Abstract
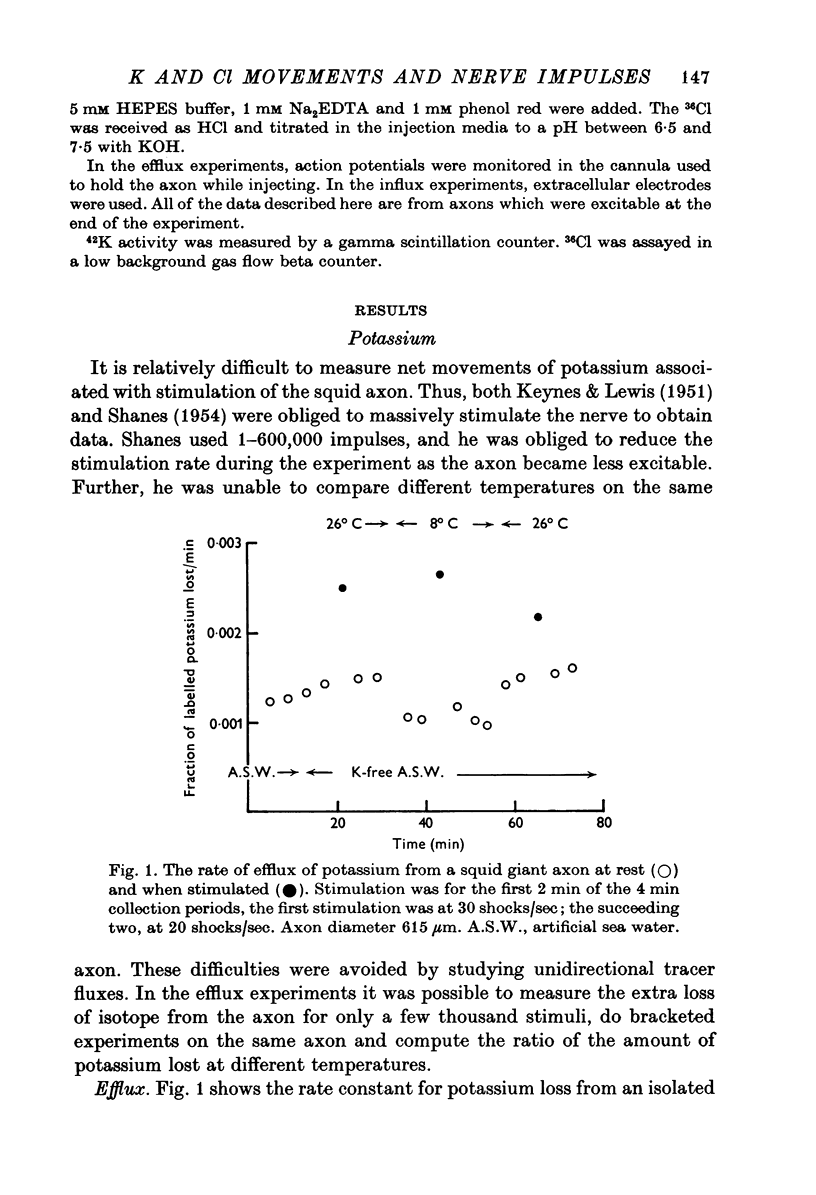
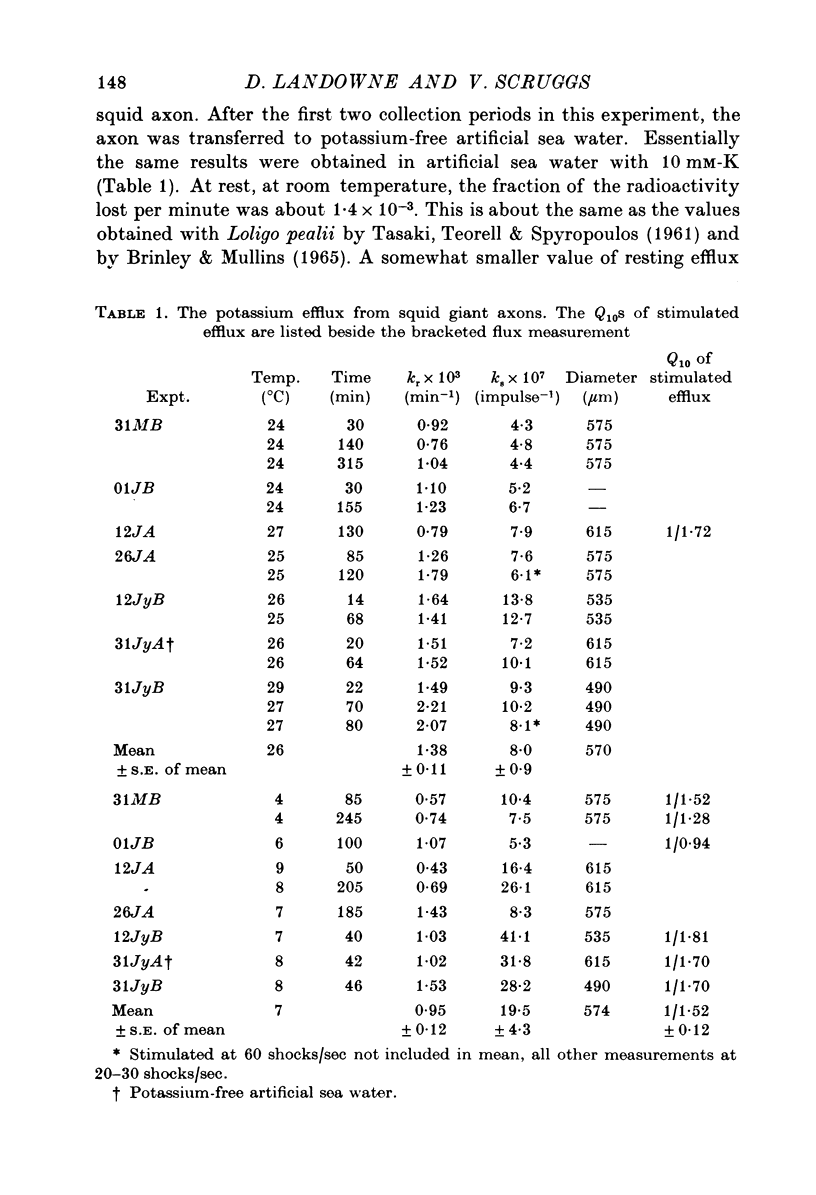
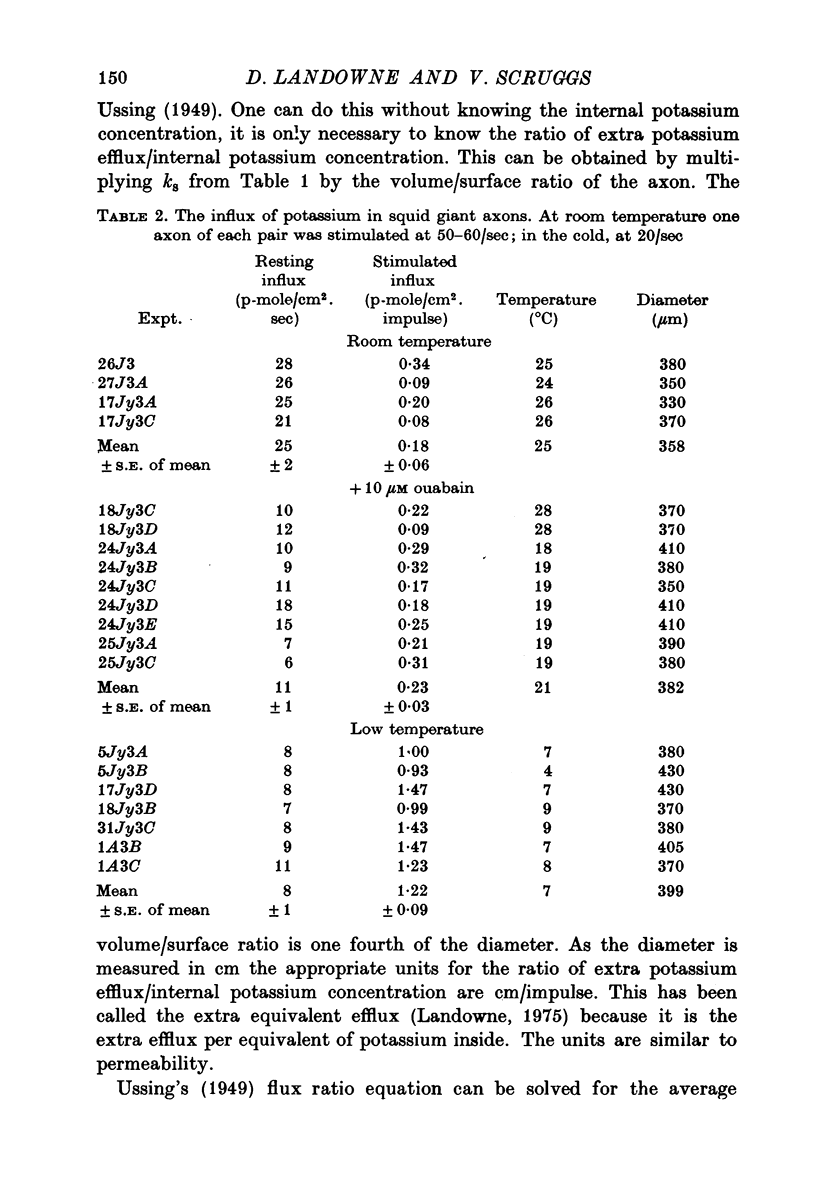
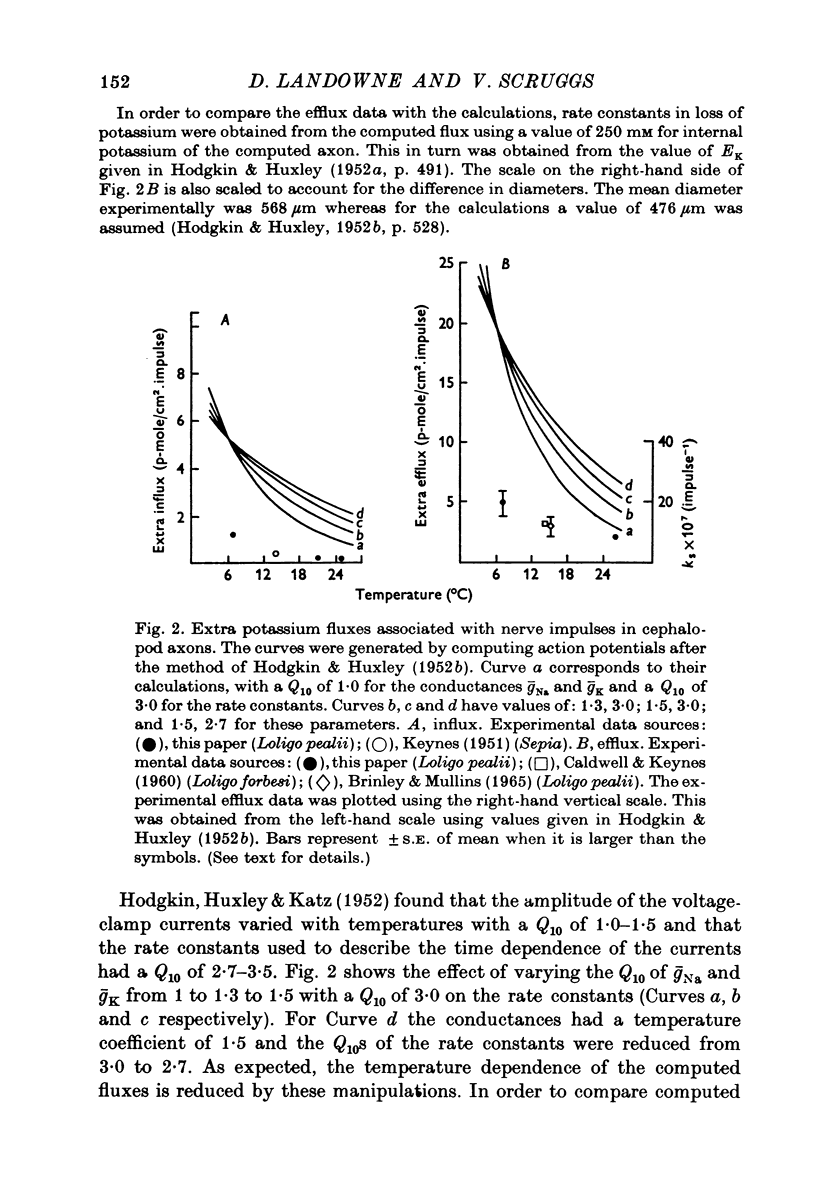
1. The influx and efflux of radioactive potassium and chloride across the membrane of the squid giant axon were measured in resting and in stimulated nerves. The measurements were made at room temperature and at 6-8 degrees C. 2. At room temperature all eight flux measurements were comparable to previously reported values. 3. When the axons were cooled the resting potassium influx decreased with a Q10 of 1-9 and the resting potassium efflux decreased with a Q10 of 1-2. 4. With cooling the resting chloride efflux decreased with a Q10 of 1-3 and the resting chloride influx decreased with a Q10 of 2-8. This latter value, together with anomalous flux ratios for resting chloride fluxes may indicate an active uptake of chloride ions into the axon. 5. Cooling increased the extra efflux of potassium associated with nerve impulses with a Q10 of 1/1-5 and increased the extra influx of potassium with a Q10 of 1/3-3. 