Abstract
Despite numerous experiments showing that administration of neuropeptide Y (NPY) to rodents stimulates feeding and obesity, whereas acute interference with NPY signaling disrupts feeding and promotes weight loss, NPY-null mice have essentially normal body weight regulation. These conflicting observations suggest that chronic lack of NPY during development may lead to compensatory changes that normalize regulation of food intake and energy expenditure in the absence of NPY. To test this idea, we used gene targeting to introduce a doxycycline (Dox)-regulated cassette into the Npy locus, such that NPY would be expressed until the mice were given Dox, which blocks transcription. Compared with wild-type mice, adult mice bearing this construct expressed ≈4-fold more Npy mRNA, which fell to ≈20% of control values within 3 days after treatment with Dox. NPY protein also fell ≈20-fold, but the half-life of ≈5 days was surprisingly long. The biological effectiveness of these manipulations was demonstrated by showing that overexpression of NPY protected against kainate-induced seizures. Mice chronically overexpressing NPY had normal body weight, and administration of Dox to these mice did not suppress feeding. Furthermore, the refeeding response of these mice after a fast was normal. We conclude that, if there is compensation for changes in NPY levels, then it occurs within the time it takes for Dox treatment to deplete NPY levels. These observations suggest that pharmacological inhibition of NPY signaling is unlikely to have long-lasting effects on body weight.
Keywords: body weight regulation, conditional knockout, feeding behavior
Numerous studies using a variety of approaches have implicated neuropeptide Y (NPY) in the regulation of body weight. Injection of NPY or NPY-related peptides intracranially (either into the hypothalamus or cerebral ventricles) stimulates robust feeding (1–6); conditions of energy deprivation (starvation) or increased energy demand (lactation) induce NPY expression in the hypothalamus (7, 8); most genetic models of obesity exhibit hyperphagia and an increase in hypothalamic NPY levels (9–13); and various pharmacological treatments that enhance feeding are accompanied by an increase in NPY (14). In addition, various methods (antisense oligonucleotides, antibodies, or receptor antagonists) aimed at blocking NPY actions in the hypothalamus have been shown to inhibit feeding (15–22). Moreover, there is anatomical and electrophysiological evidence that NPY neurons in the arcuate (ARC) region of the hypothalamus have direct inhibitory inputs onto proopiomelanocortin (POMC) cells that form part of the melanocortin-signaling pathway, which suppresses feeding (23). Thus, current models for hypothalamic control of energy balance suggest that activation of POMC cell signaling to cells bearing melanocortin-4 receptors (MC4R) suppresses feeding, whereas NPY and various other hormones and neurotransmitters stimulate feeding by antagonizing this melanocortin-signaling pathway. NPY-expressing neurons in the ARC and brainstem also project directly to the paraventricular nucleus (PVN) and other brain structures where they may influence feeding activity (see refs. 24–28 for reviews).
Although this model of opposing actions of NPY and POMC is appealing and genetic evidence for the role of the melanocortin-signaling pathway is compelling (24–28), the genetic evidence for the role of NPY does not support the model. Mice unable to make NPY have normal body weight, respond to fasting and refeeding normally, and respond like controls to a high-fat diet (refs. 29 and 30, but see below). Furthermore, disruption of individual NPY receptor genes (Npy1r, Npy2r, Npy5r) does not inhibit feeding as expected; in fact, some of these knockout mice manifest late-onset obesity (31–35). NPY-deficient mice do manifest some deficits; for example, studies indicate that they are slower to initiate feeding and their feeding response to hypoglycemic challenges is blunted transiently (36, 37). Nevertheless, even in these situations, daily food intake by NPY-null mice is normal. NPY deficiency attenuates most of the deleterious effects of leptin deficiency (38), suggesting that NPY signaling mediates some of these effects. In addition, leptin effects on feeding are exacerbated in NPY-deficient mice (29). Thus, contrary to expectations, genetic disruption of NPY signaling has little effect on feeding.
One explanation for the normal body weight regulation of NPY-null mice is that compensatory mechanisms are engaged in the chronic absence of NPY. Such compensatory mechanisms could be either cell-autonomous or circuit-based. The discovery that agouti-related protein (AgRP) is made by the same cells in the ARC that make NPY, that it is induced by starvation, and stimulates feeding by antagonizing melanocortin signaling suggested that it could mediate cell-autonomous compensation. However, mice lacking AgRP and NPY also have normal body weight regulation (39), although they fail to respond to orexigenic effects of ghrelin (40). The discovery that the NPY/AgRP neurons also make GABA leaves open the possibility of cell-autonomous compensation by GABA (23, 41) or some unidentified neuromodulator. Support for a circuit-based compensatory mechanism comes from studies showing that nearly complete ablation of hypothalamic NPY/AgRP neurons in neonatal mice has a minimal effect on body weight and food consumption, whereas ablation of these neurons in adults leads to rapid starvation (42). If compensation for the loss of NPY/AgRP neurons can occur in neonatal mice, then it follows that they could compensate for any neurotransmitter that these cells make. Thus, it becomes important to devise methods to inactivate salient signaling molecules in the adult, when the ability to compensate for the loss of NPY/AgRP neurons seems to be curtailed.
We set out to test whether inactivating NPY gene expression in the adult would have a different effect on body weight regulation than deficiency throughout life. We used gene targeting to introduce a tetracycline-regulated cassette into the Npy locus such that Npy gene is expressed until the mice are given doxycycline (Dox, a tetracycline derivative), which blocks transcription of the Npy gene in this system. We anticipated that, if NPY has a tonic effect on feeding in the adult, then inactivation of NPY synthesis in the adult would inhibit feeding. If compensation for NPY cannot occur in the adult and it is an important mediator of feeding, then mice would lose weight and perhaps perish. On the other hand, if compensation can occur, then there might be a transient period of weight loss followed by recovery. If there is no effect of inactivating NPY expression in the adult, then NPY may not be important for feeding or compensation takes place simultaneously with loss of NPY.
