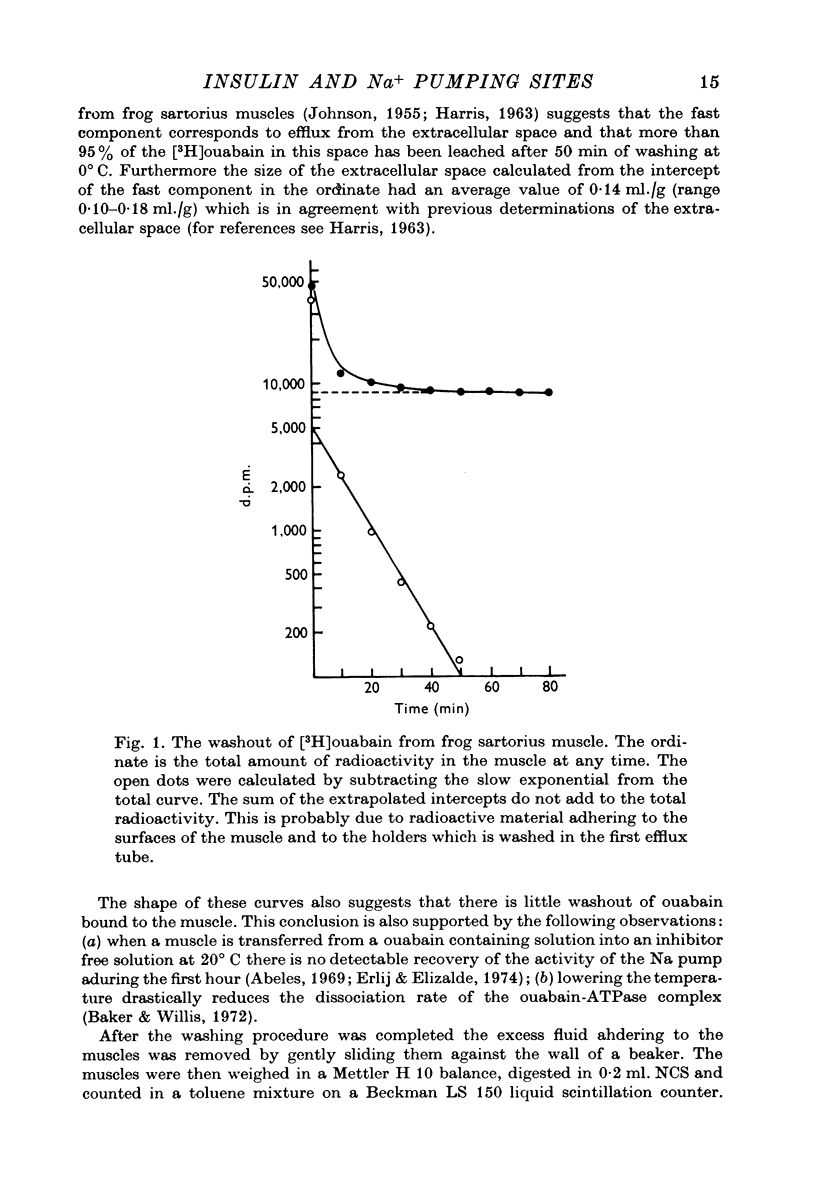
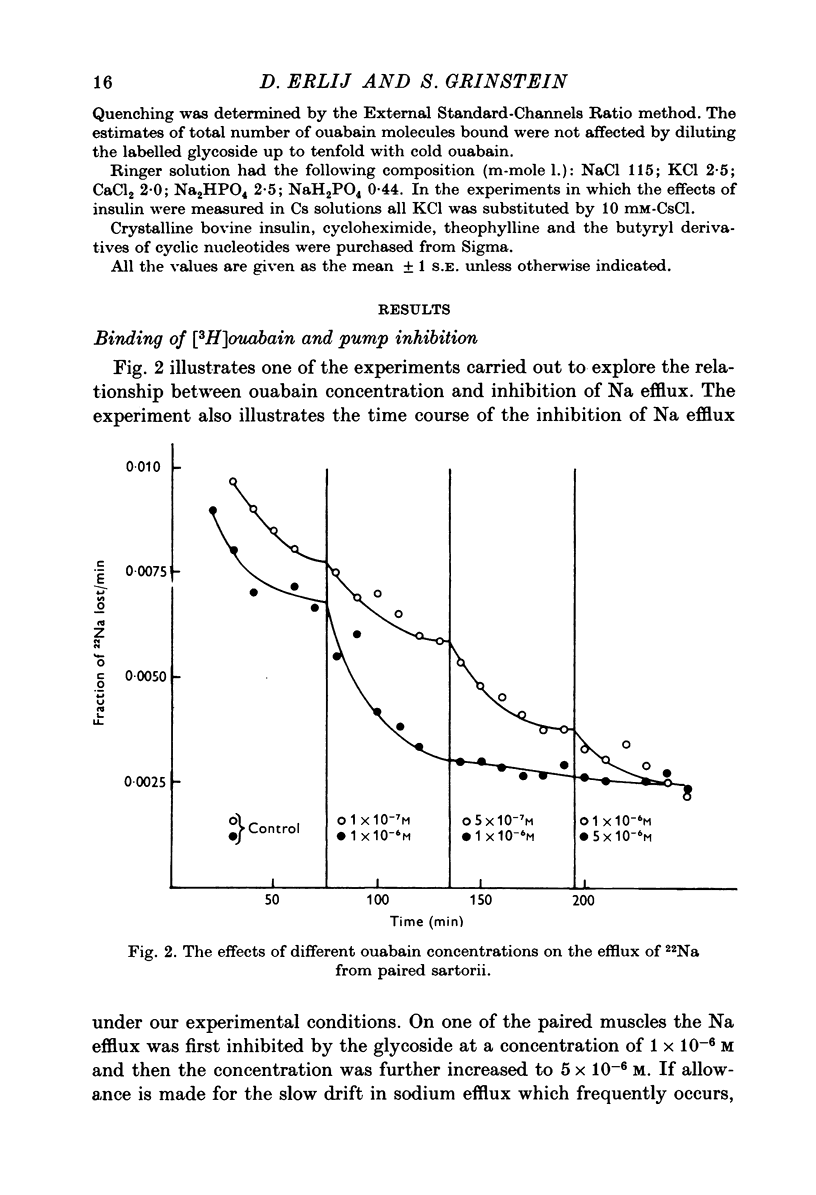
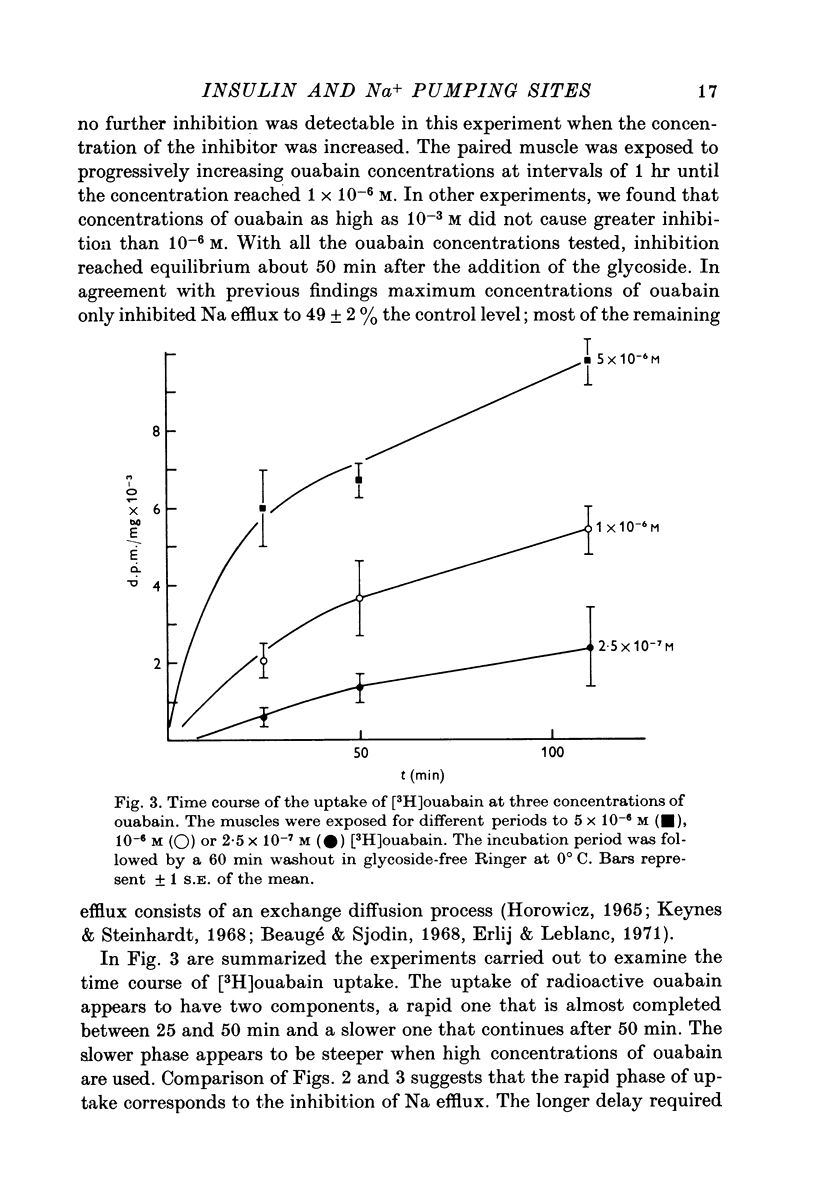
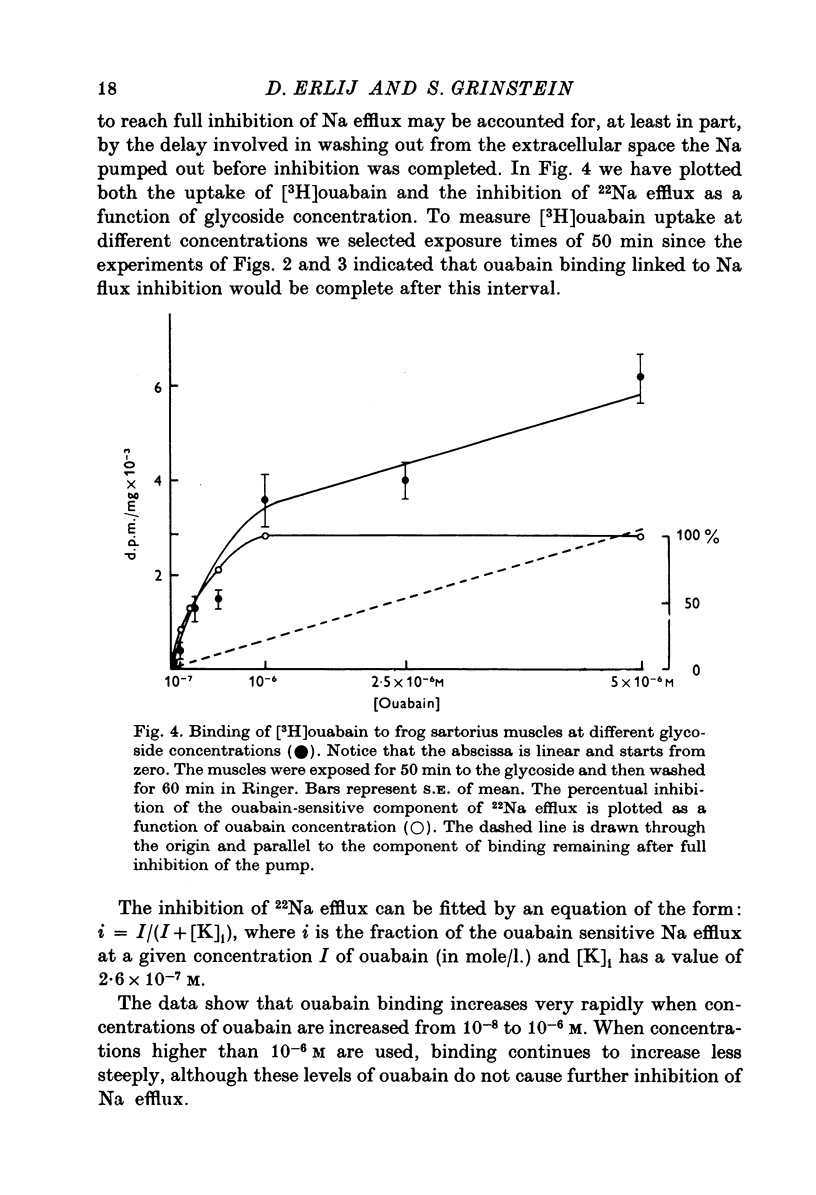
Abstract
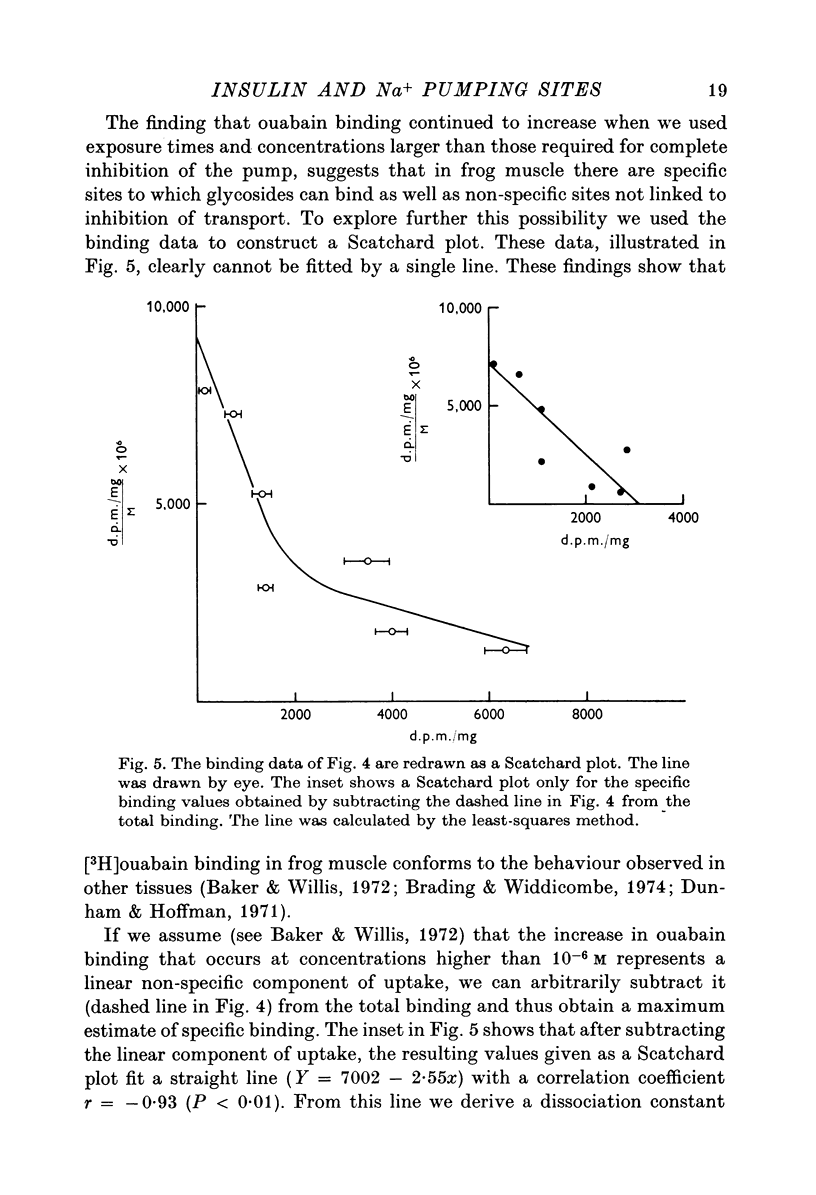
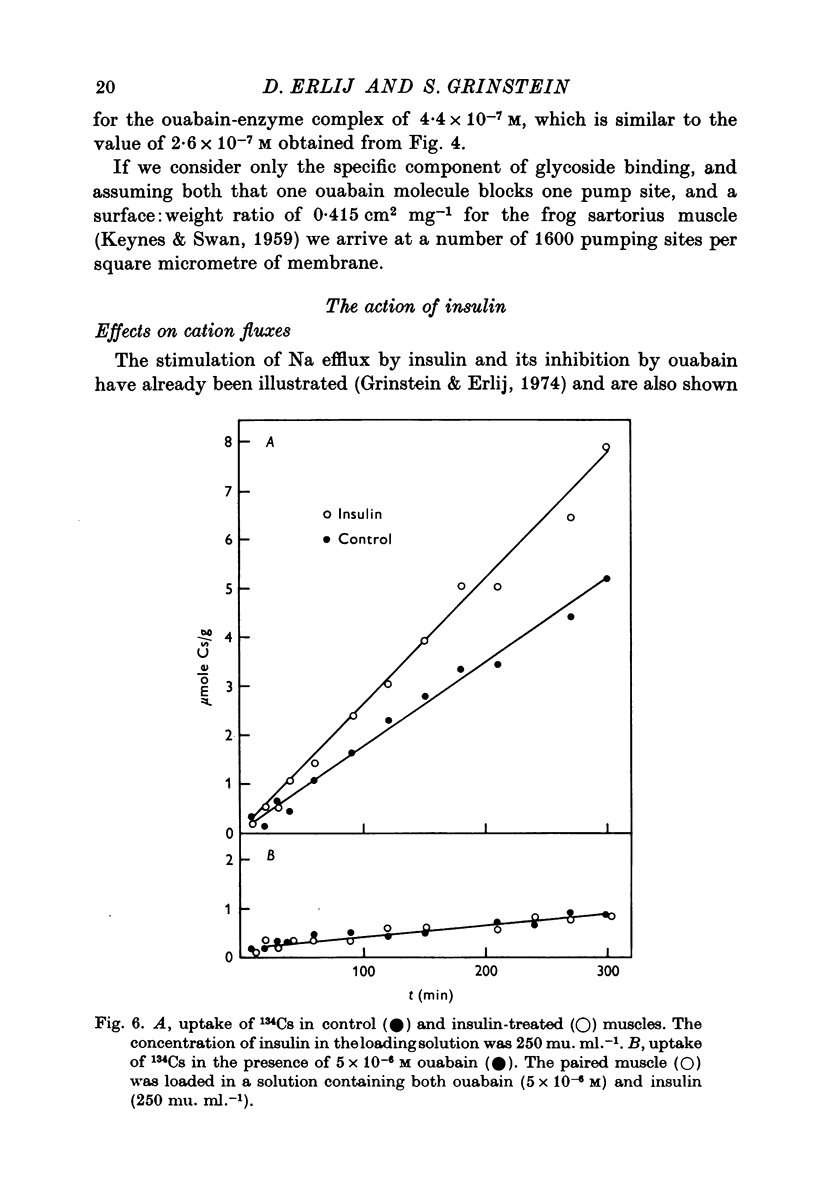
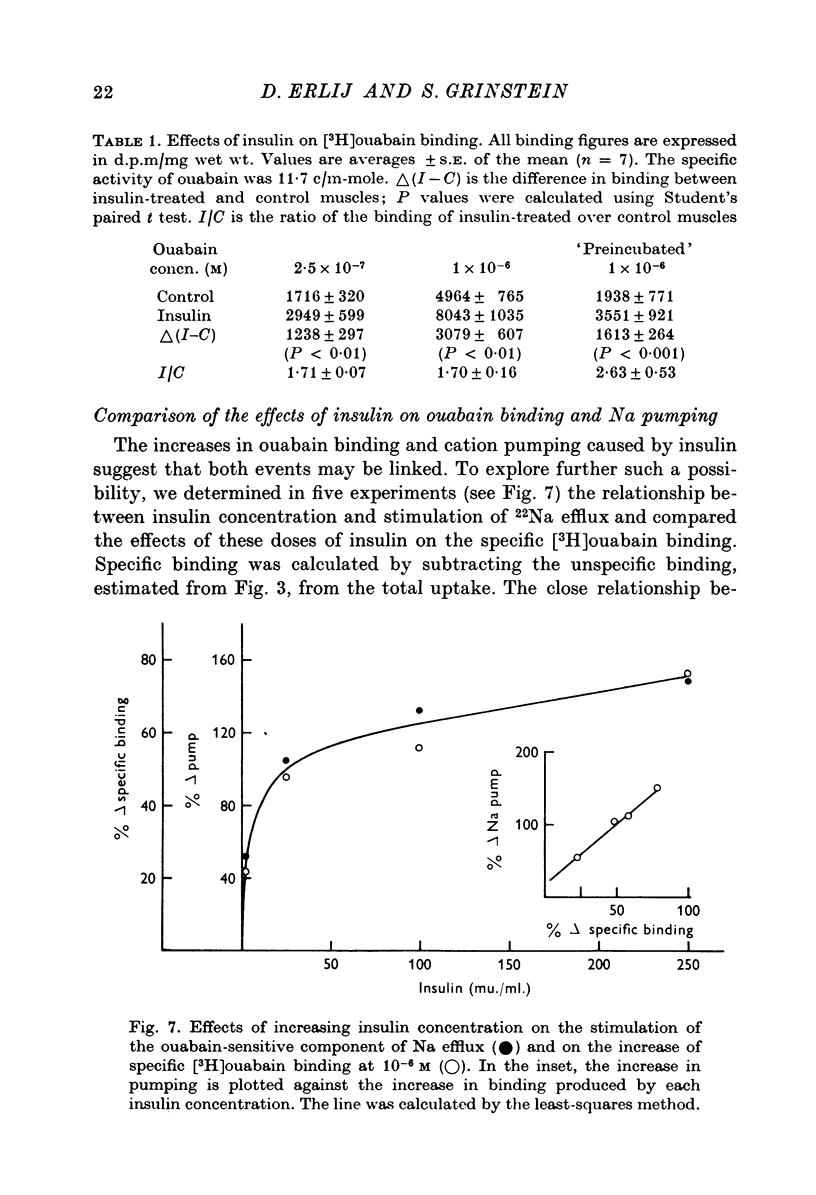
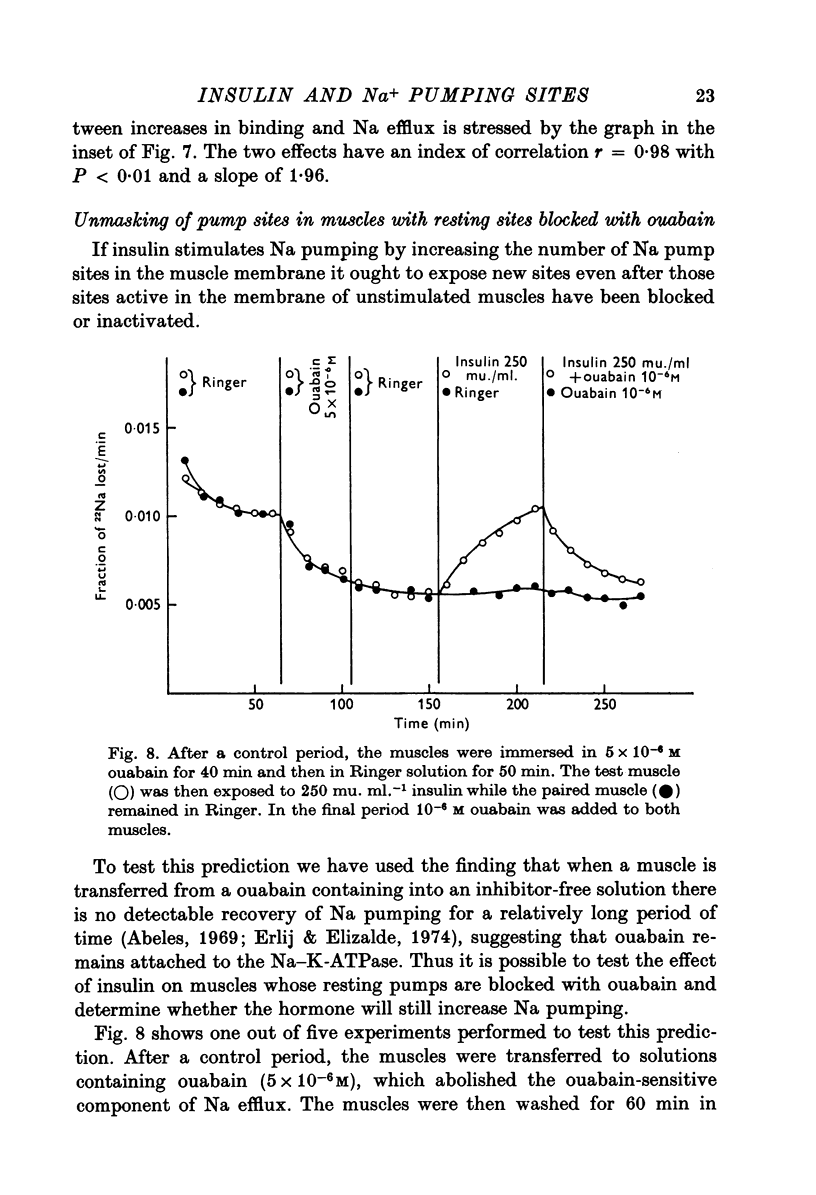
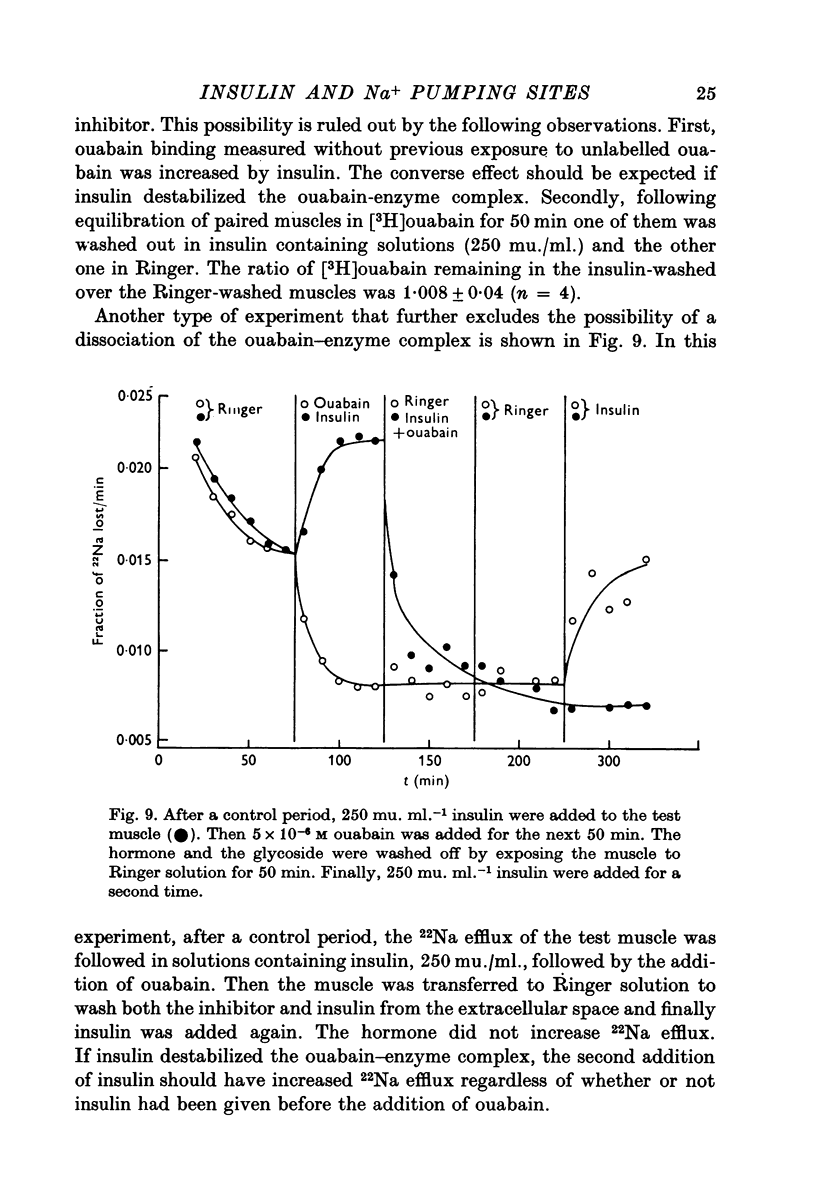
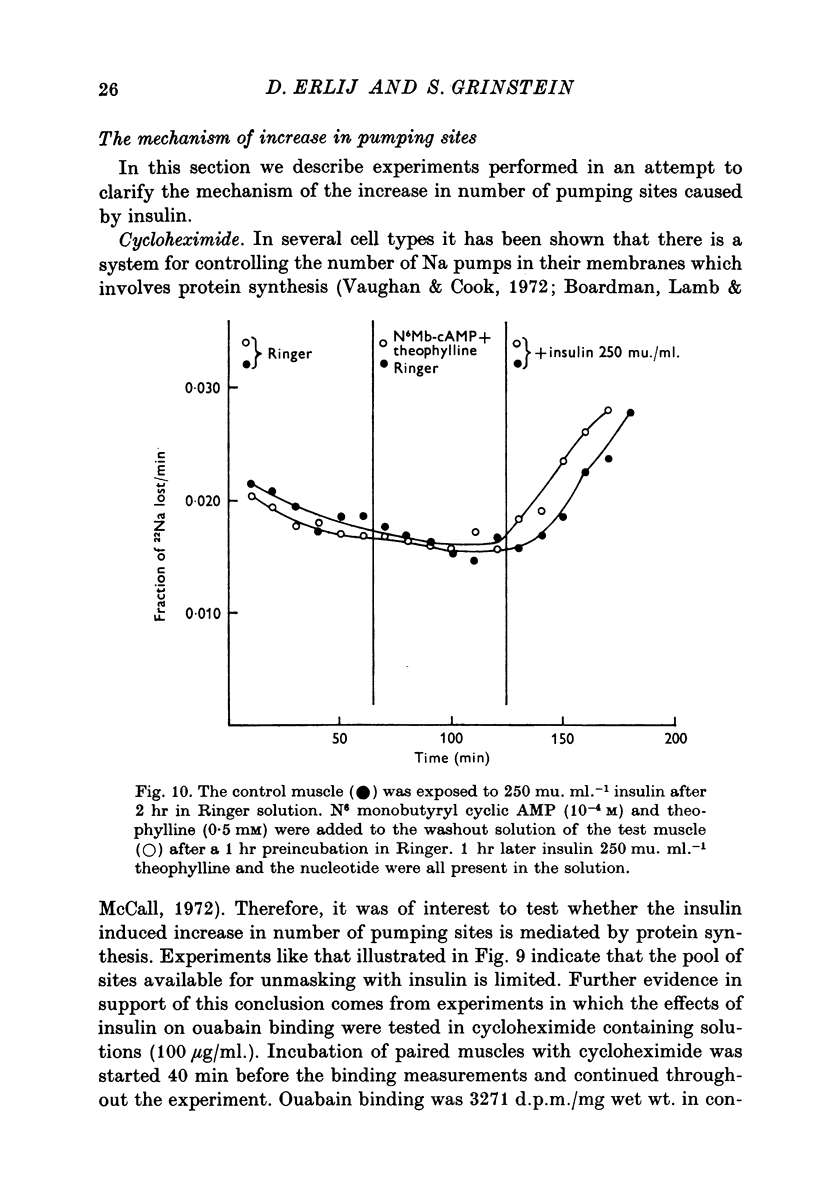
1. [3H]ouabain binding by frog sartorius muscles shows at least two components: one linked to inhibition of the pump and another not related to transport inhibition. This is suggested by the finding that [3H]ouabain uptake continued to increase when (a) the glycoside concentration was increased beyond that causing maximum transport inhibition, and (b) exposure times longer than those required to produce full inhibition were used. 2. A number of 1600 pumping sites per mum2 of membrane was estimated considering only the cylindrical surface of the muscle. 3. Insulin stimulated the ouabain-sensitive components of 22Na efflux and 134Cs influx. It also increased [3H]ouabain binding to a level of 1-7 times the total resting value. The increases in [3H]ouabain binding and in 22Na efflux followed a similar relationship with respect to insulin concentration. 