [PubMed] [Google Scholar]
- Baba W. I., Smith A. J., Townshend M. M. The effects of vasopressin, theophylline and cyclic 3'-5'-adenosine monophosphate (cyclic AMP) on sodium transport across the frog skin. Q J Exp Physiol Cogn Med Sci. 1967 Oct;52(4):416–421. doi: 10.1113/expphysiol.1967.sp001936. [DOI] [PubMed] [Google Scholar]
- Barry E., Hall W. J. Stimulation of sodium movement across frog skin by prostaglandin E1. J Physiol. 1969 Jan;200(1):83P–84P. [PubMed] [Google Scholar]
- Bastide F., Jard S. Actions de la noradrénaline et de l'oxytocine sur le transport actif de sodium et la permeabilité à l'eau de la peau de grenouille. Rôle du 3',5'-AMP cyclique. Biochim Biophys Acta. 1968 Jan 3;150(1):113–123. doi: 10.1016/0005-2736(68)90014-x. [DOI] [PubMed] [Google Scholar]
- Bieck P., Stock K., Westermann E. Wirkung von cyclischem adenosin-3',5'-Monophosphat (3'5/-AMP) und seinem Dibutyrylderivat (DBA) auf lipolyse, Glykogenolyse und Corticosteron (DBA) auf lipolyse, Glykogenolyse und Corticosteronsynthese. Naunyn Schmiedebergs Arch Exp Pathol Pharmakol. 1969;263(3):387–405. [PubMed] [Google Scholar]
- Brown B. L., Albano J. D., Ekins R. P., Sgherzi A. M. A simple and sensitive saturation assay method for the measurement of adenosine 3':5'-cyclic monophosphate. Biochem J. 1971 Feb;121(3):561–562. doi: 10.1042/bj1210561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciosek C. P., Jr, Ortel R. W., Thanassi N. M., Newcombe D. S. Indomethacin potentiates PGE1 stimulated cyclic AMP accumulation in human synoviocytes. Nature. 1974 Sep 13;251(5471):148–150. doi: 10.1038/251148a0. [DOI] [PubMed] [Google Scholar]
- Cooper R. H., McPherson M., Schofield J. G. The effect of prostaglandins on ox pituitary content of adenosine 3':5'-cyclic monophosphate and the release of growth hormone. Biochem J. 1972 Mar;127(1):143–154. doi: 10.1042/bj1270143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert A. W., Painter E. Independent action of antidiuretic hormone, theophylline and cyclic 3',5'-adenosine monophosphate on cell membrane permeability in frog skin. J Physiol. 1968 Dec;199(3):593–612. doi: 10.1113/jphysiol.1968.sp008670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert A. W., Painter E., Prince W. T. The effects of anions on sodium transport. Br J Pharmacol. 1969 May;36(1):97–106. doi: 10.1111/j.1476-5381.1969.tb08307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert A. W., Painter E. The effect of theophylline on chloride permeability and active chloride transport in various epithelia. J Pharm Pharmacol. 1968 Jun;20(6):492–495. doi: 10.1111/j.2042-7158.1968.tb09795.x. [DOI] [PubMed] [Google Scholar]
- Eckenfels A., Vane J. R. Prostaglandins, oxygen tension and smooth muscle tone. Br J Pharmacol. 1972 Jul;45(3):451–462. doi: 10.1111/j.1476-5381.1972.tb08101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fassina G., Carpenedo F., Santi R. Effect of prostaglandin E1 on isolated short-circuited frog skin. Life Sci. 1969 Feb 1;8(3):181–187. doi: 10.1016/0024-3205(69)90092-7. [DOI] [PubMed] [Google Scholar]
- Ferreira S. H., Moncada S., Vane J. R. Indomethacin and aspirin abolish prostaglandin release from the spleen. Nat New Biol. 1971 Jun 23;231(25):237–239. doi: 10.1038/newbio231237a0. [DOI] [PubMed] [Google Scholar]
- Ferreira S. H., Vane J. R. Prostaglandins: their disappearance from and release into the circulation. Nature. 1967 Dec 2;216(5118):868–873. doi: 10.1038/216868a0. [DOI] [PubMed] [Google Scholar]
- Flores A. G., Sharp G. W. Endogenous prostaglandins and osmotic water flow in the toad bladder. Am J Physiol. 1972 Dec;223(6):1392–1397. doi: 10.1152/ajplegacy.1972.223.6.1392. [DOI] [PubMed] [Google Scholar]
- Flower R., Gryglewski R., Herbaczyńska-Cedro K., Vane J. R. Effects of anti-inflammatory drugs on prostaglandin biosynthesis. Nat New Biol. 1972 Jul 26;238(82):104–106. doi: 10.1038/newbio238104a0. [DOI] [PubMed] [Google Scholar]
