Abstract
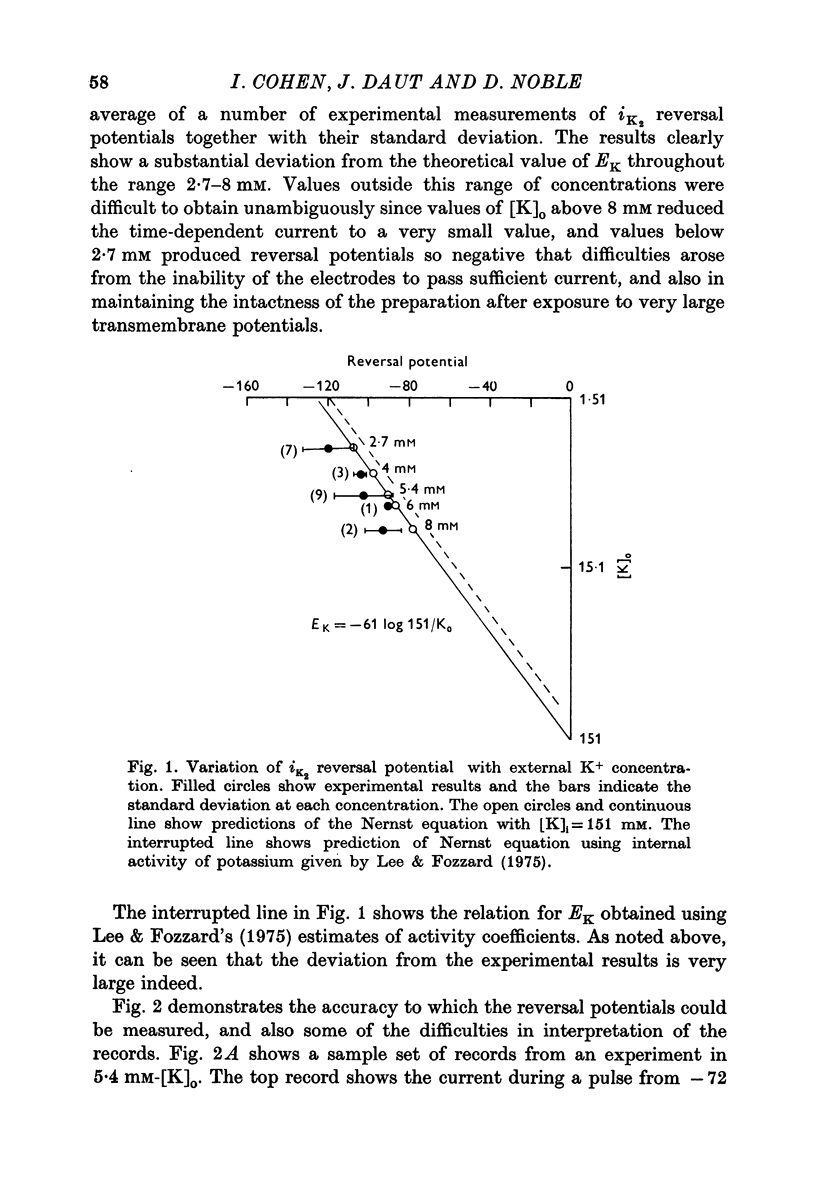
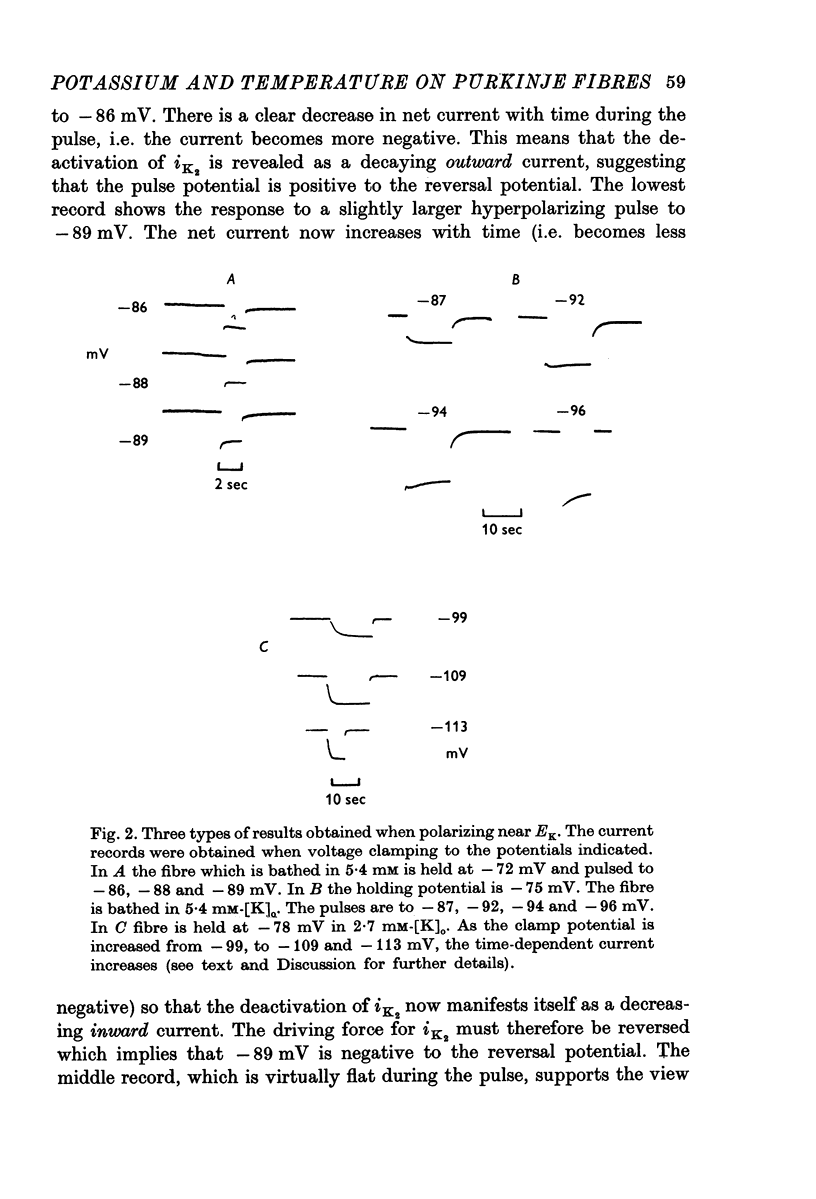
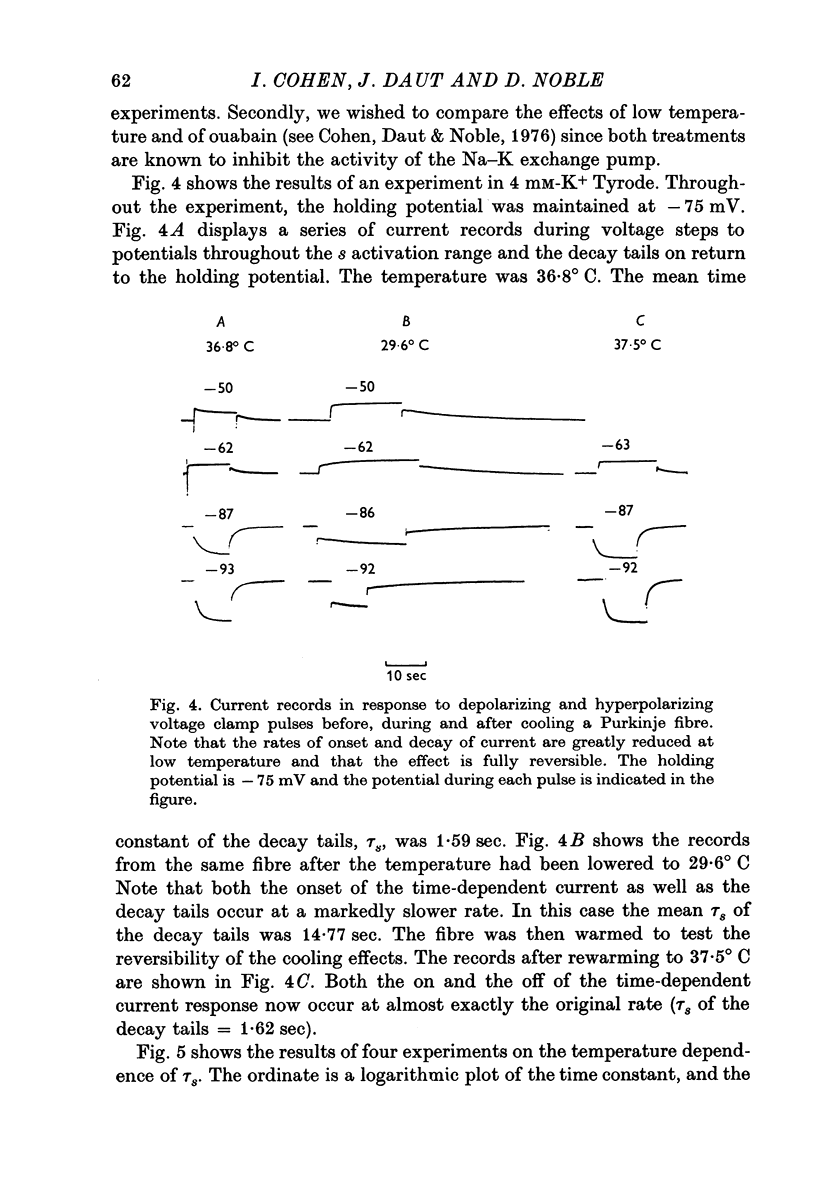
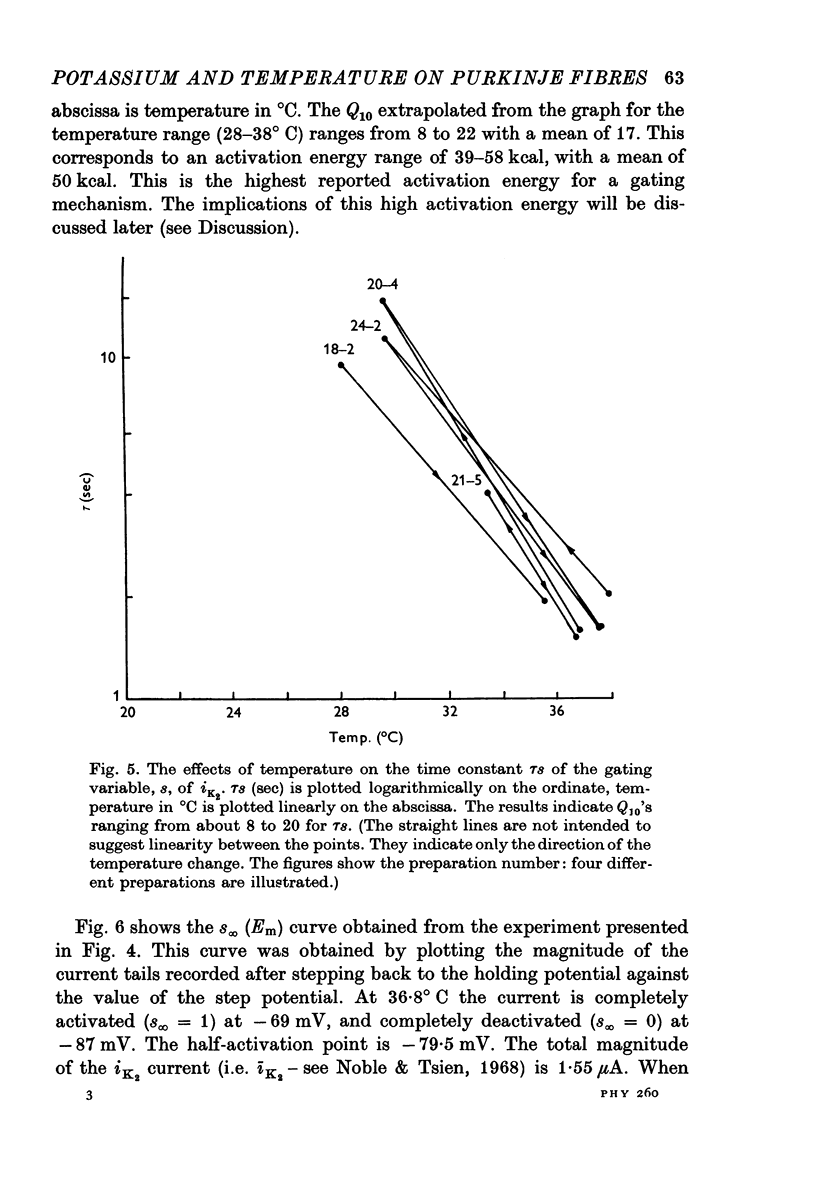
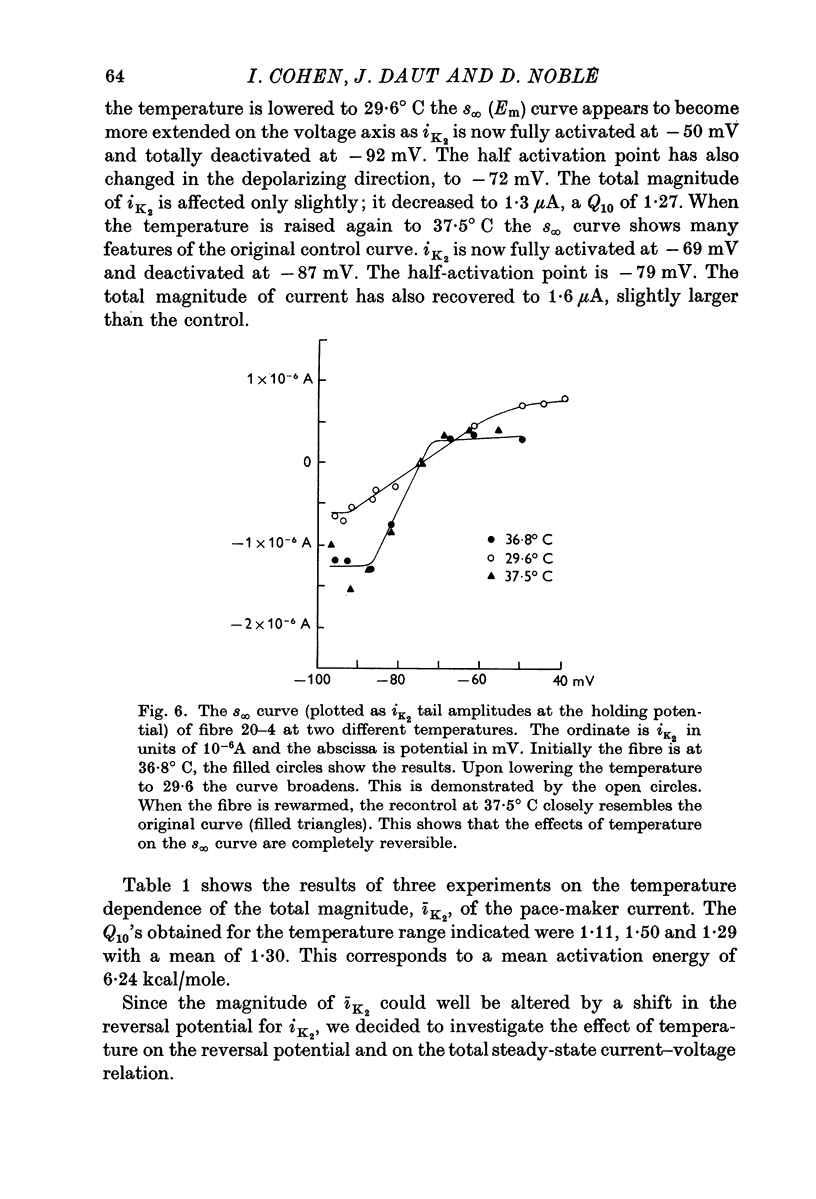
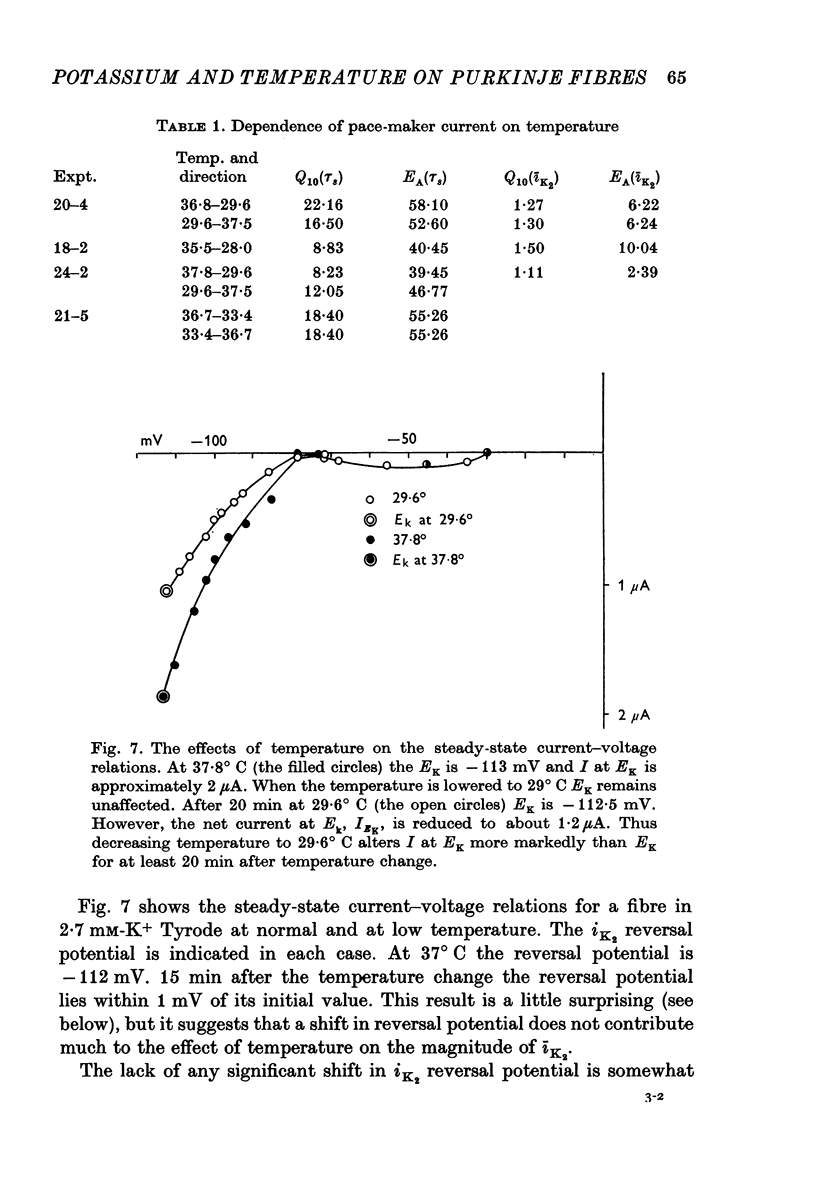
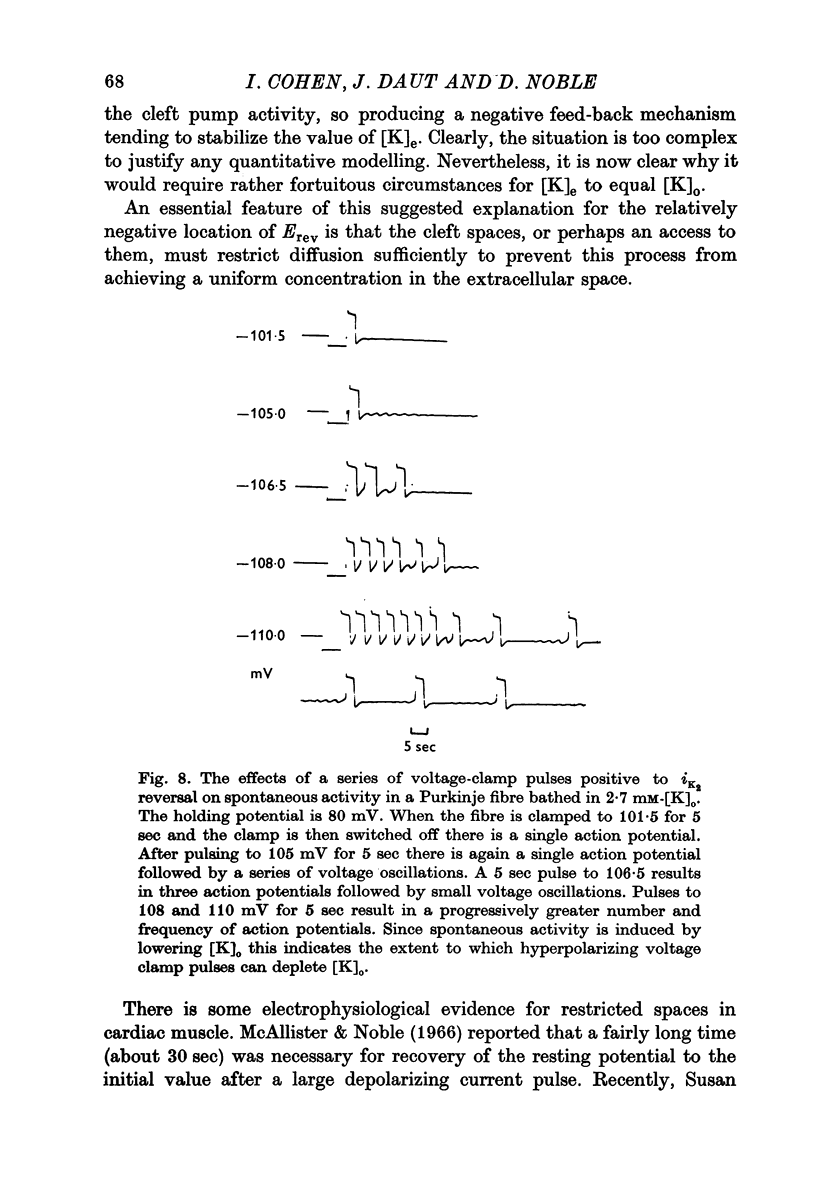
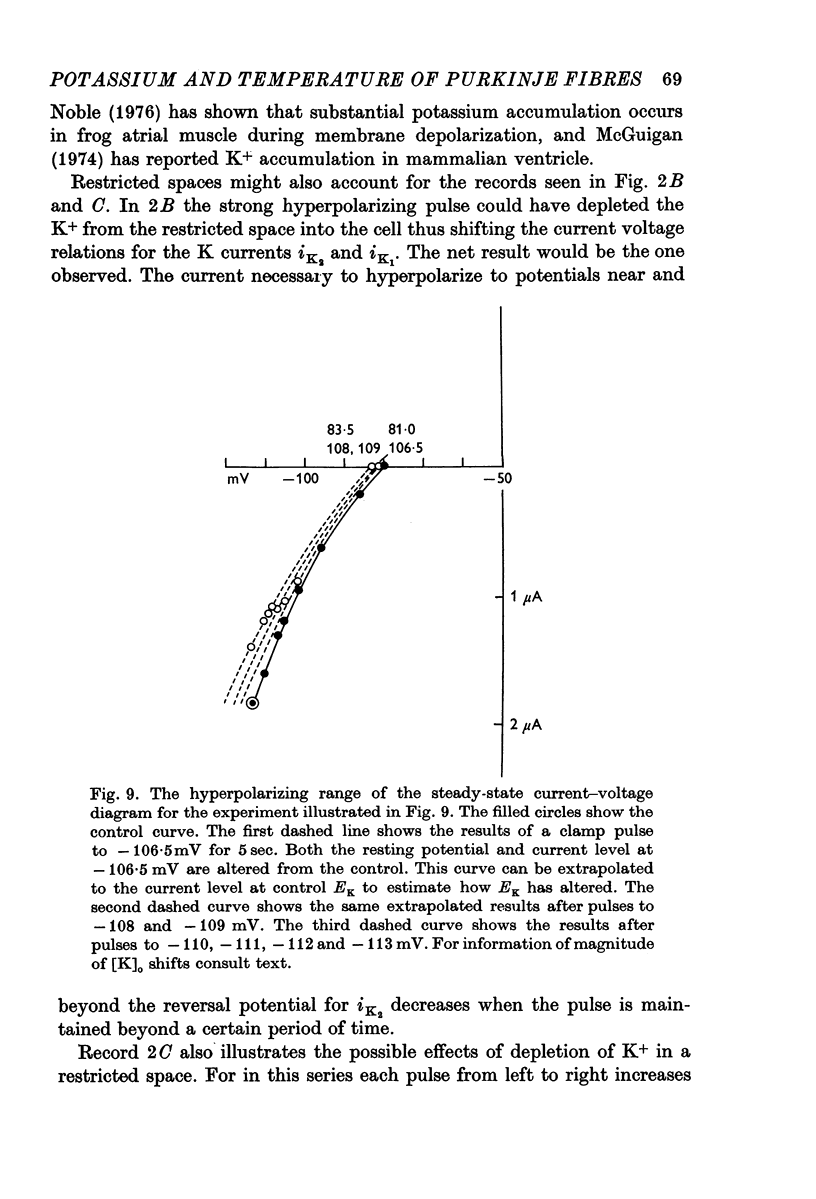
1. The reversal potential for the pace-maker K current, iK2, was measured in sheep cardiac Purkinje fibres at extracellular K concentrations, [K]O, between 2-7 and 8 mM. The reversal potentials were found to be significantly more negative than the values predicted using the Nernst equation for any reasonable value of intracellular K+ concentration or activity. 