4. Insulin stimulated the Na pump in muscles whose pumping sites had been inhibited by ouabain and then transferred to a glycoside-free solution. This stimulation was observed before detecting any recovery of the initial pumping activity. 5. When both the resting and the insulin-stimulated 22Na efflux had been blocked by ouabain, an additional dose of insulin, in a ouabain-free solution, had no further effects on 22Na efflux. 6. The effects of insulin were unaffected by cycloheximide or by high concentrations of butyryl derivatives of cyclic AMP and cyclic GMP. 7. We conclude that there are two pools of Na pumping sites in muscle cells: one active and another inactive. Insulin unmasks the inactive pumping sites by a mechanism that is independent of protein synthesis, increases in intracellular [Na] or decreases in intracellular [K].
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abeles A. L. Structure-activity relationships of several cardiotonic steroids with respect to inhibition of ion transport in frog muscle. J Gen Physiol. 1969 Aug;54(2):268–284. doi: 10.1085/jgp.54.2.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Willis J. S. Binding of the cardiac glycoside ouabain to intact cells. J Physiol. 1972 Jul;224(2):441–462. doi: 10.1113/jphysiol.1972.sp009904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaugé L. A., Sjodin R. A. Transport of caesium in frog muscle. J Physiol. 1968 Jan;194(1):105–123. doi: 10.1113/jphysiol.1968.sp008397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boardman L. J., Lamb J. F., McCall D. Uptake of ( 3 H)ouabain and Na pump turnover rates in cells cultured in ouabain. J Physiol. 1972 Sep;225(3):619–635. doi: 10.1113/jphysiol.1972.sp009960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brading A. F., Widdicombe J. H. An estimate of sodium-potassium pump activity and the number of pump sites in the smooth muscle of the guinea-pig taenia coli, using (3H)ouabain. J Physiol. 1974 Apr;238(2):235–249. doi: 10.1113/jphysiol.1974.sp010521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creese R. Sodium fluxes in diaphragm muscle and the effects of insulin and serum proteins. J Physiol. 1968 Jul;197(2):255–278. doi: 10.1113/jphysiol.1968.sp008558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DESMEDT J. E. Electrical activity and intracellular sodium concentration in frog muscle. J Physiol. 