Abstract
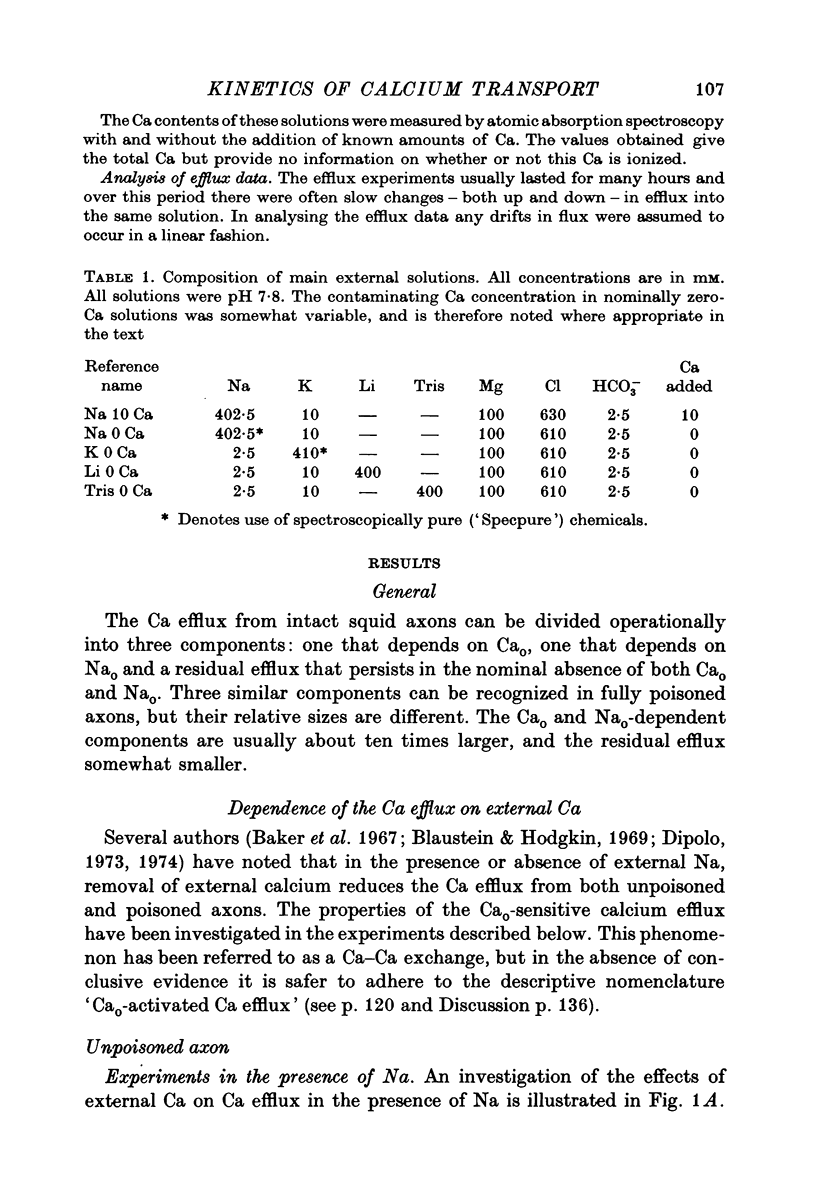
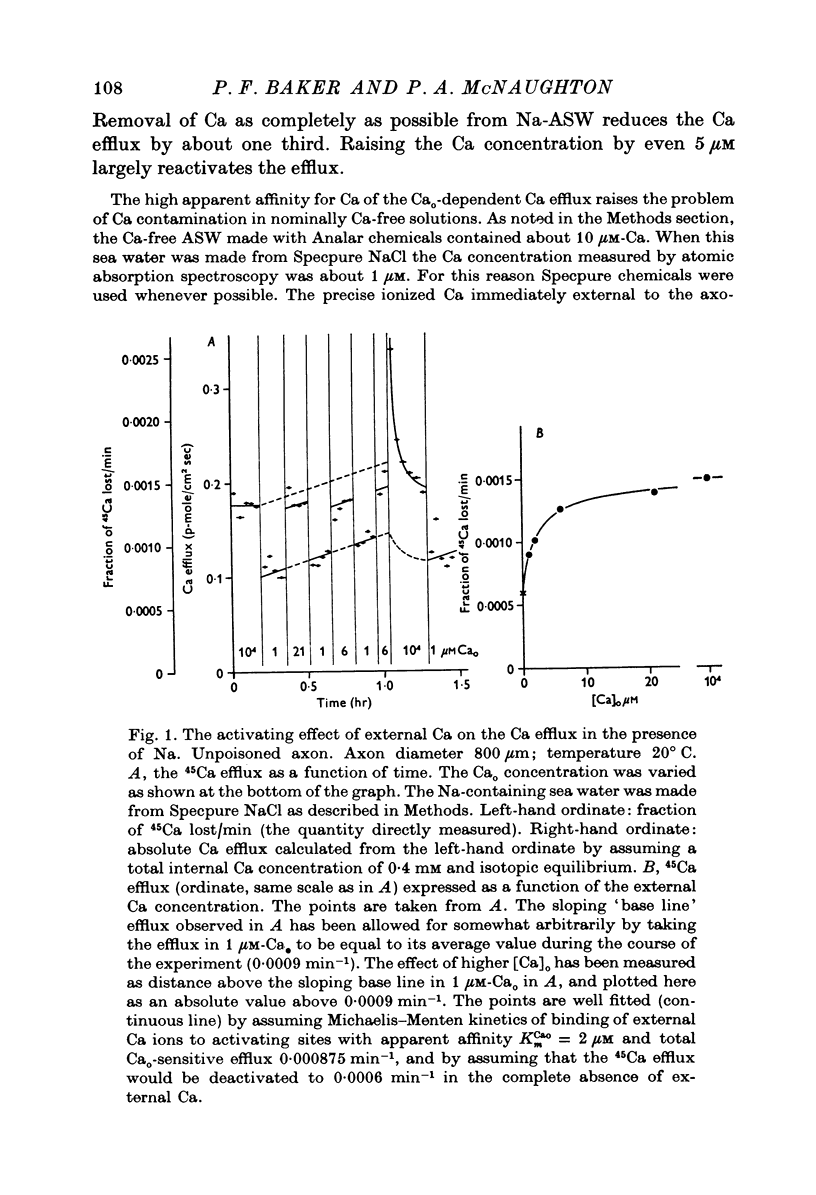
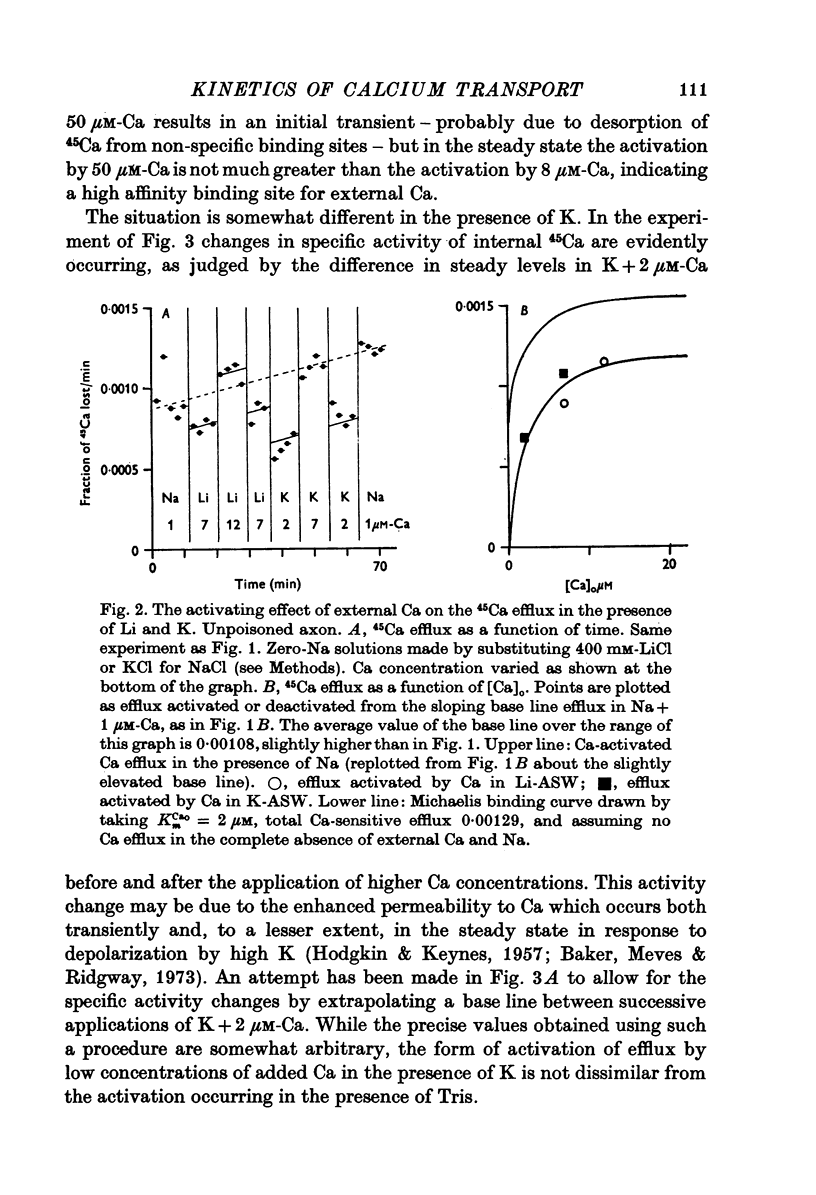
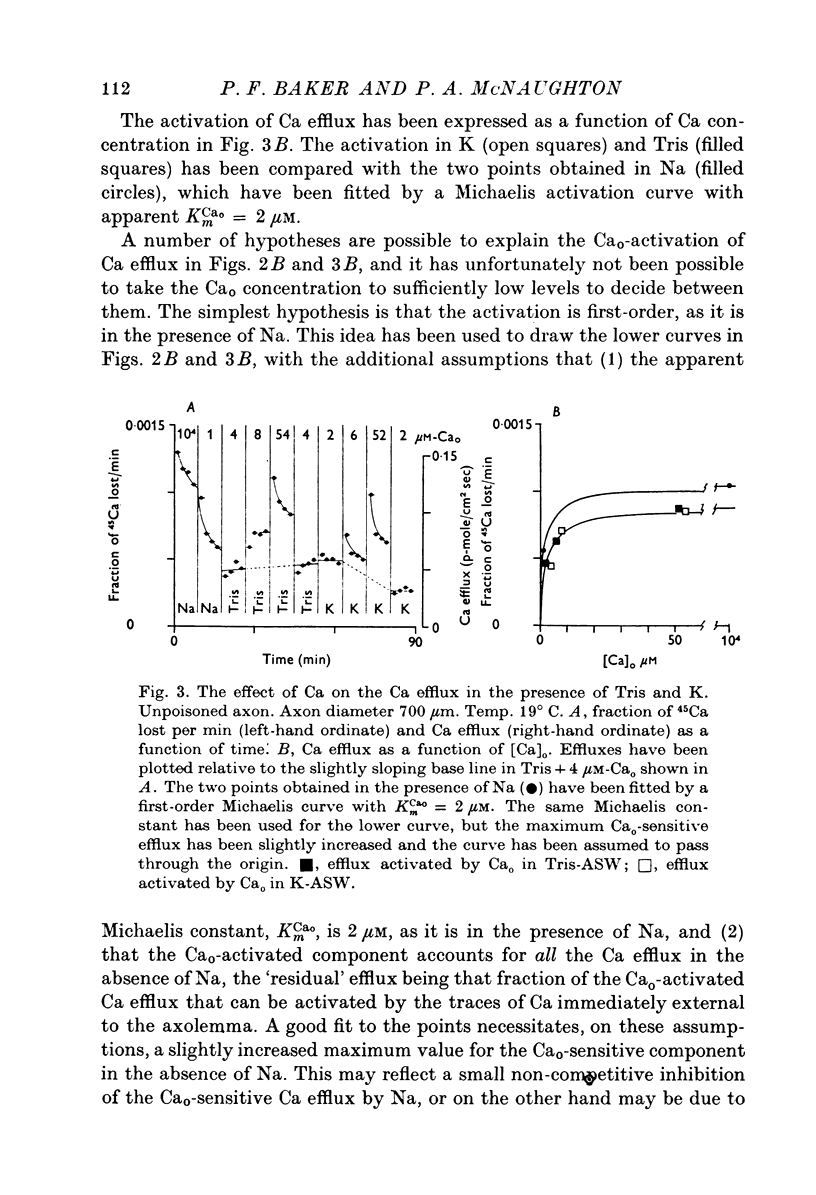
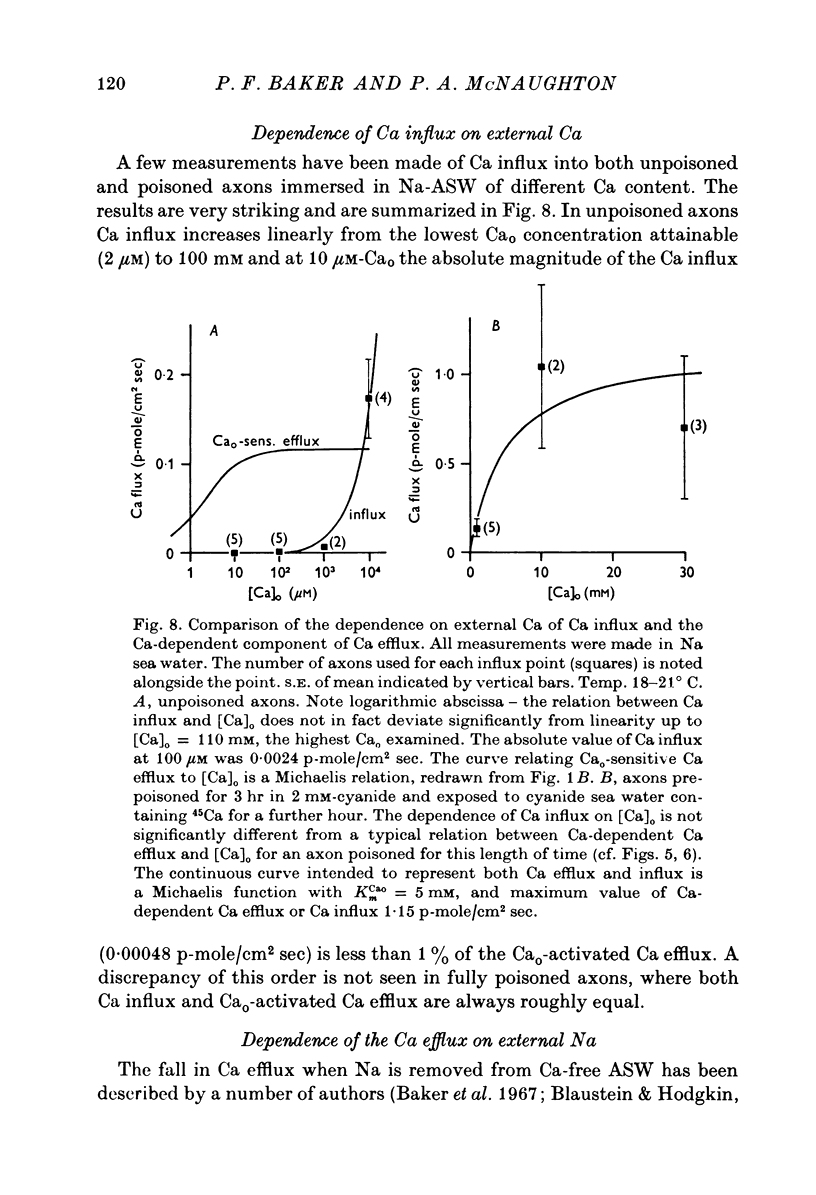
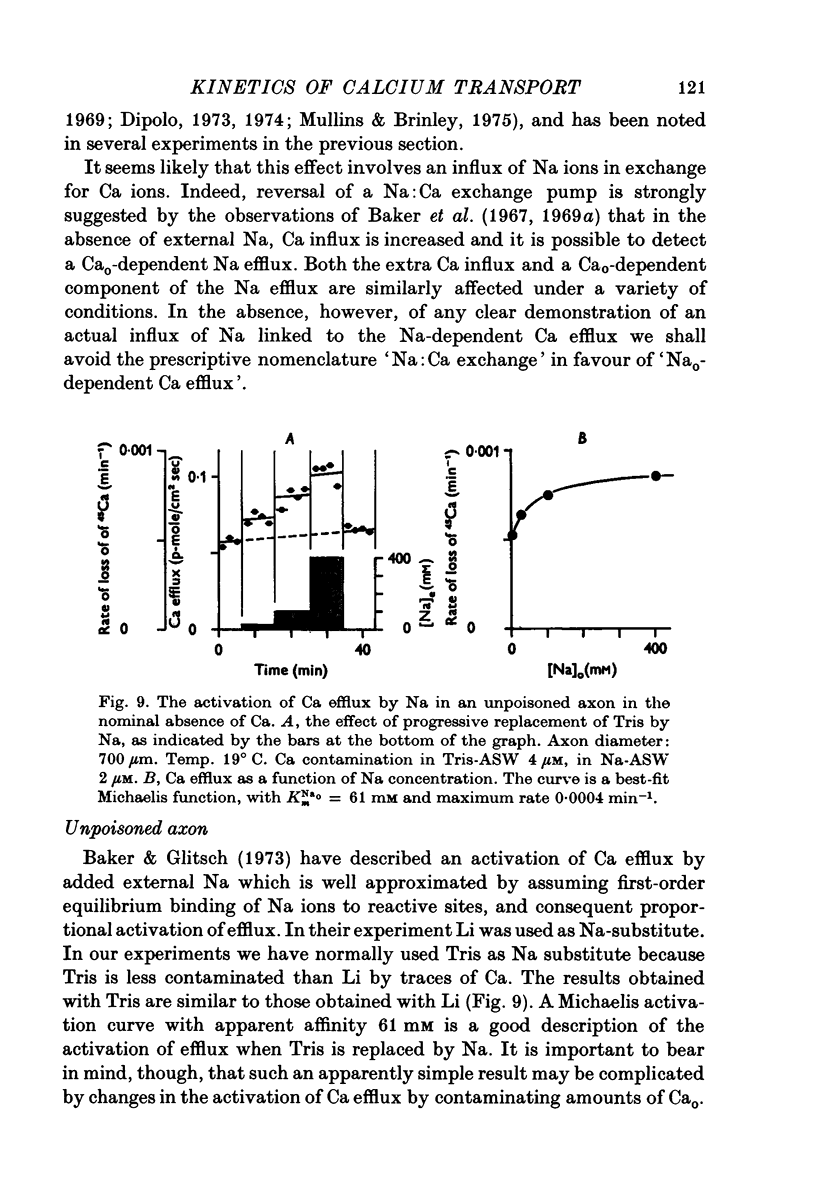
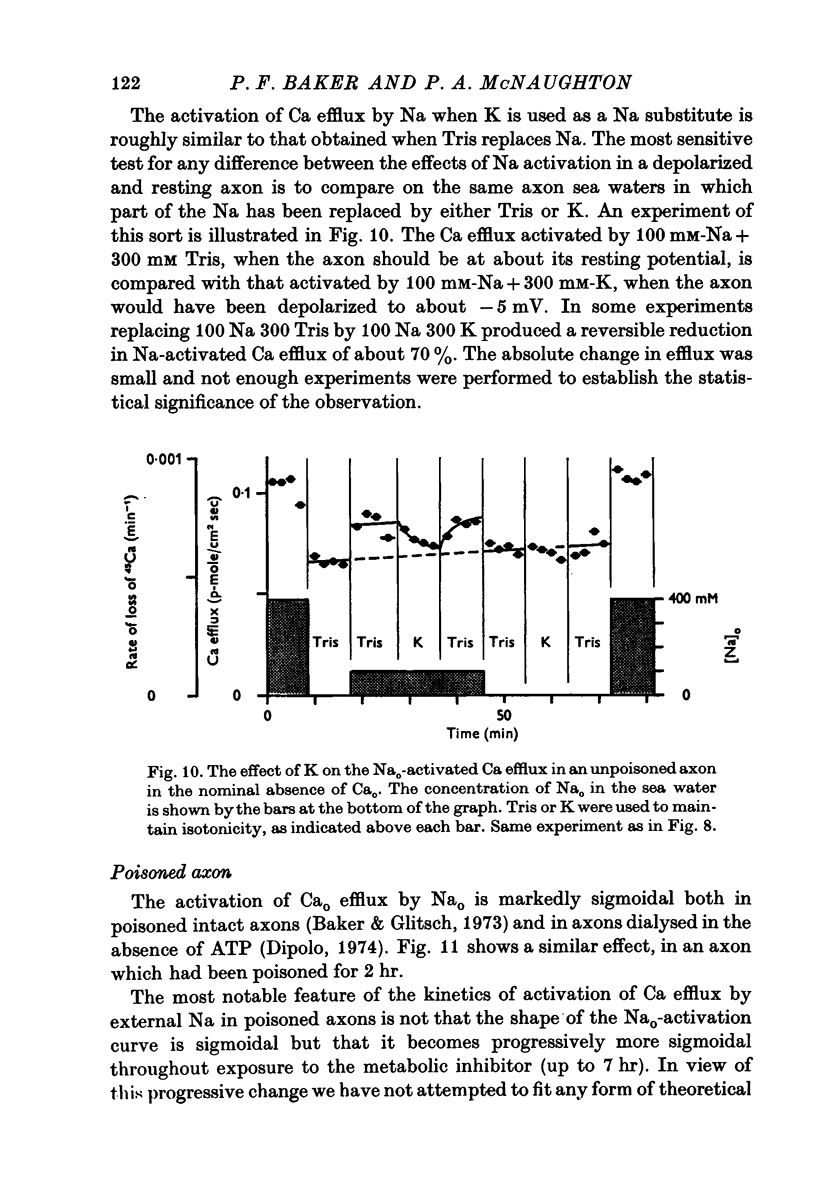
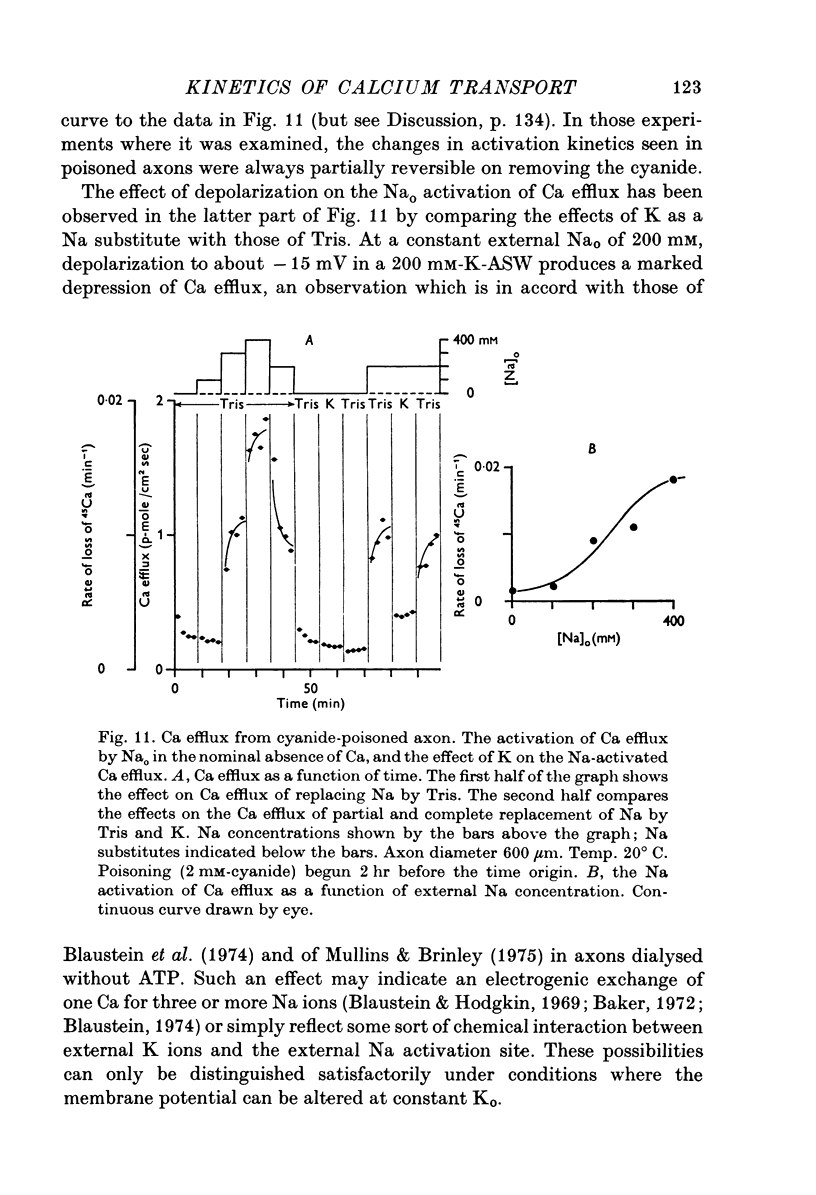
The Ca efflux from intact squid axons consists of three major components: one that is activated by Cao, one that is activated by Nao and a residual flux that persists in the nominal absence of both Cao and Nao. The properties of these components have been investigated in unpoisoned axons and in axons poisoned with cyanide. 2. Under all conditions the shape of the curve relating Cao to Cao-activated Ca efflux approximates to a section of a rectangular hyperbola, consistent with simple Michaelis activation. 3. The external Ca concentration giving half-maximal activation of Cao-activated Ca efflux is about 2 muM in unpoisoned axons immersed in Na-ASW, but on poisoning changes progressively to values in the range 1-10 mM. The residual efflux from unpoisoned axons may reflect activation by traces of Ca present immediately external to the axolemma. 