Materials and Methods
Npytet/tet Mice. A targeting construct was prepared with ≈12 kb of Npy 5′-flanking sequence, ≈8.5 kb of Npy 3′-flanking sequence, a Tet cassette (triTAUBi, generously provided by John Adelman, Oregon Health & Science University, Portland) in between, and a distal Pgk-DTA gene for negative selection (See Fig. 1C). The construct was linearized and electroporated into either AK18.1 (129/Sv) embryonic stem cells. Clones with homologous recombination (12 of 371 screened) were identified by Southern blotting (using a probe shown in Fig. 1 A) after digestion with BamHI, which gave a band of ≈15 kb for a targeted allele and >25 kb for the endogenous allele. Several clones were injected into C57BL/6 blastocysts, and resulting chimeric mice were bred with C57BL/6 mice; two clones were transmitted through the germ line. Mice with the targeted allele were identified by using a three-primer PCR strategy. The approximate locations of primers a (5′-GAGCGGCAGTGGCTCCAG), b (5′-CACTGGCGTCTGGGAGCC), and c (5′-CAGATGGACGCGGCCACC) are shown in Fig. 1. Mice carrying the targeted allele were bred with mice expressing Mox2-Cre (43) to remove the TK-Neo and Ura3 genes, and then bred with WT mice to segregate the Npytet allele and Mox2-Cre genes. The resulting heterozygous Npytet/+ mice were bred together to generate Npytet/tet and WT mice used for the experiments described here.
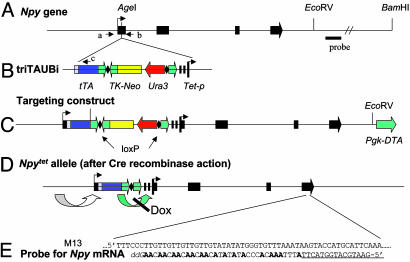
Fig. 1.
Mice with Npytet allele. (A) The Npy gene spans ≈6.7 kb and has 4 exons shown as solid boxes. (B) A tetracycline cassette, triTAUBi, contains the following components: a tripartite leader sequence from adenovirus, a tetracycline DNA-binding region fused to a strong transcriptional activator (tTA), SV40 poly(A), loxP site (diamond), a neomycin resistance gene driven by HSV thymidine kinase (TK) promoter and with TK poly(A) (TK-Neo), a Ura3 gene for selection in yeast, a loxP site, a human growth hormone poly(A) region, and multiple copies of a tTA binding site upstream of a minimal promoter from cytomegalovirus (Tet-p). This cassette was inserted into the AgeI site in exon 1 of Npy, which lies between the transcriptional and translational start sites. (C) The targeting construct, with ≈12 kb of Npy 5′ flanking sequence (not to scale) and ≈8.5 kb of 3′ flanking sequence also carried a Pgk-DTA gene for negative selection. (D) Mice carrying the correctly targeted allele were bred with mice carrying Mox2-Cre to delete the TK-Neo and Ura3 genes. (E) To measure Npy mRNA, a restriction fragment including Npy exon 4 was cloned into M13 such that the sense strand would be produced. A probe was synthesized by hybridizing a primer (underlined) to M13 and then extending it with DNA polymerase in the presence of [32P]dATP, unlabeled dTTP, and dCTP, and dideoxy-GTP. The products were denatured with NaOH and separated on an acrylamide gel.
Initially, a different Tet cassette was used; most of the components were the same, but there were variations in the promoters and polyadenylation sequences chosen. In addition, it was not possible to remove the Neo gene. Npy mRNA in those Npytet/tet mice was ≈10% of WT levels. Histological sections revealed few NPY-positive cells in the ARC compared with WT mice, suggestive of Npy gene silencing in most cells. The same result occurred when that cassette was introduced into another gene locus. Removing the TK-Neo and Ura3 genes from the cassette described here improved expression considerably, indicating that they also interfere with expression or promote silencing.
Animal Care. Mice were reared in a specific-pathogen-free facility with a 12:12 h light:dark cycle. A chow diet (PicoLab Rodent Diet 5053, ≈ 12% of calories from fat and ≈65% from carbohydrates) and water were available ad libitum unless otherwise indicated. Dox (Sigma) was dissolved in water (2 mg/ml) with saccharin (5 mM) and substituted for the drinking water. The Dox solution was made fresh and replaced every other day. All procedures were conducted in accordance with guidelines established by the National Institutes of Health and the University of Washington Animal Care Committee.
NPY Protein and mRNA Measurements. To measure NPY protein, brains of CO2-asphyxiated mice were isolated during the afternoon and frozen at -80°C. A 5% homogenate was prepared in 0.1 M HCl, an aliquot was taken for protein determination, and the remainder was centrifuged to remove insoluble material. A 0.45-ml aliquot of the supernatant was neutralized by adding 25 μl of 0.5 M sodium phosphate buffer (pH 7.2) and ≈25 μl of 2 M NaOH, by using phenol red as a pH indicator. NPY content was measured in aliquots (5–40 μl) by using a RIA kit (RK-049-03) from Phoenix Pharmaceuticals (Belmont, CA).
To measure Npy mRNA, total nucleic acids (TNA) were isolated during the afternoon from brains by homogenizing the tissue in 1× SET (1% SDS/100 mM NaCl/10 mM Tris/1 mM EDTA, pH 7.4) containing proteinase K (100 μg/ml), incubating for several hours at 37°C, and then extracting with phenol/chloroform, and the aqueous phase was precipitated with two volumes of ethanol. TNA was dissolved in 0.2× SET, and aliquots (10–30 μg) were incubated overnight with ≈20,000 cpm of 32P-labeled probe prepared as described in Fig. 1E. The nonhybridized probe was degraded with S1 nuclease, the protected products were precipitated with 3% trichloracetic acid in the presence of carrier DNA, and the radioactivity was measured by using a Beckman scintillation counter. The number of molecules per cell was calculated by using an M13 DNA standard and knowing the DNA content of the TNA samples; see ref. 44 for details.
