Abstract
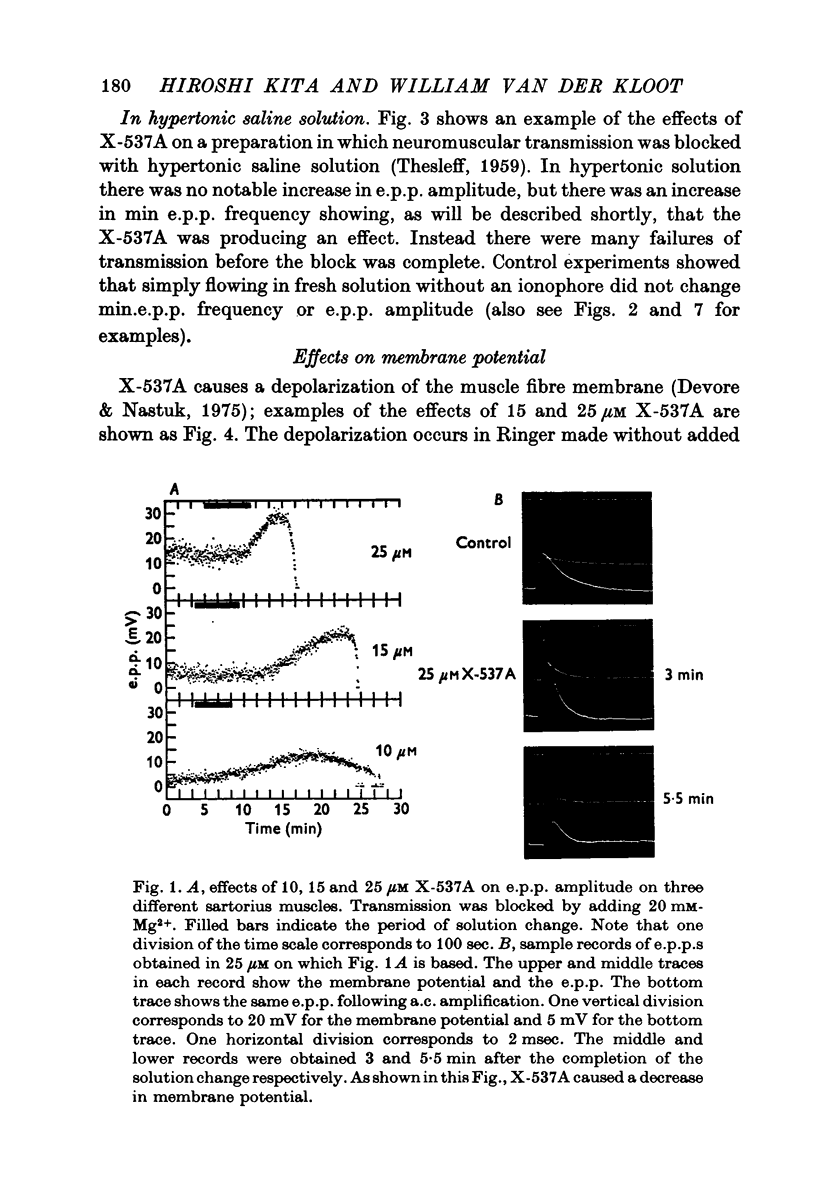
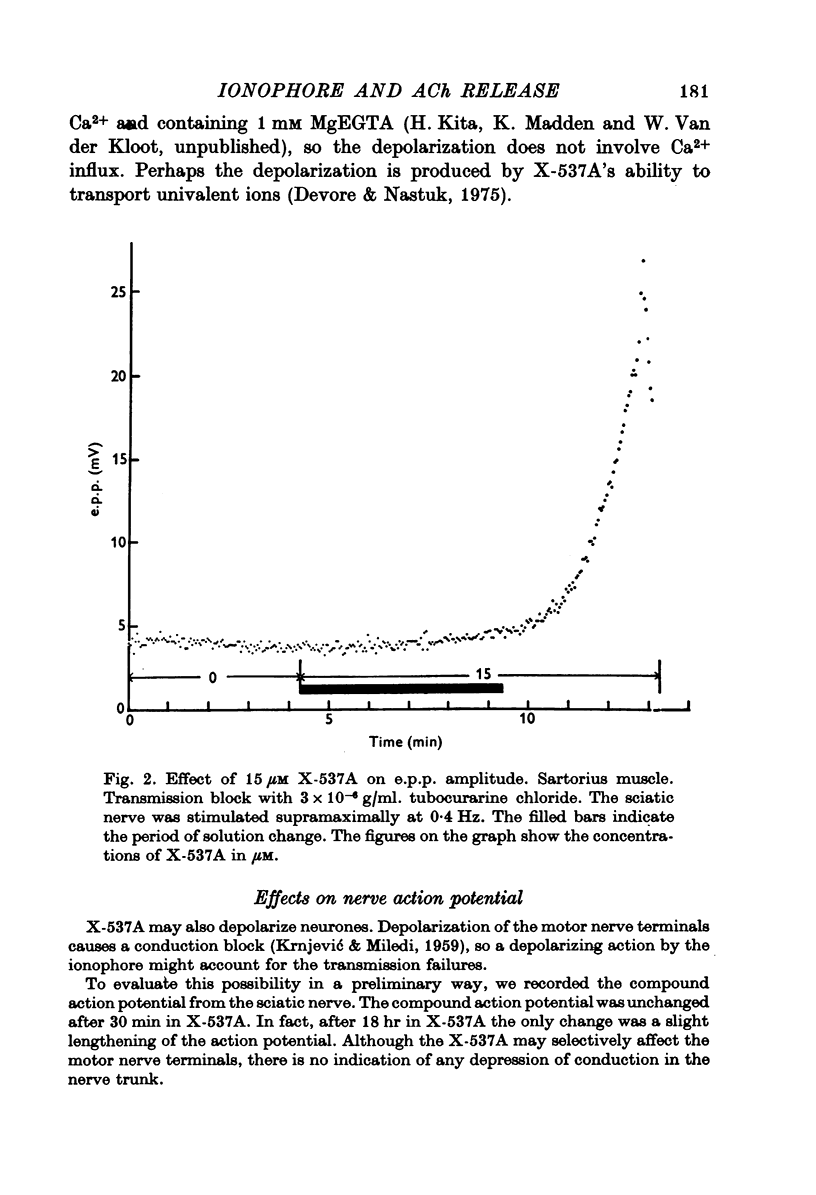
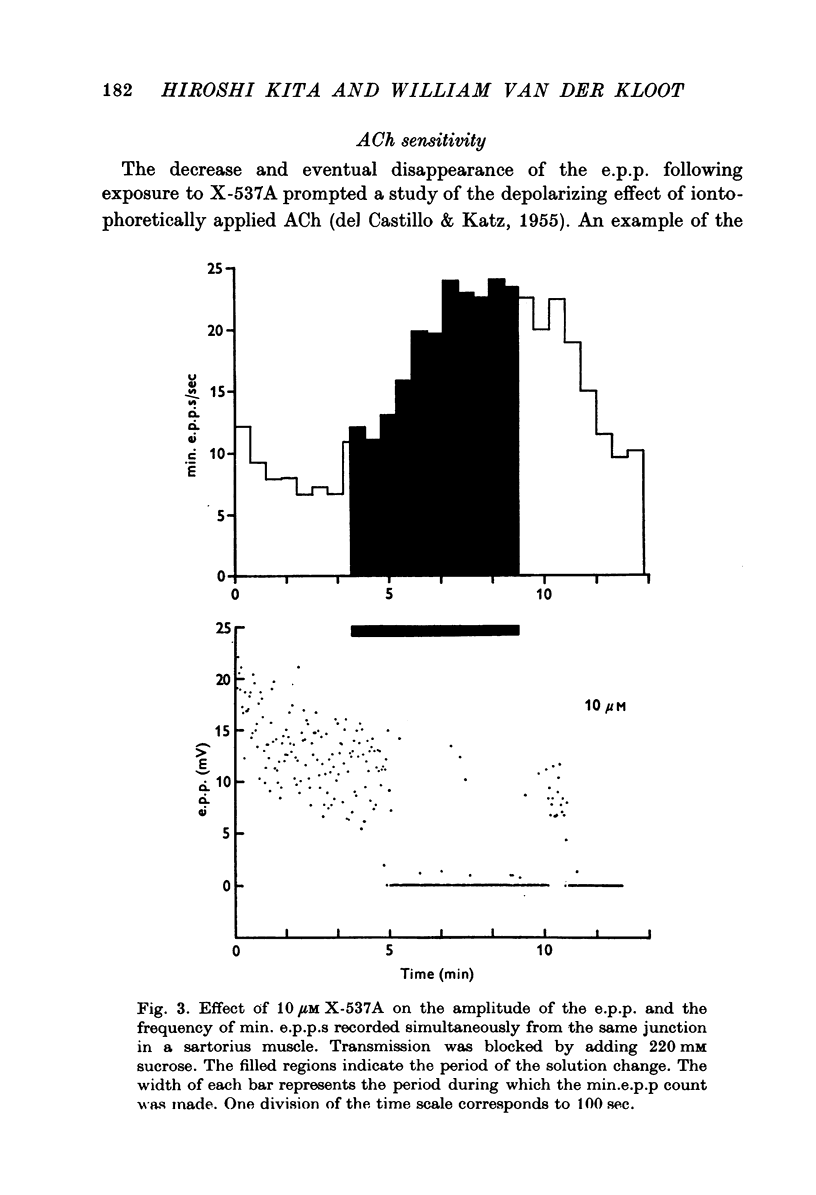
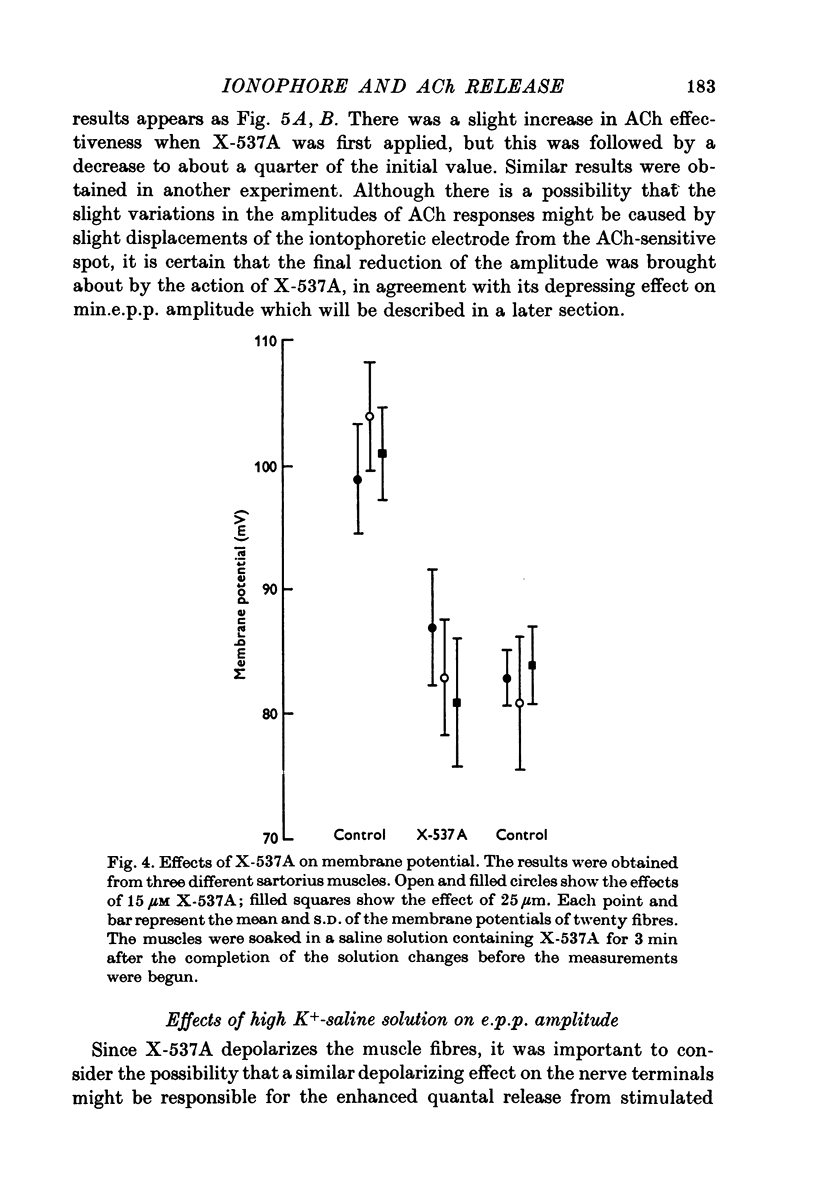
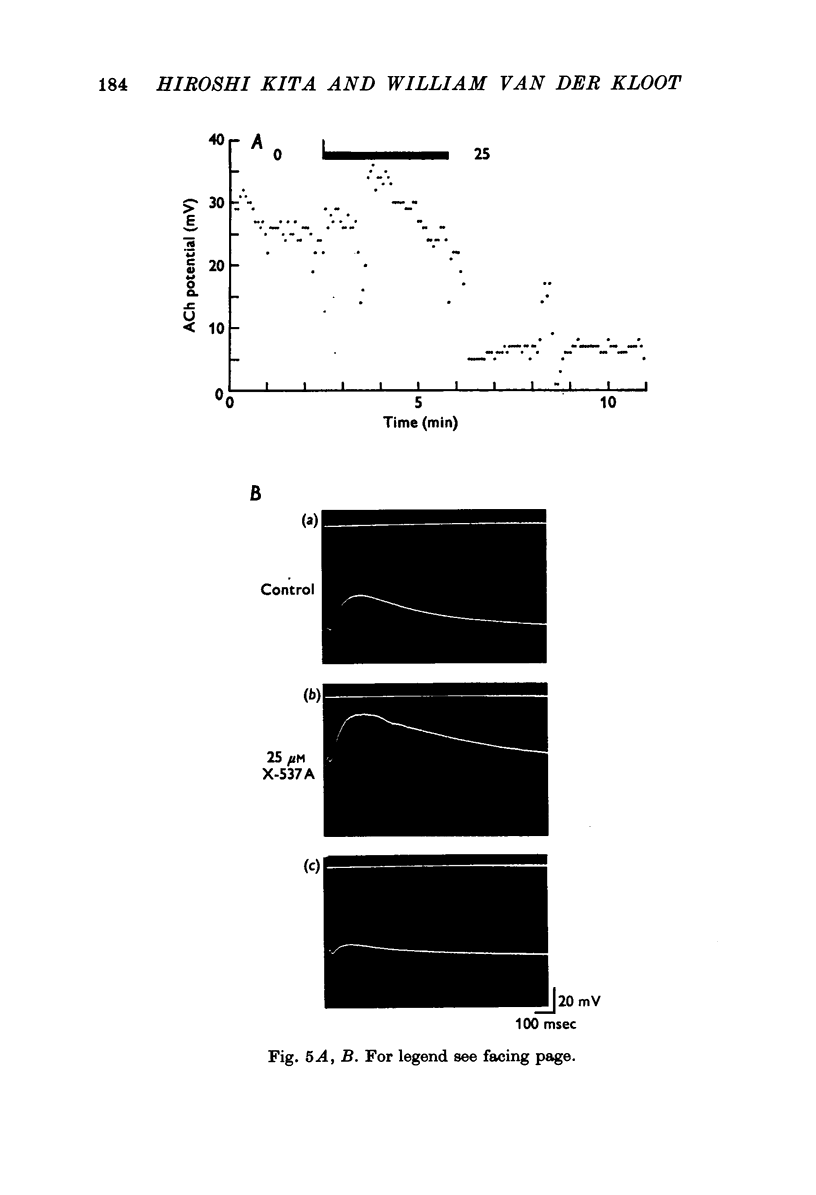
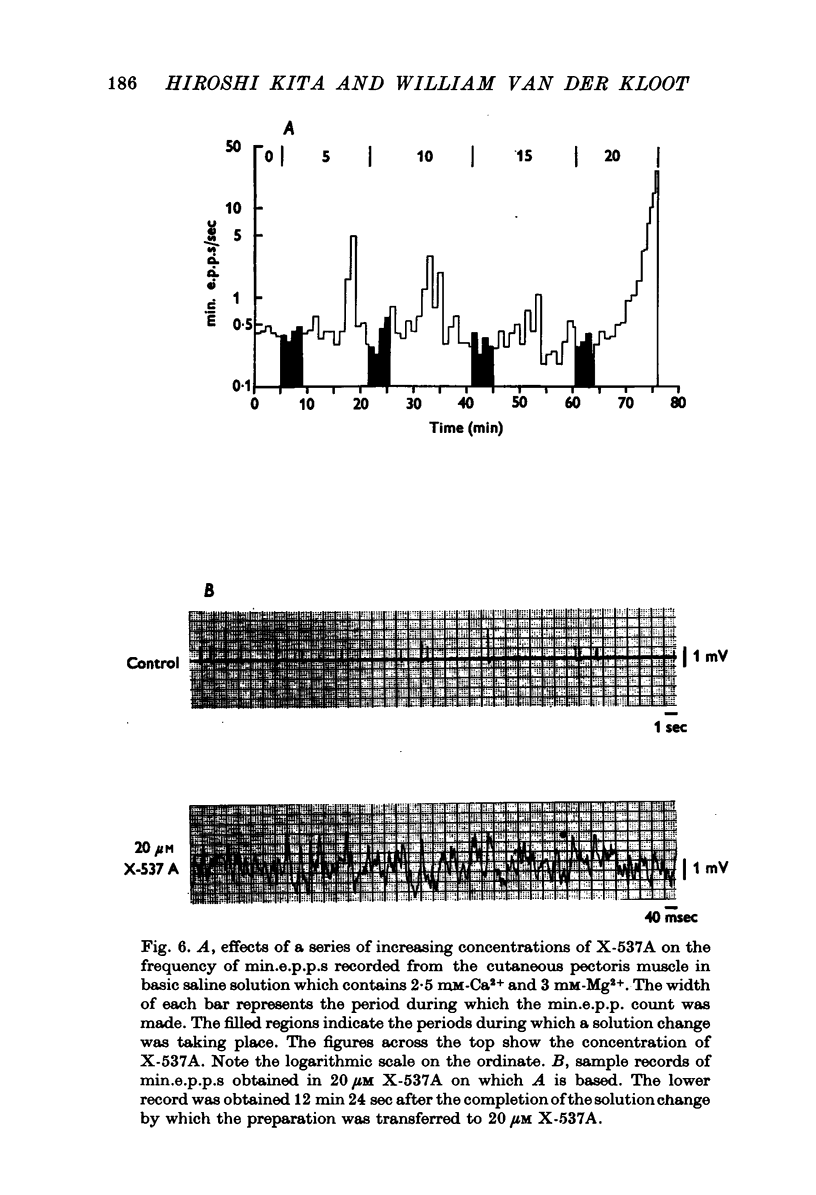
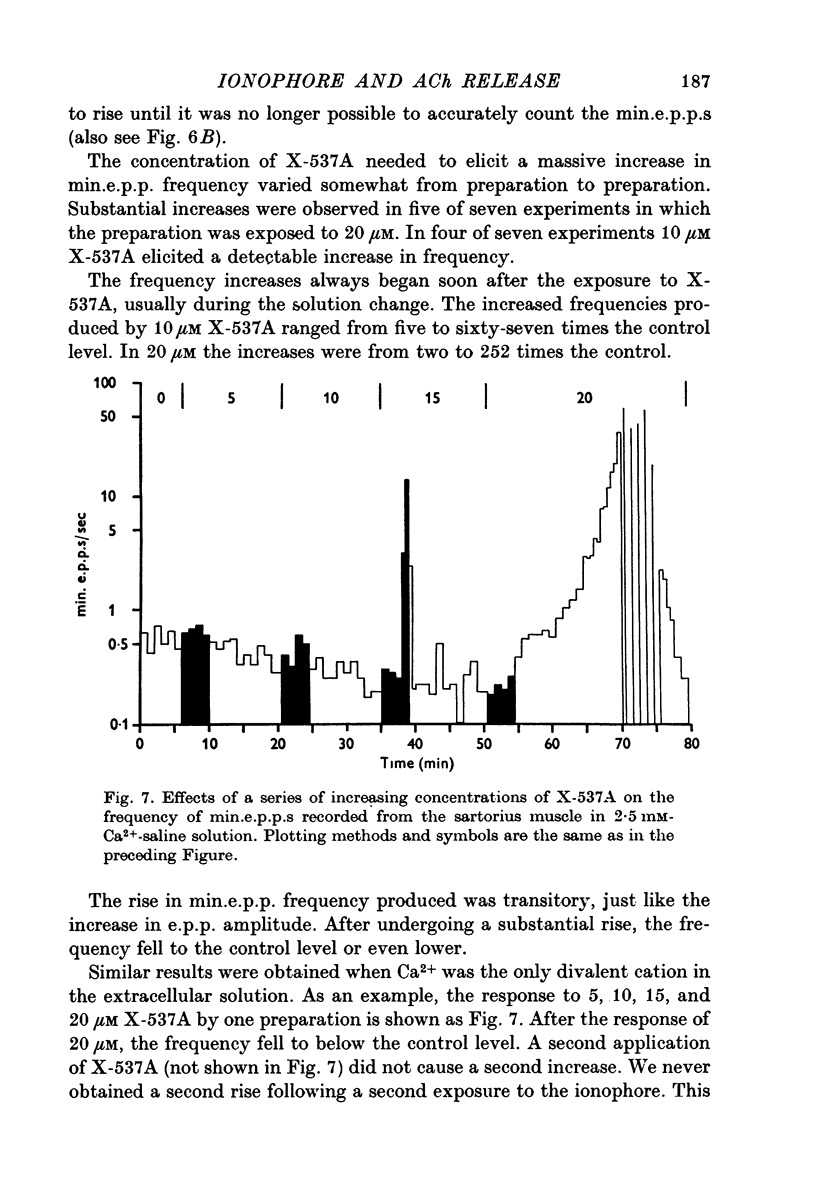
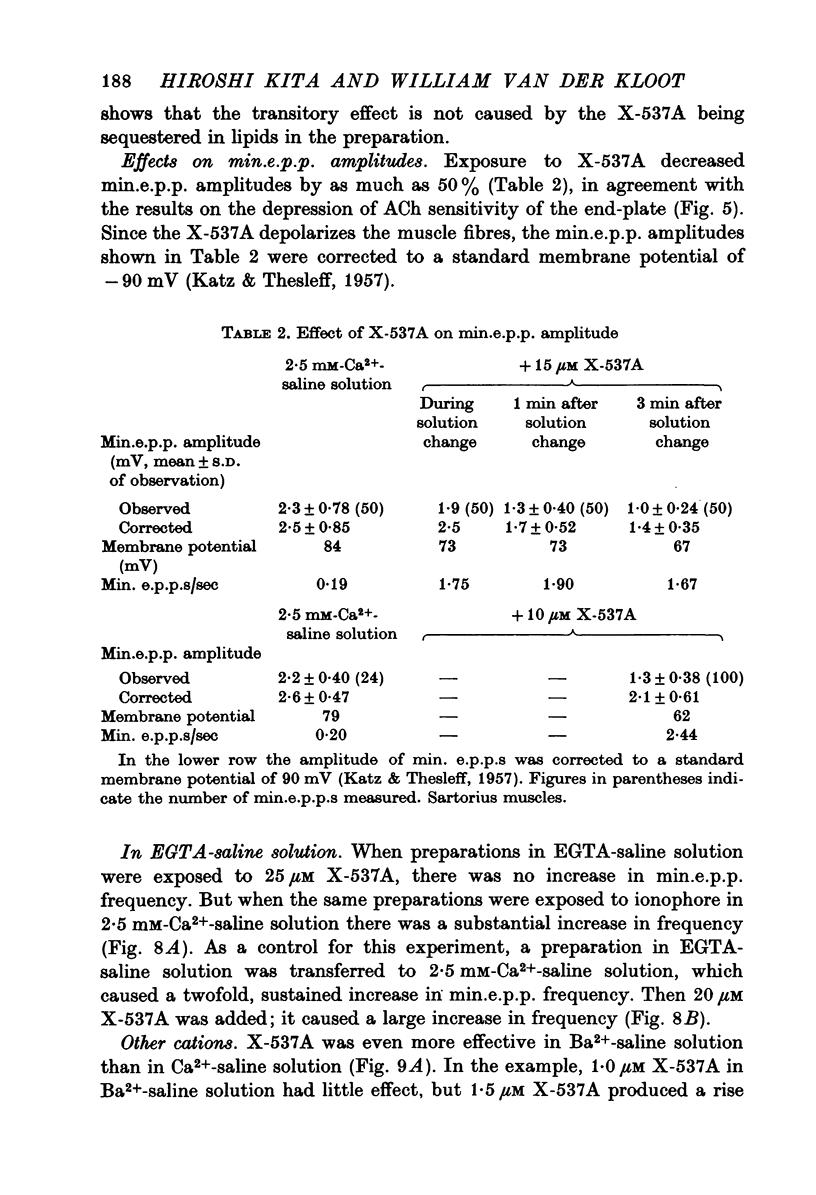
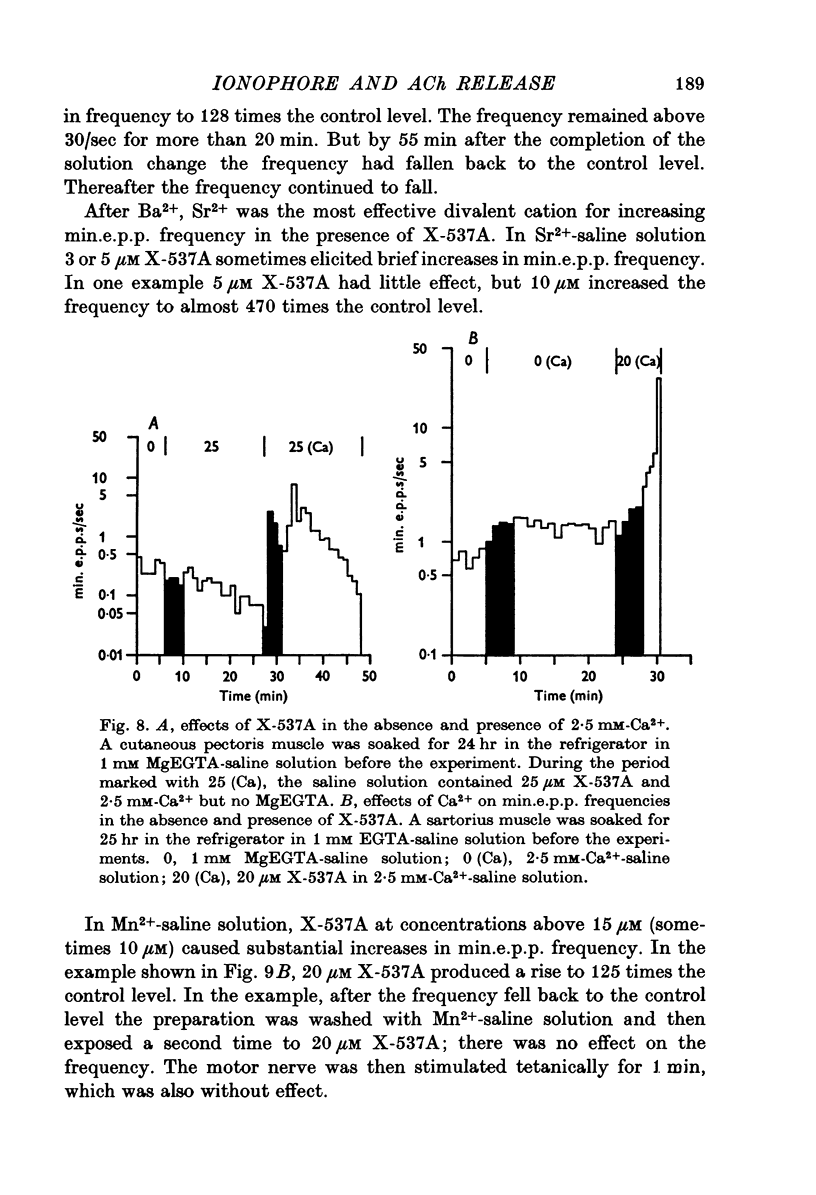
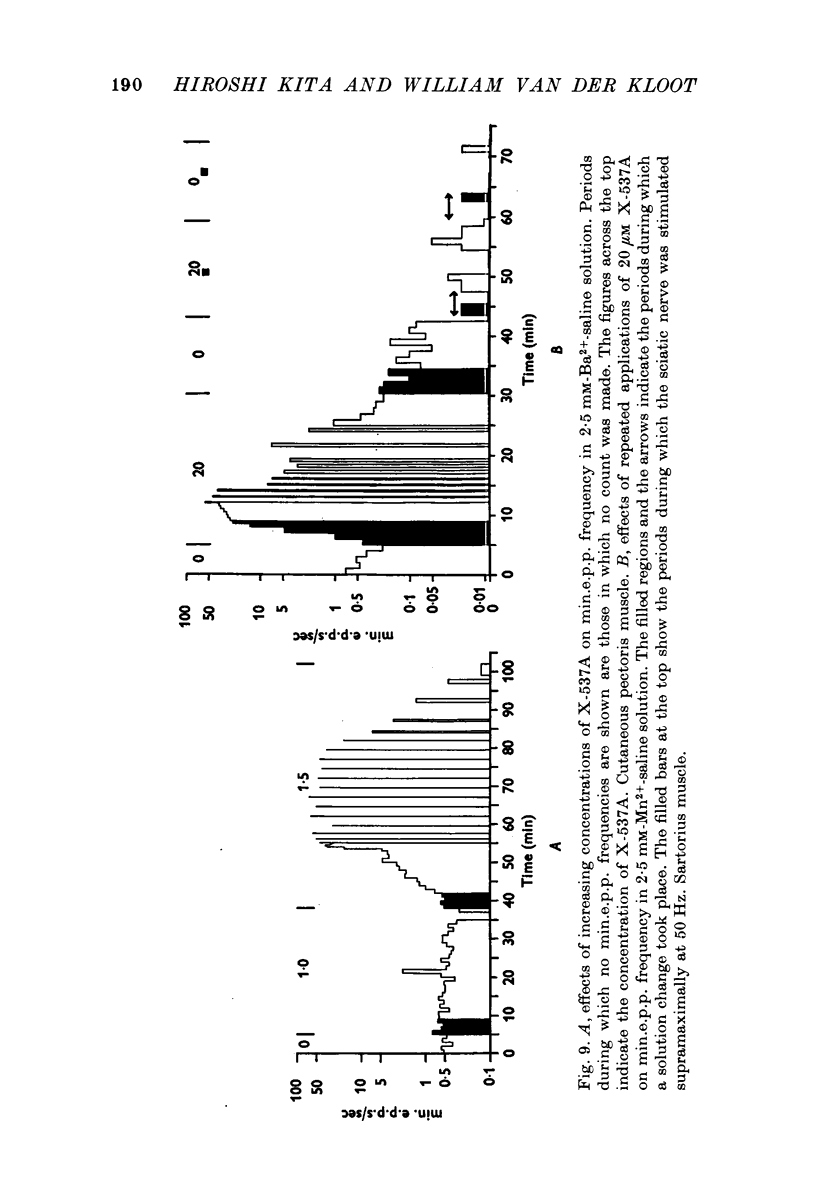
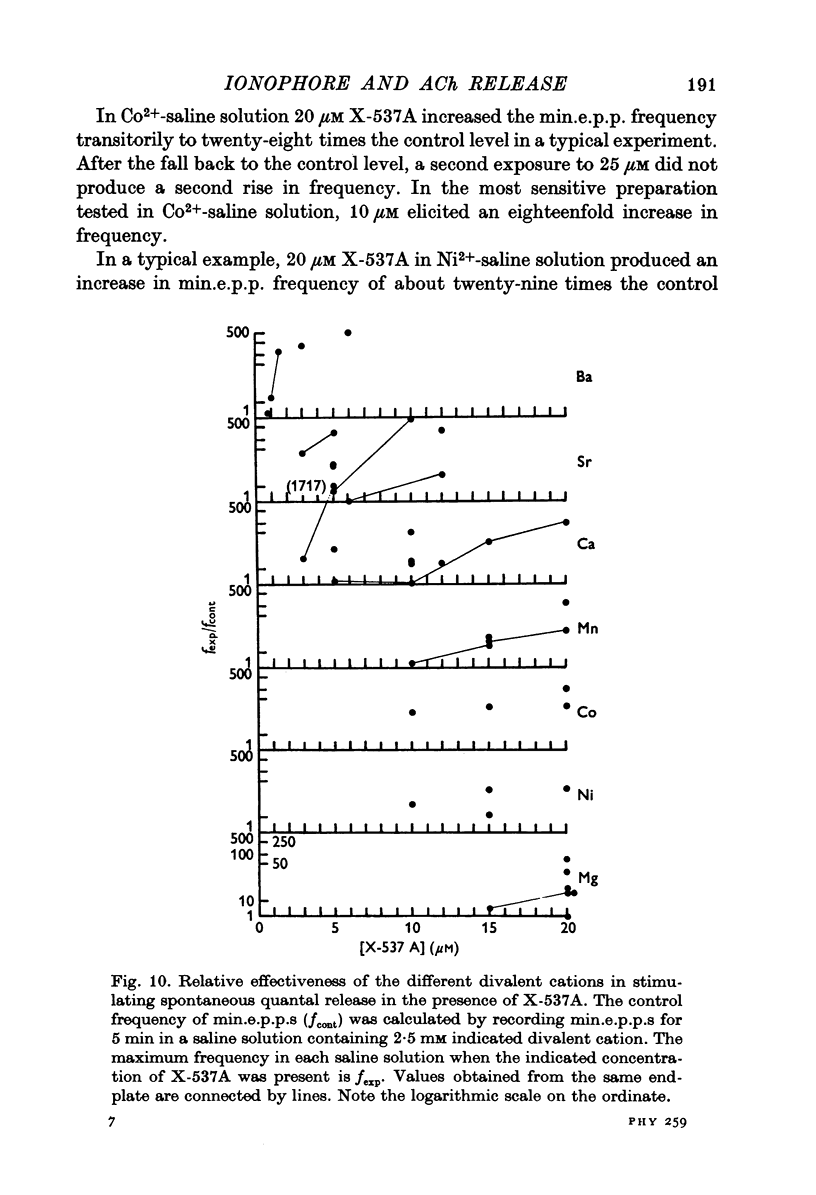
1. X-537A is an ionophore that can carry cations across cell membranes. We studied its effects on spontaneous and stimulated quantal acetylcholine (ACh) release at the frog neuromuscular junction. 2. When neuromuscular transmission was blocked with high Mg2+ or with curare, X-537A markedly increased the end-plate potential (e.p.p.) amplitude. Then a few minutes later the e.p.p. disappeared. 3. When neuromuscular transmission was blocked with hypertonic saline solution, X-537A did not increase e.p.p. amplitude; it did produce many transmission failures. 