1953 Jul;121(1):191–205. doi: 10.1113/jphysiol.1953.sp004940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunham P. B., Hoffman J. F. Active cation transport and ouabain binding in high potassium and low potassium red blood cells of sheep. J Gen Physiol. 1971 Jul;58(1):94–116. doi: 10.1085/jgp.58.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta S., Rhee H. M., Marks B. H. Effect of metabolic inhibitors on the accumulation of digitaloids by the isolated guinea-pig heart. J Pharmacol Exp Ther. 1972 Feb;180(2):351–358. [PubMed] [Google Scholar]
- Erlij D., Elizalde A. Rapidly reversible inhibition of frog muscle sodium pump caused by cardiotonic steroids with modified lactone rings. Biochim Biophys Acta. 1974 Apr 12;345(1):49–54. doi: 10.1016/0005-2736(74)90244-2. [DOI] [PubMed] [Google Scholar]
- Erlij D., Leblanc G. The effects of ethacrynic acid and other sulphydryl reagents on sodium fluxes in frog muscle. J Physiol. 1971 Apr;214(2):327–347. doi: 10.1113/jphysiol.1971.sp009435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exton J. H., Lewis S. B., Ho R. J., Robison G. A., Park C. R. The role of cyclic AMP in the interaction of glucagon and insulin in the control of liver metabolism. Ann N Y Acad Sci. 1971 Dec 30;185:85–100. doi: 10.1111/j.1749-6632.1971.tb45239.x. [DOI] [PubMed] [Google Scholar]