2. It is suggested that this discrepancy may be explained by postulating that the extracellular K+ concentration [K]e in the cleft spaces between cells is smaller than [K]o as a result of ion pumping and restricted diffusion from the bulk extracellular medium. 3. In conformity with this hypothesis, it was shown that the value of [K]e may be further reduced by hyperpolarizing pulses, presumably as a consequence of K+ depletion during the passage of inward current. 4. The influence of temperature on the kinetics of the gating mechanism, s, controlling iK2 was investigated. The Q10 for the time constant, pis, of current change following voltage clamp steps was found to be about 17. This corresponds to an apparent activation enthalpy of 50 kcal/mole. 5. The Q10 of the maximum amplitude iotaK2, was found to 1-3. 6. The activation curve, s infinity (Em), spread slightly to more negative potentials by cooling from 37 to 30 degrees C and the curve became less steep. 7. There is a large decrease in the inward background current on cooling, as estimated by measuring the net membrane current when iK is presumed to be zero, i.e. at the reversal potential for iK2.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown R. H., Jr Membrane surface charge: discrete and uniform modelling. Prog Biophys Mol Biol. 1974;28:341–370. doi: 10.1016/0079-6107(74)90021-2. [DOI] [PubMed] [Google Scholar]
- Carmeliet E. E., Van der Walt J. J. Duration of the action potential as a function of the membrane potential in cardiac Purkinje fibres. J Physiol. 1968 Feb;194(2):88P–88P. [PubMed] [Google Scholar]
- Cohen I., Daut J., Noble D. An analysis of the actions of low concentrations of ouabain on membrane currents in Purkinje fibres. J Physiol. 1976 Aug;260(1):75–103. doi: 10.1113/jphysiol.1976.sp011505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen I., Daut J., Noble D. Proceedings: The influence of extracellular potassium ions on the action of ouabain on membrane currents in sheep Purkinje fibres. J Physiol. 1975 Jul;249(1):42P–43P. [PubMed] [Google Scholar]