4. The apparent affinity for Cao of Cao-activated Ca efflux is very similar in unpoisoned axons immersed in sea waters containing Na, Li, Tris or K as major cation, whereas in poisoned axons the affinity in Na and Li is about the same but higher than that in choline and Tris. 5. In unpoisoned axons Ca influx increases linearly as Cao is increased from 2 muM to 110 mM. The absolute value of the Ca influx from 10 muM-Cao is less than 1% of the Cao-activated Ca efflux at this external Ca concentration. In poisoned axons the sizes of Cao-activated Ca efflux and Ca influx were similar at all Ca concentrations examined. 6. The shape of the curve relating Nao to Nao-activated Ca efflux approximates to a section of rectangular hyperbola in unpoisoned axons but is clearly sigmoidal in axons that have been fully poisoned with cyanide. The sigmoidal shape develops progressively during poisoning. ...
Full text
PDF







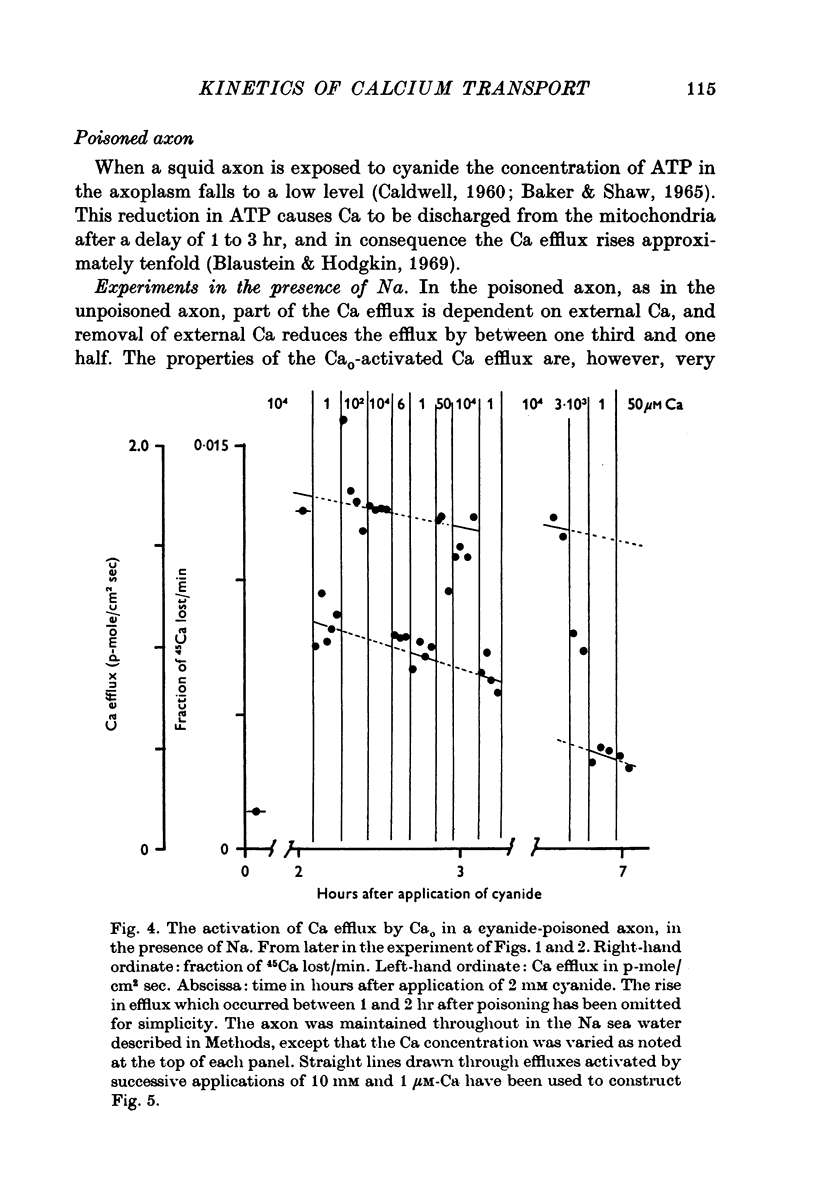
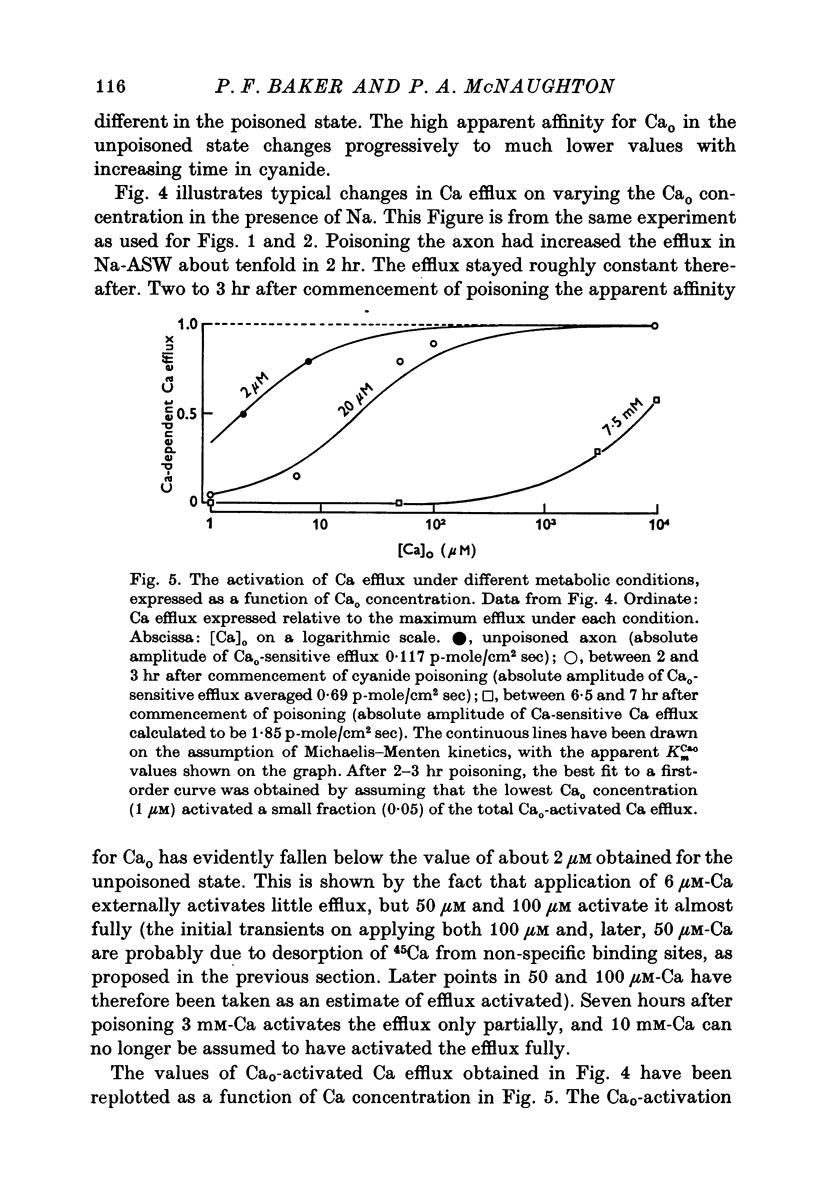

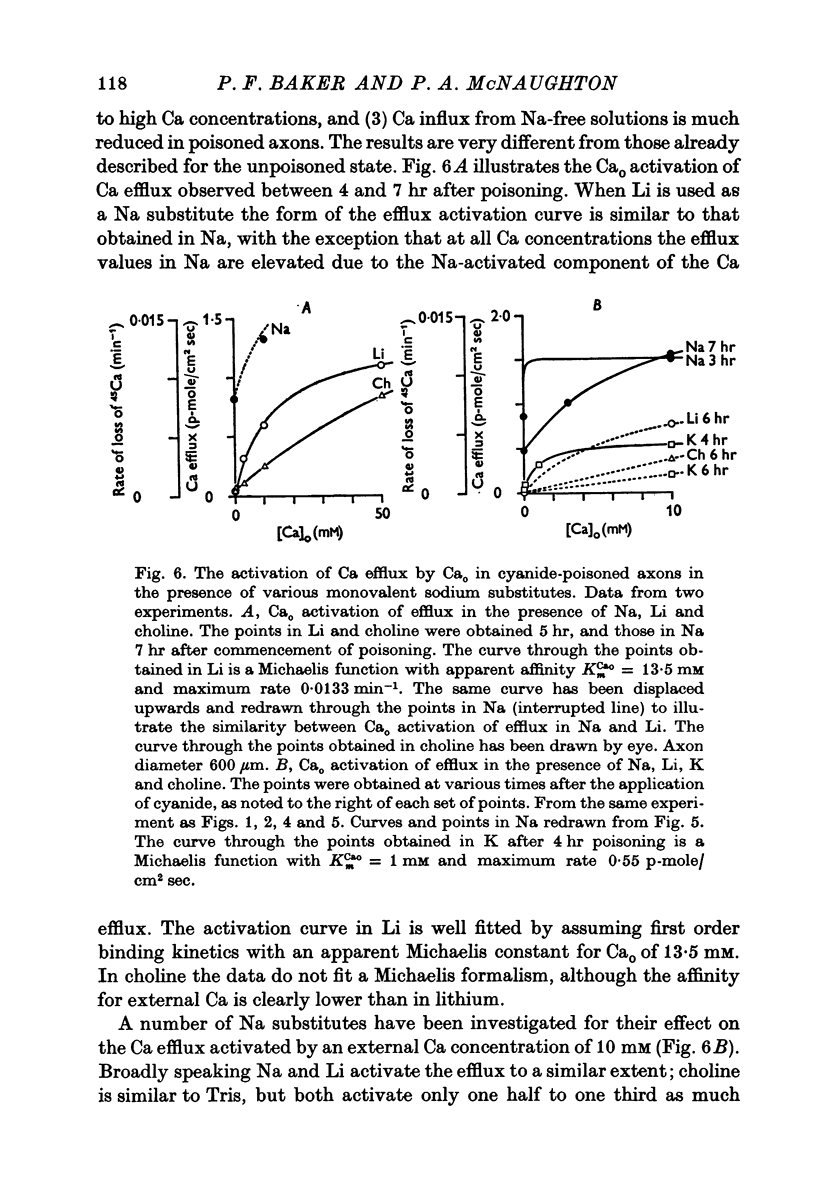
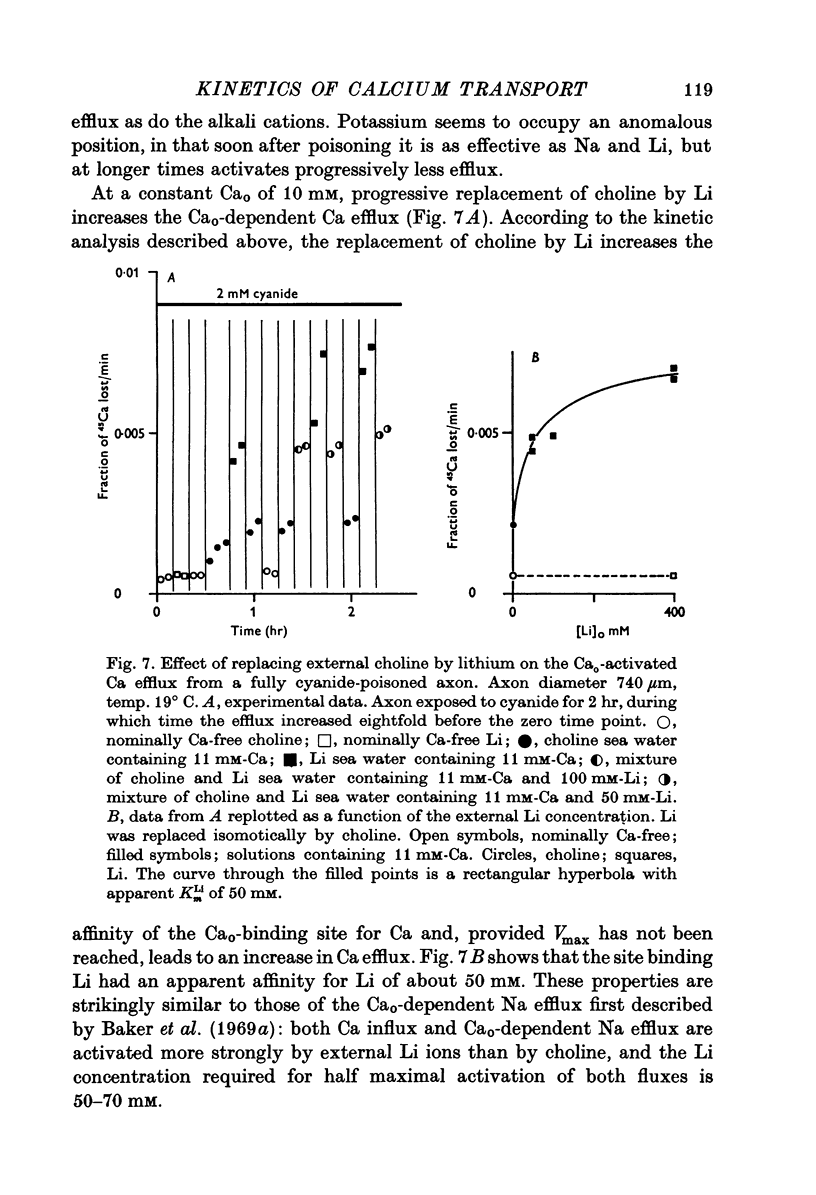