4. X-537A decreased the depolarization of the end-plate produced by iontophoretically applied ACh. this may account in part for the disappearance of the e.p.p. in solutions containing the ionophore. 5. X-537A depolarized muscle fibres by about 15 mV. 6. When the extracellular divalent cation concentration was very low, X-537A had little or no effect on miniature end-plate potential (min.e.p.p.) frequency. 7. When a divalent cation was present in the extracellular fluid, X-537A increased the frequency of the min.e.p.p.s. The sequence of effectiveness of the divalent ions we have tested is: Ba2+ greater than Sr2+ greater than Ca2+ greater than Mn2+ congruent to Co2+ congruent to Ni2+ greater than Mg2+. There is a rough parallel between these results and the reported affinity of X-537A for various divalent ions. 8. The increase in min.e.p.p. frequency caused by X-537A was transitory, following the increase min.e.p.p. frequency fell to a very low rate or to zero. Then nerve stimulation did not cause quantal release. A second application of X-537A was without effect. 9. X-537A decreased min.e.p.p. amplitude, in accord with the effect on the sensitivity of the end-plate to ACh. 10. The results support the idea that increases in intracellular divalent cation concentrations trigger quantal release from nerve terminals and are involved in the disensitization of end plate receptors to ACh.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker P. F., Crawford A. C. Mobility and transport of magnesium in squid giant axons. J Physiol. 1972 Dec;227(3):855–874. doi: 10.1113/jphysiol.1972.sp010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein M. P., Hodgkin A. L. The effect of cyanide on the efflux of calcium from squid axons. J Physiol. 1969 Feb;200(2):497–527. doi: 10.1113/jphysiol.1969.sp008704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blioch Z. L., Glagoleva I. M., Liberman E. A., Nenashev V. A. A study of the mechanism of quantal transmitter release at a chemical synapse. J Physiol. 1968 Nov;199(1):11–35. doi: 10.1113/jphysiol.1968.sp008637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carafoli E., Rossi C. S. Calcium transport in mitochondria. Adv Cytopharmacol. 1971 May;1:209–227. [PubMed] [Google Scholar]