- GOURLEY D. R. EFFECT OF INSULIN ON POTASSIUM EXCHANGE IN NORMAL AND OUABAIN-TREATED SKELETAL MUSCLE. J Pharmacol Exp Ther. 1965 Jun;148:339–347. [PubMed] [Google Scholar]
- García M., Leblanc G., Erlij D. Automatic collector of samples for determining isotope washout curves in muscle tissues. Pflugers Arch. 1969;311(3):278–282. doi: 10.1007/BF00590533. [DOI] [PubMed] [Google Scholar]
- Grinstein S., Erlij D. Insulin unmasks latent sodium pump sites in frog muscle. Nature. 1974 Sep 6;251(5470):57–58. doi: 10.1038/251057a0. [DOI] [PubMed] [Google Scholar]
- HARRIS E. J. Distribution and movement of muscle chloride. J Physiol. 1963 Apr;166:87–109. doi: 10.1113/jphysiol.1963.sp007092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARRIS E. J. Permeation and diffusion of K ions in frog muscle. J Gen Physiol. 1957 Sep 20;41(1):169–195. doi: 10.1085/jgp.41.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowicz P., Taylor J. W., Waggoner D. M. Fractionation of sodium effux in frog sartorius muscles by strophanthidin and removal of external sodium. J Gen Physiol. 