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker P. F., Blaustein M. P., Hodgkin A. L., Steinhardt R. A. The influence of calcium on sodium efflux in squid axons. J Physiol. 1969 Feb;200(2):431–458. doi: 10.1113/jphysiol.1969.sp008702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Blaustein M. P., Keynes R. D., Manil J., Shaw T. I., Steinhardt R. A. The ouabain-sensitive fluxes of sodium and potassium in squid giant axons. J Physiol. 1969 Feb;200(2):459–496. doi: 10.1113/jphysiol.1969.sp008703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Blaustein M. P. Proceedings: Apparatus for the internal dialysis of giant axons of Loligo forbesi: a comparison of calcium efflux from intact and dialysed axons. J Physiol. 1974 Oct;242(2):52P–54P. [PubMed] [Google Scholar]
- Baker P. F., Glitsch H. G. Does metabolic energy participate directly in the Na+-dependent extrusion of Ca2+ -Ca2+ ions from squid giant axons? J Physiol. 1973 Aug;233(1):44P–46P. [PubMed] [Google Scholar]
- Baker P. F., Hodgkin A. L., Ridgway E. B. Depolarization and calcium entry in squid giant axons. J Physiol. 1971 Nov;218(3):709–755. doi: 10.1113/jphysiol.1971.sp009641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Meves H., Ridgway E. B. Calcium entry in response to maintained depolarization of squid axons. J Physiol. 1973 Jun;231(3):527–548. doi: 10.1113/jphysiol.1973.sp010247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Potashner S. J. The role of metabolic energy in the transport of glutamate by invertebrate nerve. Biochim Biophys Acta. 1973 Aug 9;318(1):123–139. doi: 10.1016/0005-2736(73)90342-8. [DOI] [PubMed] [Google Scholar]
- Baker P. F., Shaw T. I. A comparison of the phosphorus metabolism of intact squid nerve with that of the isolated axoplasm and sheath. J Physiol. 1965 Sep;180(2):424–438. doi: 10.1113/jphysiol.1965.sp007710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Stone A. J. A kinetic method for investigating hypothetical models of the sodium pump. Biochim Biophys Acta. 1966 Oct 10;126(2):321–329. doi: 10.1016/0926-6585(66)90069-0. [DOI] [PubMed] [Google Scholar]