- Célis H., Estrada S., Montal M. Model translocators for divalent and monovalent ion transport in phospholipid membranes. I. The ion permeability induced in lipid bilayers by the antibiotic X-537A. J Membr Biol. 1974;18(2):187–199. doi: 10.1007/BF01870111. [DOI] [PubMed] [Google Scholar]
- DANFORTH W. H., HELMREICH E. REGULATION OF GLYCOLYSIS IN MUSCLE. I. THE CONVERSION OF PHOSPHORYLASE BETA TO PHOSPHORYLASE ALPHA IN FROG SARTORIUS MUSCLE. J Biol Chem. 1964 Oct;239:3133–3138. [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Changes in end-plate activity produced by presynaptic polarization. J Physiol. 1954 Jun 28;124(3):586–604. doi: 10.1113/jphysiol.1954.sp005131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. On the localization of acetylcholine receptors. J Physiol. 1955 Apr 28;128(1):157–181. doi: 10.1113/jphysiol.1955.sp005297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degani H., Friedman H. L. Ion binding by X-537A. Formulas, formation constants, and spectra of complexes. Biochemistry. 1974 Nov 19;13(24):5022–5032. doi: 10.1021/bi00721a025. [DOI] [PubMed] [Google Scholar]
- Devore D. I., Nastuk W. L. Effects of 'calcium ionophore' X537A on frog skeletal muscle. Nature. 1975 Feb 20;253(5493):644–646. doi: 10.1038/253644a0. [DOI] [PubMed] [Google Scholar]
- Dodge F. A., Jr, Rahamimoff R. Co-operative action a calcium ions in transmitter release at the neuromuscular junction. J Physiol. 1967 Nov;193(2):419–432. doi: 10.1113/jphysiol.1967.sp008367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage P. W. The effect of methyl, ethyl and n-propyl alcohol on neuromuscular transmission in the rat. J Pharmacol Exp Ther. 1965 Nov;150(2):236–243. [PubMed] [Google Scholar]
- HUBBARD J. I., WILLIS W. D. Reduction of transmitter output by depolarization. Nature. 1962 Mar 31;193:1294–1295. doi: 10.1038/1931294a0. [DOI] [PubMed] [Google Scholar]
- Heuser J., Katz B., Miledi R. Structural and functional changes of frog neuromuscular junctions in high calcium solutions. Proc R Soc Lond B Biol Sci. 1971 Sep 28;178(1053):407–415. doi: 10.1098/rspb.1971.0072. [DOI] [PubMed] [Google Scholar]