6. No extra efflux of chloride was detected at either temperature. Cooling produced no statistically significant change in the extra chloride influx but there was considerable scatter in the data. 7. Fluxes were computed as a function of temperature for standard action potentials with a variety of temperature coefficients for the conductances and rate constants. No single curve could match either the influx or the efflux data.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRINLEY F. J., Jr, MULLINS L. J. ION FLUXES AND TRANSFERENCE NUMBER IN SQUID AXONS. J Neurophysiol. 1965 May;28:526–544. doi: 10.1152/jn.1965.28.3.526. [DOI] [PubMed] [Google Scholar]
- CALDWELL P. C., KEYNES R. D. The permeability of the squid giant axon to radioactive potassium and chloride ions. J Physiol. 1960 Nov;154:177–189. doi: 10.1113/jphysiol.1960.sp006572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FITZHUGH R., COLE K. S. THEORETICAL POTASSIUM LOSS FROM SQUID AXONS AS A FUNCTION OF TEMPERATURE. Biophys J. 1964 Jul;4:257–265. doi: 10.1016/s0006-3495(64)86781-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F., KATZ B. Measurement of current-voltage relations in the membrane of the giant axon of Loligo. J Physiol. 1952 Apr;116(4):424–448. doi: 10.1113/jphysiol.1952.sp004716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. The components of membrane conductance in the giant axon of Loligo. J Physiol. 