- Blaustein M. P., Hodgkin A. L. The effect of cyanide on the efflux of calcium from squid axons. J Physiol. 1969 Feb;200(2):497–527. doi: 10.1113/jphysiol.1969.sp008704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein M. P., Oborn C. J. The influence of sodium on calcium fluxes in pinched-off nerve terminals in vitro. J Physiol. 1975 Jun;247(3):657–686. doi: 10.1113/jphysiol.1975.sp010951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein M. P., Russell J. M. Sodium-calcium exchange and calcium-calcium exchange in internally dialyzed squid giant axons. J Membr Biol. 1975 Jul 24;22(3-4):285–312. doi: 10.1007/BF01868176. [DOI] [PubMed] [Google Scholar]
- Blaustein M. P., Russell J. M., Weer P. Calcium efflux from internally dialyzed squid axons: the influence of external and internal cations. J Supramol Struct. 1974;2(5-6):558–581. doi: 10.1002/jss.400020505. [DOI] [PubMed] [Google Scholar]
- Blaustein M. P. The interrelationship between sodium and calcium fluxes across cell membranes. Rev Physiol Biochem Pharmacol. 1974;70:33–82. doi: 10.1007/BFb0034293. [DOI] [PubMed] [Google Scholar]
- Brinley F. J., Jr, Spangler S. G., Mullins L. J. Calcium and EDTA fluxes in dialyzed squid axons. J Gen Physiol. 1975 Aug;66(2):223–250. doi: 10.1085/jgp.66.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CALDWELL P. C. The phosphorus metabolism of squid axons and its relationship to the active transport of sodium. J Physiol. 1960 Jul;152:545–560. doi: 10.1113/jphysiol.1960.sp006508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dipolo R. Calcium efflux from internally dialyzed squid giant axons. J Gen Physiol. 1973 Nov;62(5):575–589. doi: 10.1085/jgp.62.5.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dipolo R. Effect of ATP on the calcium efflux in dialyzed squid giant axons. J Gen Physiol. 1974 Oct;64(4):503–517. doi: 10.1085/jgp.64.4.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKENHAEUSER B., HODGKIN A. L. The after-effects of impulses in the giant nerve fibres of Loligo. J Physiol. 1956 Feb 28;131(2):341–376. doi: 10.1113/jphysiol.1956.sp005467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garay R. P., Garrahan P. J. The interaction of sodium and potassium with the sodium pump in red cells. J Physiol. 