- Hurlbut W. P., Longenecker H. B., Jr, Mauro A. Effects of calcium and magnesium on the frequency of miniature end-plate potentials during prolonged tetanization. J Physiol. 1971 Dec;219(1):17–38. doi: 10.1113/jphysiol.1971.sp009647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATZ B., THESLEFF S. On the factors which determine the amplitude of the miniature end-plate potential. J Physiol. 1957 Jul 11;137(2):267–278. doi: 10.1113/jphysiol.1957.sp005811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KRNJEVIC K., MILEDI R. Presynaptic failure of neuromuscular propagation in rats. J Physiol. 1959 Dec;149:1–22. doi: 10.1113/jphysiol.1959.sp006321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kafka M. S., Holz R. W. Ionophores X537A and A23187. Effects on the permeability of lipid bimolecular membranes to dopamine and calcium. Biochim Biophys Acta. 1976 Feb 19;426(1):31–37. doi: 10.1016/0005-2736(76)90426-0. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. A study of synaptic transmission in the absence of nerve impulses. J Physiol. 1967 Sep;192(2):407–436. doi: 10.1113/jphysiol.1967.sp008307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. Spontaneous and evoked activity of motor nerve endings in calcium Ringer. J Physiol. 1969 Aug;203(3):689–706. doi: 10.1113/jphysiol.1969.sp008887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. The role of calcium in neuromuscular facilitation. J Physiol. 1968 Mar;195(2):481–492. doi: 10.1113/jphysiol.1968.sp008469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita H., Van der Kloot W. Action of Co and Ni at the frog neuromuscular junction. Nat New Biol. 1973 Sep 12;245(141):52–53. doi: 10.1038/newbio245052a0. [DOI] [PubMed] [Google Scholar]
- Kita H., Van der Kloot W. Calcium ionophore X-537A increases spontaneous and phasic quantal release of acetylcholine at frog neuromuscular junction. Nature. 1974 Aug 23;250(5468):658–660. doi: 10.1038/250658a0. [DOI] [PubMed] [Google Scholar]
- Llinás R., Blinks J. R., Nicholson C. Calcium transient in presynaptic terminal of squid giant synapse: detection with aequorin. Science. 1972 Jun 9;176(4039):1127–1129. doi: 10.1126/science.176.4039.1127. [DOI] [PubMed] [Google Scholar]