1970 Mar;55(3):401–425. doi: 10.1085/jgp.55.3.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illiano G., Tell G. P., Siegel M. E., Cuatrecasas P. Guanosine 3':5'-cyclic monophosphate and the action of insulin and acetylcholine. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2443–2447. doi: 10.1073/pnas.70.8.2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHNSON J. A. Kinetics of release of radioactive sodium, sulfate and sucrose from the frog sartorius muscle. Am J Physiol. 1955 May;181(2):263–268. doi: 10.1152/ajplegacy.1955.181.2.263. [DOI] [PubMed] [Google Scholar]
- KEYNES R. D. SOME FURTHER OBSERVATIONS ON THE SODIUM EFFLUX IN FROG MUSCLE. J Physiol. 1965 May;178:305–325. doi: 10.1113/jphysiol.1965.sp007629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEYNES R. D., SWAN R. C. The effect of external sodium concentration on the sodium fluxes in frog skeletal muscle. J Physiol. 1959 Oct;147:591–625. doi: 10.1113/jphysiol.1959.sp006264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIPINIS D. M. Regulation of glucose uptake by muscle: functional significance of permeability and phosphorylating activity. Ann N Y Acad Sci. 1959 Sep 25;82:354–365. doi: 10.1111/j.1749-6632.1959.tb44916.x. [DOI] [PubMed] [Google Scholar]