1973 Jun;231(2):297–325. doi: 10.1113/jphysiol.1973.sp010234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrahan P. J., Garay R. P. A kinetic study of the Na pump in red cells: its relevance to the mechanism of active transport. Ann N Y Acad Sci. 1974;242(0):445–458. doi: 10.1111/j.1749-6632.1974.tb19108.x. [DOI] [PubMed] [Google Scholar]
- Glynn I. M., Karlish S. J. The sodium pump. Annu Rev Physiol. 1975;37:13–55. doi: 10.1146/annurev.ph.37.030175.000305. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., KEYNES R. D. Experiments on the injection of substances into squid giant axons by means of a microsyringe. J Physiol. 1956 Mar 28;131(3):592–616. doi: 10.1113/jphysiol.1956.sp005485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KEYNES R. D. Movements of labelled calcium in squid giant axons. J Physiol. 1957 Sep 30;138(2):253–281. doi: 10.1113/jphysiol.1957.sp005850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman P. G., Tosteson D. C. Active sodium and potassium transport in high potassium and low potassium sheep red cells. J Gen Physiol. 1971 Oct;58(4):438–466. doi: 10.1085/jgp.58.4.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jundt H., Porzig H., Reuter H., Stucki J. W. The effect of substances releasing intracellular calcium ions on sodium-dependent calcium efflux from guinea-pig auricles. J Physiol. 1975 Mar;246(1):229–253. doi: 10.1113/jphysiol.1975.sp010888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins L. J., Brinley F. J., Jr Sensitivity of calcium efflux from squid axons to changes in membrane potential. J Gen Physiol. 1975 Feb;65(2):135–152. doi: 10.1085/jgp.65.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas E., Hidalgo C. Effect of temperature and metabolic inhibitors on 45Ca outflow from squid giant axons. Biochim Biophys Acta. 1968 Dec 10;163(4):550–556. doi: 10.1016/0005-2736(68)90084-9. [DOI] [PubMed] [Google Scholar]
- Russell J. M., Blaustein M. P. Calcium efflux from barnacle muscle fibers. Dependence on external cations. J Gen Physiol. 1974 Feb;63(2):144–167. doi: 10.1085/jgp.63.2.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons T. J. The interaction of ATP-analogues possessing a blocked gamma-phosphate group with the sodium pump in human red cells. J Physiol. 1975 Jan;244(3):731–739. doi: 10.1113/jphysiol.1975.sp010822. [DOI] [PMC free article] [PubMed] [Google Scholar]