- Manthey A. A. The effect of calcium on the desensitization of membrane receptors at the neuromuscular junction. J Gen Physiol. 1966 May;49(5):963–976. doi: 10.1085/jgp.49.5.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R. Transmitter release induced by injection of calcium ions into nerve terminals. Proc R Soc Lond B Biol Sci. 1973 Jul 3;183(1073):421–425. doi: 10.1098/rspb.1973.0026. [DOI] [PubMed] [Google Scholar]
- Nastuk W. L., Parsons R. L. Factors in the inactivation of postjunctional membrane receptors of frog skeletal muscle. J Gen Physiol. 1970 Aug;56(2):218–249. doi: 10.1085/jgp.56.2.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pressman B. C. Properties of ionophores with broad range cation selectivity. Fed Proc. 1973 Jun;32(6):1698–1703. [PubMed] [Google Scholar]
- Scarpa A., Baldassare J., Inesi G. The effect of calcium ionophores on fragmented sarcoplasmic reticulum. J Gen Physiol. 1972 Dec;60(6):735–749. doi: 10.1085/jgp.60.6.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THESLEFF S. Motor end-plate 'desensitization' by repetitive nerve stimuli. J Physiol. 1959 Oct;148:659–664. doi: 10.1113/jphysiol.1959.sp006314. [DOI] [PMC free article] [PubMed] [Google Scholar]