Seizure Susceptibility. Kainic acid hydrate (Cayman Chemical, Ann Arbor, MI) was dissolved in saline at 4 mg/ml, neutralized with NaOH, and injected i.p. at a dose of 25 mg/kg body weight. The mice were placed in a clear plastic cage observed for 2 h, and someone unaware of the genotype of the mice scored the latency to various seizure episodes. The maximum seizure severity was scored as described (45): 0, no response; 1, staring; 2, focal clonic convusion (myoclonic jerk); 3, forelimb clonus; 4, rearing; 5, loss of posture (falling); 6, status epilepticus leading to death.
Immunohistochemistry. Adult female mice were fasted for 24 h before euthanasia by CO2 inhalation, followed by cardiac perfusion with 0.9% saline at 22°C, then by ice-cold 4% paraformaldehyde in 0.1 M PBS (pH 7.0). Brains were immediately removed and immersion fixed overnight in the same fixative at 4°C. Paraffin-embedded sections (8 μm) were dewaxed in xylene and a graded series of ethanol, rinsed twice in PBS, and then subjected to microwave antigen retrieval by heating at ≈95°C in 1 mM EDTA (pH 7.5) for 15 min. Rabbit polyclonal anti-NPY (Peninsula Laboratory, San Carlos, CA) was used at 1/1,000 dilution in TBS (150 mM NaCl/50 mM Tris·HCl, pH 7.5) containing 0.05% Triton X-100, 3% donkey serum, and visualized with CY3-labeled secondary antibody (1/500, Jackson Immunolaboratory, West Grove, PA). Images were captured by using a CoolSnap camera attached to a Nikon microscope; all paired photomicrographs were obtained by using the same image acquisition settings.
Other Procedures. Body weights of mice were measured daily for 4 weeks starting at 8 weeks of age. Daily food intake of individually housed mice was measured as the weight of chow that disappeared every 24 h. Percent adiposity was determined by adding the weights of the scapular, inguinal, retro-peritoneal, and reproductive fat pads and dividing this value by the animal's body weight. To measure plasma leptin and insulin, mice were fasted for 16 h before blood collection from the saphenous vein. All samples were collected, placed on ice, centrifuged, and stored at -20°C until further analysis. Plasma leptin was determined by using a mouse leptin ELISA (Crystal Chem, Downers Grove, IL). Plasma insulin was determined by using an ultrasensitive mouse insulin ELISA (Alpco Diagnostics, Windham, NH). Blood glucose levels were determined in blood collected from the saphenous vein of mice fasted for 16 h by using a blood glucose analyzer (Hemocue, Lake Forest, CA).
Statistics. Significance was evaluated by Student's t test; values shown are means ± SE M; *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Results
Mice with a Regulatable Npy Gene. A cassette was inserted into exon 1of the Npy locus (Fig. 1 A) between the transcription and translation start sites. This cassette has a transcriptional activator [tetracycline-controlled transcriptional activator (tTA)] that was predicted to be driven by adjacent Npy regulatory elements and a promoter [tetracycline promoter DNA sequence (Tet-p)] upstream of the Npy coding region. Thus, tTA should bind to Tet-p to drive expression of Npy mRNA; Dox should bind to tTA and prevent its binding to Tet-p (Fig. 1D). This cassette (Fig. 1B) was used previously to achieve regulated expression of other genes (46, 47). The cassette also carries a TK-Neo for selection in embryonic stem cells (Fig. 1C) that can be removed along with the flanking Ura3 gene by Cre-mediated recombination, to generate the Npytet allele shown in Fig. 1D. In the absence of Dox, expression from the allele bearing this cassette may exceed that of the WT allele because tTA has a strong activation domain (from VP16) and there are multiple binding sites in Tet-p.
Expression and Regulation of the Npytet Allele. Breeding heterozygotes generated homozygous Npytet/tet mice and WT controls. Npy mRNA abundance was determined by solution hybridization by using a radioactive probe complementary to the 3′ untranslated region of Npy mRNA. The probe was synthesized from single-stranded M13 DNA by using a primer adjacent to a region that would favor incorporation of [32P]dATP and that could be terminated with dideoxy-GTP (Fig. 1E).
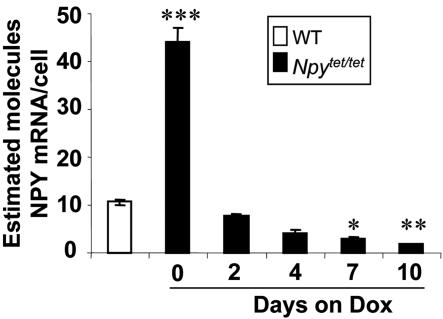
There was ≈4-fold more Npy mRNA in whole brain of Npytet/tet mice compared with WT controls (Fig. 2). Heterozygotes had intermediate levels of Npy mRNA (data not shown). Before removal of the TK-Neo and Ura3 genes by Cre-mediated recombination (see Fig. 1 C and D), the amount of Npy mRNA produced from the Npytet allele was slightly less than the WT allele (data not shown), indicating that these DNA elements interfere with Npy gene expression. After 2 days of treatment with Dox (2 mg/ml in drinking water), Npy mRNA level fell ≈5-fold, and after a week it fell to ≈20% of WT levels (Fig. 2). Treatment with Dox had no effect on Npy mRNA in WT mice (data not shown).
Fig. 2.
Npy mRNA levels in control and Npytet/tet mice after treatment with Dox. Npy mRNA content in whole brain of Npytet/tet and WT mice was measured at various times after treatment with Dox (2 mg/ml) by solution hybridization. *, P < 0.05 relative to WT level.