- KIPNIS D. M., NOALL M. W. Stimulation of amino acid transport by insulin in the isolated rat diaphragm. Biochim Biophys Acta. 1958 Apr;28(1):226–227. doi: 10.1016/0006-3002(58)90466-9. [DOI] [PubMed] [Google Scholar]
- Keynes R. D., Steinhardt R. A. The components of the sodium efflux in frog muscle. J Physiol. 1968 Oct;198(3):581–599. doi: 10.1113/jphysiol.1968.sp008627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim N. D., Bailey L. E., Dresel P. E. The effect of insulin on the subcellular distribution and the inotropic effect of 3H-digoxin in the guinea pig heart. Life Sci I. 1970 Oct 1;9(19):1135–1139. doi: 10.1016/0024-3205(70)90145-1. [DOI] [PubMed] [Google Scholar]
- Krolenko S. A. Effect of fluxes of sugars and mineral ions on the light microscopic structure of frog fast muscle fibres. Nature. 1971 Feb 5;229(5284):424–426. doi: 10.1038/229424a0. [DOI] [PubMed] [Google Scholar]
- LEVINE R., GOLDSTEIN M. The action of insulin on the distribution of galactose in eviscerated nephrectomized dogs. J Biol Chem. 1949 Jun;179(2):985–985. [PubMed] [Google Scholar]