1952 Apr;116(4):473–496. doi: 10.1113/jphysiol.1952.sp004718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KATZ B. The effect of temperature on the electrical activity of the giant axon of the squid. J Physiol. 1949 Aug;109(1-2):240–249. doi: 10.1113/jphysiol.1949.sp004388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KEYNES R. D. Active transport of cations in giant axons from Sepia and Loligo. J Physiol. 1955 Apr 28;128(1):28–60. doi: 10.1113/jphysiol.1955.sp005290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KEYNES R. D. Movements of labelled calcium in squid giant axons. J Physiol. 1957 Sep 30;138(2):253–281. doi: 10.1113/jphysiol.1957.sp005850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KEYNES R. D. The potassium permeability of a giant nerve fibre. J Physiol. 1955 Apr 28;128(1):61–88. doi: 10.1113/jphysiol.1955.sp005291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEYNES R. D. CHLORIDE IN THE SQUID GIANT AXON. J Physiol. 1963 Dec;169:690–705. doi: 10.1113/jphysiol.1963.sp007289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEYNES R. D., LEWIS P. R. The sodium and potassium content of cephalopod nerve fibers. J Physiol. 1951 Jun;114(1-2):151–182. doi: 10.1113/jphysiol.1951.sp004609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEYNES R. D. The ionic movements during nervous activity. J Physiol. 1951 Jun;114(1-2):119–150. doi: 10.1113/jphysiol.1951.sp004608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landowne D. A comparison of radioactive thallium and potassium fluxes in the giant axon of the squid. J Physiol. 1975 Oct;252(1):79–96. doi: 10.1113/jphysiol.1975.sp011135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore J. W., Ramon F. On numerical integration of the Hodgkin and Huxley equations for a membrane action potential. J Theor Biol. 1974 May;45(1):249–273. doi: 10.1016/0022-5193(74)90054-x. [DOI] [PubMed] [Google Scholar]
- Mullins L. J., Brinley F. J., Jr Potassium fluxes in dialyzed squid axons. J Gen Physiol. 1969 Jun;53(6):704–740. doi: 10.1085/jgp.53.6.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins L. J. Ion and molecule fluxes in squid axons. Ann N Y Acad Sci. 1966 Jul 14;137(2):830–836. doi: 10.1111/j.1749-6632.1966.tb50203.x. [DOI] [PubMed] [Google Scholar]
- TASAKI I., TEORELL T., SPYROPOULOS C. S. Movement of radioactive tracers across squid axon membrane. Am J Physiol. 1961 Jan;200:11–22. doi: 10.1152/ajplegacy.1961.200.1.11. [DOI] [PubMed] [Google Scholar]