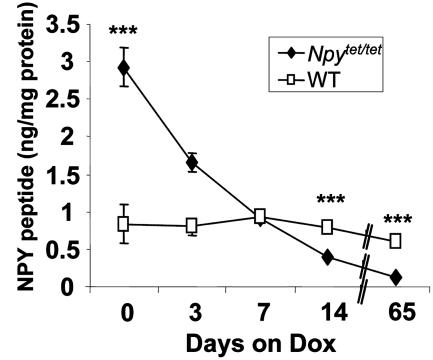
An RIA was used to measure NPY peptide levels. In agreement with the mRNA data, NPY was overexpressed in the brains of Npytet/tet mice compared with WT mice, and fell to ≈50% of WT levels after 2 wk treatment with Dox and to ≈15% of WT levels after 9 wk of Dox treatment (Fig. 3). The half-life of NPY peptide in whole brain was ≈5 days. As expected, peptide levels fell more slowly than mRNA levels (compare Figs. 2 and 3). Dox had little effect on NPY levels in WT mice (Fig. 3). RIA of NPY from dissected hypothalamus after 9 weeks of Dox treatment revealed that Npytet/tet mice had 1/7th the amount of NPY compared with WT mice (0.13 vs. 0.97 ng/mg protein).
Fig. 3.
Brain NPY peptide content of control and Npytet/tet mice before and after DOX treatment. Each value is mean of two to five brains from WT or Npytet/tet mice that were treated with Dox (2 mg/kg) for the time indicated.
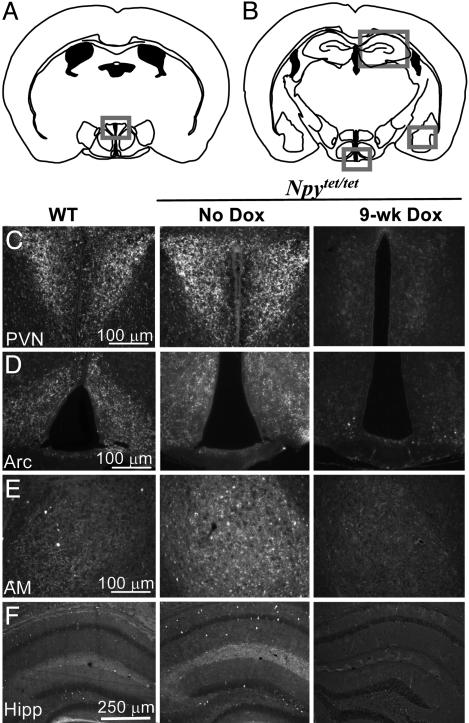
Immunohistochemistry was used to assess whether the NPY peptide was regulated similarly in different brain regions. Brain sections from WT and Npytet/tet mice that were either untreated or treated with Dox for 1 or 9 weeks were compared. There was more NPY staining in the cortex, hippocampus, hypothalamus (ARC and PVN), and amygdala of Npytet/tet mice (no Dox) compared with WT controls (Fig. 4 C–F). After a week of Dox treatment, the staining patterns were similar (data not shown), in agreement with normalization of total NPY levels at that time (Fig. 3). After 9 weeks of Dox treatment, the NPY signal was greatly reduced in all regions of the brain of Npytet/tet mice that were examined (Fig. 4 C–F) compared with WT controls, in agreement with the depletion of NPY protein (Fig. 3).
Fig. 4.
NPY immunohistochemistry of brain sections from control and Npytet/tet mice before and after treatment with Dox. (A) Diagram of coronal section at level of PVN. (B) Diagram of coronal section at level of ARC. (C) Immunohistochemistry of PVN (boxed region in A) from WT and Npytet/tet mice that were either untreated or given Dox (2 mg/kg) for 9 weeks. (D, E, and F) Immunocytochemistry of ARC, amygdala, and hippocampus (boxed regions in B), respectively, from WT and Npytet/tet mice that were either untreated or given DOX (2 mg/kg) for 9 weeks. The mice were fasted for 24 h before euthanizing to maximize NPY expression in the ARC and PVN.
Body Weight of Mice Expressing Excess NPY Is Normal. Comparison of WT and Npytet/tet male mice at 12–16 weeks of age that had been group housed and reared on regular mouse chow revealed that their body weights were not different (Table 1). Similar results were obtained with females (data not shown). Food consumption of individually housed mice was also the same. Likewise, the amounts of leptin, insulin, and glucose in the blood were the same (Table 1). Surprisingly, the dissected fat pads of Npytet/tet male mice weighed less than the WT controls (Table 1). All of these measurements indicate that chronic NPY excess does not promote weight gain or adiposity when mice are reared on normal chow diet.
Table 1. Body weight, adiposity, leptin, insulin, and glucose are normal in Npytet/tet mice.
| WT | Npytet/tet | |
|---|---|---|
| Body weight, g | 31.18 ± 0.74 (12) | 28.92 ± 0.7 (13) |
| Percent adiposity | 5.8 ± 0.52 (12) | 4.4 ± 0.46 (13)* |
| Leptin, ng/ml | 7.7 ± 1.3 (12) | 6.0 ± 1.7 (12) |
| Insulin, ng/ml | 0.29 ± 0.05 (12) | 0.20 ± 0.04 (10) |
| Blood glucose, mg/dl | 107.5 ± 5.3 (12) | 97.2 ± 6.5 (10) |
Parameters were measured from male mice 12–16 weeks old. The number of mice used to generate each value appears in parentheses (*, P < 0.05).