- MANCHESTER K. L., YOUNG F. G. The effect of insulin in vitro on the accumulation of amino acids by isolated rat diaphragm. Biochem J. 1960 Jun;75:487–495. doi: 10.1042/bj0750487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore R. D. Effect of insulin upon the sodium pump in frog skeletal muscle. J Physiol. 1973 Jul;232(1):23–45. doi: 10.1113/jphysiol.1973.sp010255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robison G. A., Sutherland E. W. Cyclic AMP and the function of eukaryotic cells: an introduction. Ann N Y Acad Sci. 1971 Dec 30;185:5–9. doi: 10.1111/j.1749-6632.1971.tb45229.x. [DOI] [PubMed] [Google Scholar]
- Sjodin R. A. Some Cation Interactions in Muscle. J Gen Physiol. 1961 May 1;44(5):929–962. doi: 10.1085/jgp.44.5.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan G. L., Cook J. S. Regeneration of cation-transport capacity in HeLa cell membranes after specific blockade by ouabain. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2627–2631. doi: 10.1073/pnas.69.9.2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZIERLER K. L. Effect of insulin on membrane potential and potassium content of rat muscle. Am J Physiol. 1959 Sep;197:515–523. doi: 10.1152/ajplegacy.1959.197.3.515. [DOI] [PubMed] [Google Scholar]