- Daut J., Spindler A. J. Proceedings: A mounting device for short Purkinje fibres. J Physiol. 1975 Nov;252(2):1P–2P. [PubMed] [Google Scholar]
- Dudel J., Rüdel R. Voltage and time dependence of excitatory sodium current in cooled sheep Purkinje fibres. Pflugers Arch. 1970;315(2):136–158. doi: 10.1007/BF00586657. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellam D. C., Studt J. W. A core-conductor model of the cardiac Purkinje fibre based on structural analysis. J Physiol. 1974 Dec;243(3):637–660. doi: 10.1113/jphysiol.1974.sp010770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iggo A. Cutaneous thermoreceptors in primates and sub-primates. J Physiol. 1969 Feb;200(2):403–430. doi: 10.1113/jphysiol.1969.sp008701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. O., Fozzard H. A. Activities of potassium and sodium ions in rabbit heart muscle. J Gen Physiol. 1975 Jun;65(6):695–708. doi: 10.1085/jgp.65.6.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister R. E., Noble D. The time and voltage dependence of the slow outward current in cardiac Purkinje fibres. J Physiol. 1966 Oct;186(3):632–662. doi: 10.1113/jphysiol.1966.sp008060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuigan J. A. Some limitations of the double sucrose gap, and its use in a study of the slow outward current in mammalian ventricular muscle. J Physiol. 