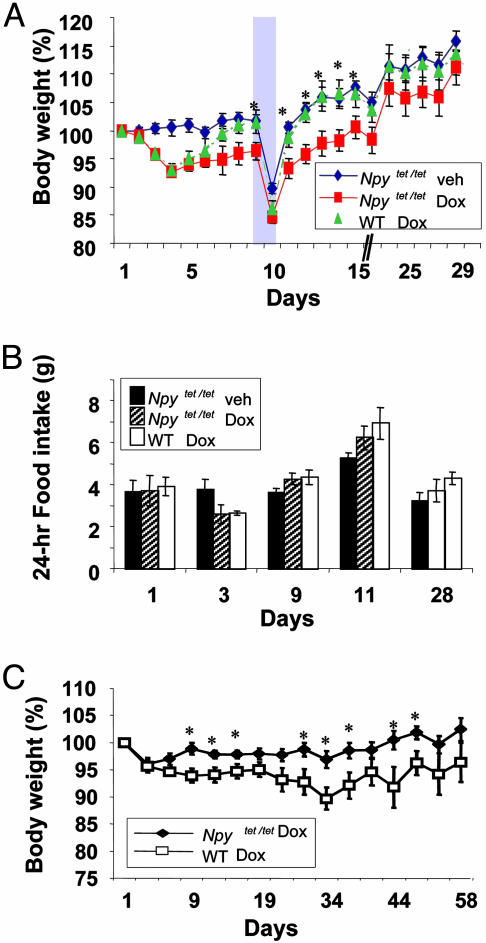
Reduction of NPY Expression in Adult Mice Does Not Affect Appetite. WT and Npytet/tet mice were treated with Dox (2 mg/ml); food consumption and body weights were monitored daily. All groups of mice had the same body weight at the outset of the experiment (Fig. 5, legend). There was a small decrease in body weight (Fig. 5A) and food consumption (Fig. 5B) by both groups during the first few days of Dox treatment compared with mice given vehicle alone, but the WT mice recovered their body weight by day 9 whereas the Npytet/tet mice did not (Fig. 5A). Nevertheless, food consumption on day 9 was normal (Fig. 5B). When food was removed for 36 h, both groups lost ≈15% of their prefasting body weights (Fig. 5A), and their refeeding responses were the same after food was returned (day 11, Fig. 5B). The mice were maintained on Dox for another 20 days to allow sufficient time for NPY protein to be cleared. By day 24, the body weights of all of the groups converged (Fig. 5A) and food consumption remained the same for all groups (Fig. 5B). In a separate experiment, Npytet/tet and control mice were given DOX for 2 months (Fig. 5C). The data were evaluated by repeated measures analysis of variance (ANOVA); a groups-by-days comparison revealed a genotype effect (F = 6.22, df 1/18, P < 0.03). Body weighs of both groups fell during the first few days, but the weight of control animals returned to normal, whereas body weight of the Npytet/tet mice remained depressed for about a month before returning to normal. Food consumption was not measured in this experiment.
Fig. 5.
Effect of Dox on body weight and food consumption. Female WT and Npytet/tet mice were treated with Dox (2 mg/kg) starting at ≈8 wk of age. A control group of Npytet/tet mice received only the vehicle. Body weight (A) and 24-hr food consumption (B) were monitored daily for 4 weeks, but only selected days are shown. On day 9, the mice were fasted for 36 h (shading); food consumption during the following 24 h was recorded. Average body weights (± SEM) at the start of DOX treatment were: WT (Dox), 19.8 ± 1.47 g; Npytet/tet mice (Dox), 19.70 ± 1.17 g; Npytet/tet mice (vehicle), 19.37 ± 0.80 g (n = 5 for all groups). In a separate experiment, ≈ 16-wk-old, male Npytet/tet mice (n = 9; average initial weight 29.82 ± 1.48 g) and WT mice (n = 13; average initial weight 27.74 ± 1.38 g) were treated with DOX for 2 months, and body weight was recorded. *, Significant difference (P < 0.05) between WT and Npytet/tet mice treated with Dox based on t tests at individual time points.
Restoration of NPY in the Adult Does Not Affect Body Weight. We also did the inverse experiment, by rearing pregnant Npytet/tet and WT females on Dox (2 mg/ml) and maintaining them and their pups on Dox until 1 wk after weaning; then the pups were switched to normal water. Food consumption and body weights were monitored before after Dox treatment ceased. There were no significant effects of genotype on either food consumption or body weight (data not shown).
Reducing NPY Expression Affects Seizure Severity. Previous studies showed that overexpression of NPY using an adeno-associated virus protects rats against seizures induced by kainic acid and that mice lacking NPY or its receptors are more sensitive to seizures (29, 48, 49). Therefore, we evaluated sensitivity to kainate-induced seizures to determine whether changing NPY expression affects neuronal excitability. Groups of WT and Npytet/tet mice (with or without Dox for 1 week) were exposed to kainate (25 mg/kg) and monitored for 2 h for signs of seizure activity. Comparison of WT and Npytet/tet mice indicates that overexpression of NPY decreased sensitivity to kainate-induced seizures. Table 2 shows that the latencies to onset of stage 3 and stage 5 seizures were greater, and the amount of time spent in stages 3, 4, and 5 seizures were reduced in mice overexpressing NPY. Treatment of Npytet/tet mice with Dox for 1 wk increased kainate sensitivity such that latencies to stage 3 and 5 seizures and the amount of time spend in stages 3, 4, or 5 seizures were no longer significantly different from in WT controls (Table 2). Similar results were obtained with Npytet/tet mice exposed to Dox for 9 wk (data not shown).
Table 2. NPY overexpression protects against kainate-induced seizures.
| WT | Npytet/tet | Npytet/tet + Dox | |
|---|---|---|---|
| Latency to stage 3, min | 28.1 ± 3.4 | 61.9 ± 12** | 51.8 ± 13 |
| Latency to stage 5, min | 46.7 ± 8.1 | 83.5 ± 12* | 56.6 ± 13 |
| Time in stage 3–5, min | 21.9 ± 6.1 | 5.6 ± 3.3* | 12.2 ± 6.1 |
| Avg. severity score | 4.9 ± 0.1 | 4.1 ± 0.5 | 4.6 ± 0.5 |
Mice were injected i.p. with 25 mg/kg kainic acid and monitored for 2 h. WT (n = 11), NPYtet/tet, and NPYtet/tet (n = 9) treated with Dox for 1 wk. *, P < 0.05 significant difference between NPYtet/tet and WT; **, P < 0.01 significant difference between NPYtet/tet and WT.