- Gilmore N., Vane J. R., Wyllie J. H. Prostaglandins released by the spleen. Nature. 1968 Jun 22;218(5147):1135–1140. doi: 10.1038/2181135a0. [DOI] [PubMed] [Google Scholar]
- Hall W. J., Martin J. D. Effect of calcium and vasopressin on the response of frog skin to prostaglandin E1. J Physiol. 1974 Aug;240(3):595–608. doi: 10.1113/jphysiol.1974.sp010624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall W. J., O'Regan M. G. Proceedings: The effects of indomethacin and theophylline on the response of frog skin to prostaglandin E1. J Physiol. 1974 Jan;236(1):48P–49P. [PubMed] [Google Scholar]
- Hall W. J., O'Regan M. G. The effects of indomethacin and prostaglandin E1 on cyclic AMP levels in frog skin. J Physiol. 1975 May;247(1):31P–32P. [PubMed] [Google Scholar]
- Hall W. J. Seasonal changes in the sensitivity of frog skin to prostaglandin and the effect of external sodium and chloride on the response. Ir J Med Sci. 1973 Sep;142(5):230–243. doi: 10.1007/BF02950017. [DOI] [PubMed] [Google Scholar]
- Handler J. S., Butcher R. W., Sutherland E. W., Orloff J. The effect of vasopressin and of theophylline on the concentration of adenosine 3',5'-phosphate in the urinary bladder of the toad. J Biol Chem. 1965 Nov;240(11):4524–4526. [PubMed] [Google Scholar]
- Isaacson L. C., Douglas R. J., Pepler J. Automatic measurement of voltage and short-circuit current across amphibian epithelia. J Appl Physiol. 1971 Aug;31(2):298–299. doi: 10.1152/jappl.1971.31.2.298. [DOI] [PubMed] [Google Scholar]
- Kawada J., Taylor R. E., Jr, Barker S. B. Measurement of Na--K--ATPase in the separated epidermis of Rana catesbeiana frogs and tadpoles. Comp Biochem Physiol. 1969 Sep 1;30(5):965–975. doi: 10.1016/0010-406x(69)90051-6. [DOI] [PubMed] [Google Scholar]
- Lipson L. C., Sharp G. W. Effect of prostaglandin E1 on sodium transport and osmotic water flow in the toad bladder. Am J Physiol. 1971 Apr;220(4):1046–1052. doi: 10.1152/ajplegacy.1971.220.4.1046. [DOI] [PubMed] [Google Scholar]
- Lucchesi B. R. Cardiac actions of glucagon. Circ Res. 1968 Jun;22(6):777–787. doi: 10.1161/01.res.22.6.777. [DOI] [PubMed] [Google Scholar]
- Marguerat J., de Sousa R. C. Lanthanides and amphibian epithelia: block of the hormone-induced stimulation of sodium and water transport. Experientia. 1975 Jan 15;31(1):73–75. doi: 10.1007/BF01924686. [DOI] [PubMed] [Google Scholar]
- Northover B. J. Mechanism of the inhibitory action of indomethacin on smooth muscle. Br J Pharmacol. 1971 Mar;41(3):540–551. doi: 10.1111/j.1476-5381.1971.tb08052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northover B. J. The effects of indomethacin on calcium, sodium, potassium and magnesium fluxes in various tissues of the guinea-pig. Br J Pharmacol. 1972 Aug;45(4):651–659. doi: 10.1111/j.1476-5381.1972.tb08124.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramwell P. W., Shaw J. E. Biological significance of the prostaglandins. Recent Prog Horm Res. 1970;26:139–187. doi: 10.1016/b978-0-12-571126-5.50008-x. [DOI] [PubMed] [Google Scholar]
- Rider J., Thomas S. The effect of theophylline on sodium transport across frog-skin in the absence of chloride. Br J Pharmacol. 1969 Oct;37(2):539P–540P. [PMC free article] [PubMed] [Google Scholar]
- Smith J. B., Willis A. L. Aspirin selectively inhibits prostaglandin production in human platelets. Nat New Biol. 1971 Jun 23;231(25):235–237. doi: 10.1038/newbio231235a0. [DOI] [PubMed] [Google Scholar]
- Splawinski J. A., Nies A. S., Sweetman B., Oates J. A. The effects of arachidonic acid, prostaglandin E2 and prostaglandin F2alpha on the longitudinal stomach strip of the rat. J Pharmacol Exp Ther. 1973 Dec;187(3):501–510. [PubMed] [Google Scholar]
- VANE J. R. A sensitive method for the assay of 5-hydroxytryptamine. Br J Pharmacol Chemother. 1957 Sep;12(3):344–349. doi: 10.1111/j.1476-5381.1957.tb00146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vane J. R. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat New Biol. 1971 Jun 23;231(25):232–235. doi: 10.1038/newbio231232a0. [DOI] [PubMed] [Google Scholar]