1974 Aug;240(3):775–806. doi: 10.1113/jphysiol.1974.sp010634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobley B. A., Page E. The surface area of sheep cardiac Purkinje fibres. J Physiol. 1972 Feb;220(3):547–563. doi: 10.1113/jphysiol.1972.sp009722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble D., Tsien R. W. The kinetics and rectifier properties of the slow potassium current in cardiac Purkinje fibres. J Physiol. 1968 Mar;195(1):185–214. doi: 10.1113/jphysiol.1968.sp008454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble S. J. Potassium accumulation and depletion in frog atrial muscle. J Physiol. 1976 Jul;258(3):579–613. doi: 10.1113/jphysiol.1976.sp011436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peper K., Trautwein W. A note on the pacemaker current in Purkinje fibers. Pflugers Arch. 1969 Jun 19;309(4):356–361. doi: 10.1007/BF00587758. [DOI] [PubMed] [Google Scholar]
- ROBERTSON W. V., DUNIHUE F. W. Water and electrolyte distribution in cardiac muscle. Am J Physiol. 1954 May;177(2):292–298. doi: 10.1152/ajplegacy.1954.177.2.292. [DOI] [PubMed] [Google Scholar]
- VASSALLE M. CARDIAC PACEMAKER POTENTIALS AT DIFFERENT EXTRA-AND INTRACELLULAR K CONCENTRATIONS. Am J Physiol. 1965 Apr;208:770–775. doi: 10.1152/ajplegacy.1965.208.4.770. [DOI] [PubMed] [Google Scholar]