Discussion
The main conclusion of these studies is that changing NPY levels in the brain by 20-fold (from 4-fold more than WT levels to a fraction of WT levels) has little effect on body-weight regulation. The protocol used here had a significant effect on NPY levels in the medial hypothalamus, where it is predicted to have important effects on appetite and metabolism (24–28). Because transcription, translation, storage, and decay of Npy mRNA or protein could be differentially regulated, detailed kinetics of Npy mRNA and protein levels before, during, and after Dox treatment might reveal cell-specific differences. Nevertheless, reducing NPY expression to ≈20% of WT levels had no discernible effect on appetite, although there was a transient effect on body weight. We anticipated that there would at least be a transient inhibition of feeding; it did decline during the first few days of Dox treatment, but it declined in both WT and Npytet/tet mice, presumably due to aversion to the new drinking water.
There are several caveats to interpretation of the results based on the experimental approach used here. One is that overexpression of NPY throughout development might perturb subsequent responsiveness to NPY depletion although one could also argue that overexpression of NPY might lead to exaggerated effects upon depletion. The other caveat is that NPY depletion was incomplete, even after 9 weeks of Dox treatment; therefore, one could argue that residual NPY is sufficient to maintain feeding. The latter possibility seems remote, considering that NPY receptor antagonists, antibodies, and antisense methods (15–22) have all produced transient effects on feeding, and it is unlikely that complete inhibition of NPY signaling was achieved with any of these methods. The transient effect of Dox treatment on body weight of Npytet/tet mice relative to WT controls was independent of food consumption and occurred while NPY levels were greater than those in WT mice. Locomotion was not monitored; thus, we do not know whether the difference in body weight is a consequence of enhanced activity by Dox-treated Npytet/tet mice or enhanced basal metabolism. Nevertheless, body weight of the Npytet/tet mice returned to normal after a few weeks. A third caveat is that NPY release and occupancy of NPY receptors were not measured in this study. Thus, it is possible that, despite the 20-fold reduction of NPY protein in the hypothalamus, residual NPY continued either to be released normally or to activate NPY receptors normally.
A plausible explanation for the maintenance of feeding behavior is that NPY protein decays so slowly (half-life of ≈5 days) that compensatory mechanisms have ample time to be engaged, resulting in a smooth transition without any obvious interruption in feeding. NPY neurons have Y2 autoreceptors that would probably respond to NPY loss with less feedback inhibition, which could lead to increased release of GABA, AgRP, and any other neuromodulators made by these cells. Consequently, the inhibitory tone on POMC cells or other critical cells may be maintained during the gradual decline in NPY. This logic might explain why chemical antagonists to Y1 and/or Y5 receptors affect feeding, but it does not explain the effects of antibodies to NPY or antisense oligonucleotides directed against NPY because they should also reduce NPY binding to autoreceptors. Some approaches that interfere with NPY signaling may have nonspecific effects on feeding. For example, a recent study showed that a “Y5 receptor-specific antagonist” was still effective in Npy5r-null mice lacking these receptors (50).
A more extreme possibility for maintenance of feeding by DOX-treated Npytet/tet mice is that NPY is not an important regulator of feeding in the adult, even though the cells that make NPY in the ARC are critical (41). NPY may fine tune other more critical signaling processes (e.g., GABA signaling) by these cells. The observation that mice can compensate for ablation of NPY/AgRP neurons in the ARC as neonates, but not as adults, provides an opportunity to ask what molecules elicit the compensatory mechanisms. For example, if the loss of NPY in neonates elicits the compensatory mechanism, then we predict that ablation of the NPY/AgRP neurons in adults would be tolerated by Npy-null mice.
An important observation is that modest excess (4-fold) of NPY does not result in hyperphagia or obesity. This result is consistent with results based on mice with multiple copies of a marked NPY transgene (51) and with transgenic rats that express excess NPY (52). Rodents overexpressing NPY manifest other interesting phenotypes (51–53); thus, we anticipate that these mice will as well, as indicated by the reduced seizure sensitivity. The ineffectiveness of chronic genetic augmentation of NPY expression on feeding and body weight is in stark contrast to the profound effects of either injecting or chronically perfusing NPY into the brain (e.g., see ref. 54). Approximately 1 nmol of NPY is typically injected into the brain to achieve robust feeding, although 30 pmol is effective under special conditions (55); even this lower amount of NPY would produce a local concentration >0.1 μM, considerably higher than the affinity of NPY for its receptors. At these concentrations, NPY may act nonspecifically, or it may engage NPY receptors that do not normally receive an NPY signal. The gene-targeting approach that we used should amplify NPY expression only in cells that normally express NPY because the tTA is driven by regulatory elements of the Npy gene. However, we do not know whether NPY-expressing neurons of Npytet/tet mice have more NPY per synaptic vesicle or more vesicles with the normal amount of NPY. Most importantly, we do not know whether NPY secretion is actually increased.
Although down-regulation of NPY expression in adult mice had little effect on appetite in this study with mice reared on a normal diet, the approach described here, or alternative approaches for reducing NPY expression in the adult (15, 56), can be used to distinguish between developmental and physiological roles for NPY signaling in other physiological conditions. For example, chronic NPY deficiency has a profound effect on the obesity phenotype of Lepob/ob mice, including improvement in glucose regulation, reproduction, and thyroid hormone synthesis (38). Thus, determining whether any of the severe phenotypes of Lepob/ob mice or diet-induced obesity can be ameliorated by reducing NPY expression in adults may be revealing.
Acknowledgments
We thank Aundrea Rainwater for help maintaining this mouse colony, Glenda Froelick for help with histology, and our colleagues for suggestions during the course of these experiments. This research was supported in part by National Institutes of Health Grant DK-58089.
Author contributions: L.S., S.L., and T.B.C. performed research; L.S. and S.L. analyzed data; R.D.P. and L.S. designed research; and R.D.P. wrote the paper.
Conflict of interest statement: No conflicts declared.
Abbreviations: AgRP, agouti-related protein; ARC, arcuate region of hypothalamus; Dox, doxycycline; NPY, neuropeptide Y; POMC, proopiomelanocortin; PVN, paraventricular nucleus; tTA, tetracycline-controlled transcriptional activator; Tet-p, tetracycline promoter DNA sequence.
References
- 1.Clark, J. T., Kalra, P. S., Crowley, W. R. & Kalra, S. P. (1984) Endocrinology 115, 427-429. [DOI] [PubMed] [Google Scholar]
- 2.Kalra, S. P., Dube, M. G., Fournier, A. & Kalra, P. S. (1991) Physiol. Behav. 50, 5-9. [DOI] [PubMed] [Google Scholar]
- 3.Morley, J. E., Levine, A. S., Grace, M. & Kneip, J. (1985) Brain Res. 341, 200-203. [DOI] [PubMed] [Google Scholar]
- 4.Stanley, B. G. & Leibowitz, S. F. (1985) Proc. Natl. Acad. Sci. USA 82, 3940-3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corp, E. S., Melville, L. D., Greenberg, D., Gibbs, J. & Smith, G. P. (1990) Am. J. Physiol. 259, R317-R323. [DOI] [PubMed] [Google Scholar]
- 6.Steinman, J. L., Gunion, M. W. & Morley, J. E. (1994) Pharmacol. Biochem. Behav. 47, 207-214. [DOI] [PubMed] [Google Scholar]
- 7.Brady, L. S., Smith, M. A., Gold, P. W. & Herkenham, M. (1990) Neuroendocrinology 52, 441-447. [DOI] [PubMed] [Google Scholar]
- 8.Smith, M. S. (1993) Endocrinology 133, 1258-1265. [DOI] [PubMed] [Google Scholar]
- 9.Sanacora, G., Kershaw, M., Finkelstein, J. A. & White, J. D. (1990) Endocrinology 127, 730-737. [DOI] [PubMed] [Google Scholar]
- 10.Kalra, S. P., Dube, M. G., Sahu, A. & Phelps, C. P. (1991) Proc. Natl. Acad. Sci. USA 88, 10931-10935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kesterson, R. A., Huszar, D., Lynch, C. A., Simerly, R. B. & Cone, R. D. (1997) Mol. Endocrinol. 11, 630-637. [DOI] [PubMed] [Google Scholar]
- 12.Schwartz, M. W. & Seeley, R. J. (1997) J. Am. Dietetic Assoc. 97, 54-58. [DOI] [PubMed] [Google Scholar]
- 13.Beck, B. (2000) Nutrition 16, 916-923. [DOI] [PubMed] [Google Scholar]
- 14.Li, A.-J. & Ritter, S. (2004) Eur. J. Neurosci. 19, 2147-2154. [DOI] [PubMed] [Google Scholar]
- 15.Kalra, P. S. & Kalra, S. P. (2000) Methods 22, 249-254. [DOI] [PubMed] [Google Scholar]
- 16.Burlet, A., Grouzmann, E., Musse, N., Fernette, B., Nicolas, J. P. & Burlet, C. (1995) Neuroscience 66, 151-159. [DOI] [PubMed] [Google Scholar]
- 17.Hulsey, M. G., Pless, C. M., White, B. D. & Martin, R. J. (1995) Regul. Pept. 59, 207-214. [DOI] [PubMed] [Google Scholar]
- 18.Chance, W. T., Tao, Z., Sheriff, S. & Balasubramaniam, A. (1998) Brain Res. 803, 39-43. [DOI] [PubMed] [Google Scholar]
- 19.Ishihara, A., Tanaka, T., Kanatani, A., Fukami, T., Ihara, M. & Fukuroda, T. (1998) Am. J. Physiol 274, R1500-R1504. [DOI] [PubMed] [Google Scholar]
- 20.Dube, M. G., Xu, B., Crowley, W. R., Kalra P. O. S. & Kalra, S. P. (1994) Brain Res. 646, 341-344. [DOI] [PubMed] [Google Scholar]
- 21.Daniels, A. J., Grizzle, M. K., Wiard, R. P., Matthews, J. E. & Heyeer, D. (2002) Regul. Pept. 106, 47-54. [DOI] [PubMed] [Google Scholar]
- 22.Turnbull, A. V., Ellershaw, L., Masters, D. J. Birtles, S., Boyer, S., Caroll, D., Clarkson, P., Loxham, S. J., McAulay, P., Teague J. L., et al. (2002) Diabetes 51, 2441-2449. [DOI] [PubMed] [Google Scholar]
- 23.Cowley, M. A., Smart, J. L., Rubenstein, M., Cerdan, M. G., Diano, S., Horvath, T. L., Cone, R. D. & Low, M. J. (2001) Nature 411, 480-484. [DOI] [PubMed] [Google Scholar]
- 24.Palmiter, R. D., Erickson, J. C., Hollopeter, G., Baraban, S. C. & Schwartz M. W. (1998) Recent Prog. Horm. Res. 53, 163-199. [PubMed] [Google Scholar]
- 25.Kalra, S. P., Dube, M. G., Pu, S., Xu, B., Horvath, T. L. & Kalra, P. S. (1999) Endocrinol. Rev. 20, 68-100. [DOI] [PubMed] [Google Scholar]
- 26.Spiegelman, B. M. & Flier, J. S. (2001) Cell 104, 531-543. [DOI] [PubMed] [Google Scholar]
- 27.Lin, S., Boey, D. & Herzog, H. (2004) Neuropeptides 38, 189-200. [DOI] [PubMed] [Google Scholar]
- 28.Schwartz, M. W. & Porte, D., Jr. (2005) Science 307, 375-379. [DOI] [PubMed] [Google Scholar]
- 29.Erickson, J. C., Clegg, K. E. & Palmiter, R. D. (1996) Nature 381, 415-421. [DOI] [PubMed] [Google Scholar]
- 30.Hollopeter, G., Erickson, J. C. & Palmiter, R. D. (1998) Int. J. Obesity 22, 506-512. [DOI] [PubMed] [Google Scholar]
- 31.Marsh, D. J., Hollopeter, G., Kafer, K. E. & Palmiter R. D. (1998) Nat. Med. 4, 718-721. [DOI] [PubMed] [Google Scholar]
- 32.Kushi, A., Sasai, H., Koizumi, H., Takeda, N., Yokoyama, M. & Nakamura, M. (1998) Proc. Natl. Acad. Sci. USA 95, 15659-15664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pedrazzini, T., Seydoux, J., Kunstner, P., Aubert, J. F., Grouzmann, E., Beerman, F. & Brunner, H. R. (1998) Nat. Med. 4, 722-726. [DOI] [PubMed] [Google Scholar]
- 34.Naveilhan, P., Hassani, H., Canals, J. M., Ekstrand, J., Larefalk, A., Chhajlani, V., Arenas, E., Gedda, K. Svensson, L, Thoren, P. & Ernfors, P. (1999) Nat. Med. 5, 1188-1193. [DOI] [PubMed] [Google Scholar]
- 35.Batterham, R. L., Cowley, M. A., Small, C. J., Herzog, H., Cohen, M. A., Dakin, C. L., Wren, A. M., Brynes, A. E., Low, M. J. & Ghatei, M. A., et al. (2002) Nature 418, 650-654. [DOI] [PubMed] [Google Scholar]
- 36.Sindelar, D. K., Mystkowski, P., Marsh, D. J., Palmiter, R. D. & Schwartz, M. W. (2002) Diabetes 51, 778-783. [DOI] [PubMed] [Google Scholar]
- 37.Sindelar, D. K., Ste. Marie, L., Miura, G. I., Palmiter, R. D., McMinn, J. E., Morton, G. J. & Schwartz, M. W. (2004) Endocrinology 145, 3363-3368. [DOI] [PubMed] [Google Scholar]
- 38.Erickson, J. C., Hollopeter, G. & Palmiter, R. D. (1996) Science 274, 1704-1707. [DOI] [PubMed] [Google Scholar]
- 39.Qian, S., Chen, H., Weingarth, D., Trumbauer, M. E., Novi, D. E., Guan X. M., Yu, H., Shen, Z., Feng, Y., Fraiser, E., et al. (2002) Mol. Cell. Biol. 22, 5027-5035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen, H. Y., Trumbauer, M. E., Chen, A. S., Weingarth, D. T., Adams, J. R., Fraiser, E. G., Shen, Z., Marsh, D. J., Feighner, S. D., Guan, X.-M., et al. (2004) Endocrinology 145, 2607-2612. [DOI] [PubMed] [Google Scholar]
- 41.Horvath, T. L., Bechmann, I., Naftolin, F., Kalra, S. P. & Leranth, C. (1997) Brain Res. 756, 283-286. [DOI] [PubMed] [Google Scholar]
- 42.Luquet, S., Perez, F. A., Hnasko, T. S. & Palmiter, R. D. (2005) Science 310, 683-685. [DOI] [PubMed] [Google Scholar]
- 43.Tallquist, M. D. & Soriano, P. (2000) Genesis 26, 113-115. [DOI] [PubMed] [Google Scholar]
- 44.Durnam, D. M. & Palmiter, R. D. (1983) Anal. Biochem. 131, 385-393. [DOI] [PubMed] [Google Scholar]
- 45.Racine, R. J. (1972) Electroencephalogr. Clin. Neurophysiol. 32, 281-294. [DOI] [PubMed] [Google Scholar]
- 46.Bond, C. T., Sprengel, R., Bissonnette, J. M., Kaufmann, W. A., Pribnow, D., Neelands, T., Storck, T., Baetscher, M., Jerecic, J., Maylie, J., et al. (2000) Science 289, 1942-1946. [DOI] [PubMed] [Google Scholar]
- 47.Yusa, K., Horie, K., Kondoh, G., Kuono, M., Maeda, Y., Kinoshita, T. & Takeda, J. (2004) Nature 429, 896-899. [DOI] [PubMed] [Google Scholar]
- 48.Richichi, C., Lin, E. J., Stefanin, D., Colella, D., Ravizza, T., Grignaschi, G., Veglianese, P., Sperk, G., During, M. J. & Vezzani, A. (2004) J. Neurosci. 24, 3051-3059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Marsh, D. J., Baraban, S. C., Hollopeter, G. & Palmiter, R. D. (1999). Proc. Natl. Acad. Sci. USA 96, 13518-13523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Della-Zuana, O., Revereault, L., Beck-Sickinger, A., Monge, A., Caignard, D.-H., Fauchère, J.-L., Henlin, J.-M., Audinot, V., Boutin, J. A., Chamorro, S., et al. (2004) Int. J. Obes. Relat. Metab. Disord. 28, 628-639. [DOI] [PubMed] [Google Scholar]
- 51.Thiele, T. E., Marsh, D. J., Ste. Marie, L., Bernstein, I. L. & Palmiter, R. D. (1998) Nature 396, 366-369. [DOI] [PubMed] [Google Scholar]
- 52.Thorsell, A., Michalkiewicz, M., Dumont, Y., Quirion, R., Caberlotto, L., Rimondini, R., Mathe, A. A. & Helig M. (2000) Proc. Natl. Acad. Sci. USA 97, 12852-12857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thorsell, A. & Helig, M. (2002) Neuropeptides 36, 182-193. [DOI] [PubMed] [Google Scholar]
- 54.Raposinho, P. D., Pedrazzini, T., White, R. B., Palmiter, R. D. & Aubert, M. L. (2004) Endocrinology 145, 304-310. [DOI] [PubMed] [Google Scholar]
- 55.Kalra, P. S., Dube, M. G., Xu, B. & Kalra, S. P. (1997) Regul. Pept. 72, 121-130. [DOI] [PubMed] [Google Scholar]
- 56.Gardiner, J. V., Kong, W. M., Ward, H., Murphy, K. G., Dhillo, W. S. & Bloom, S. R. (2005) Biochem. Biophy. Res. Commun. 327, 1088-1093. [DOI] [PubMed] [Google Scholar]