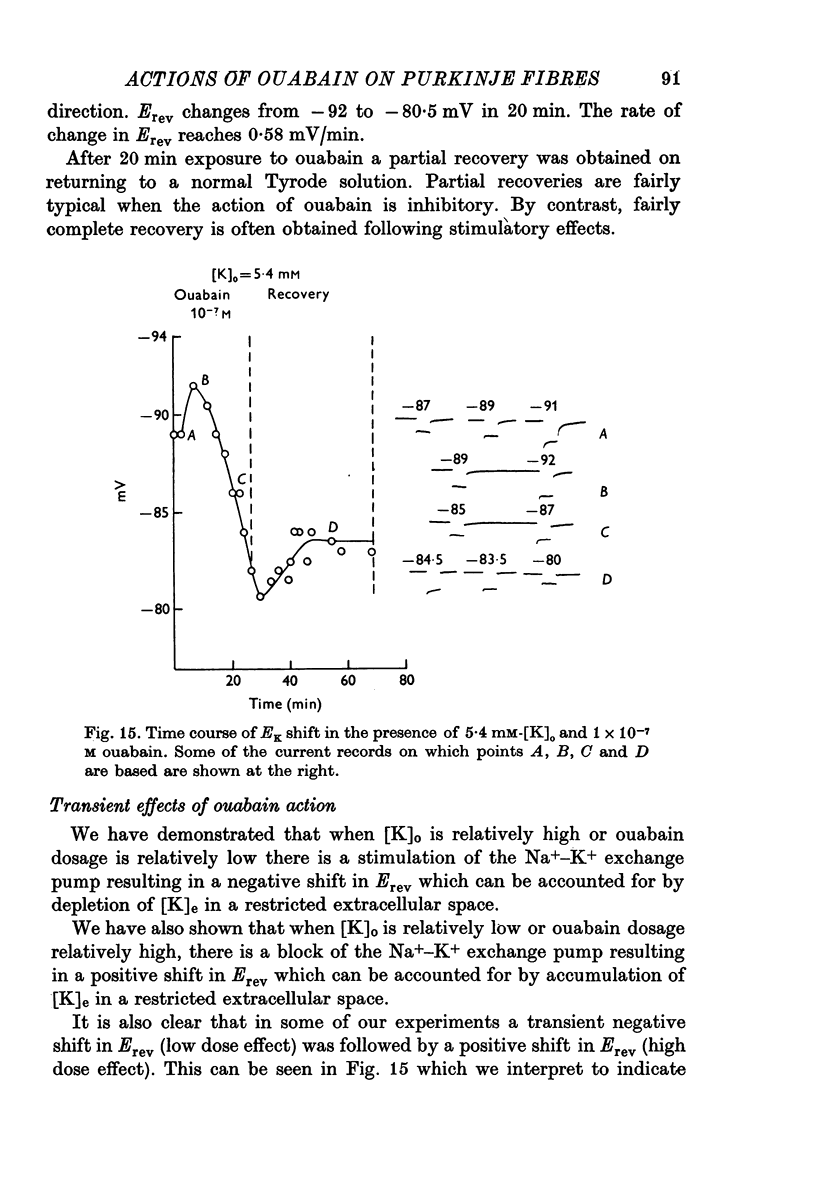
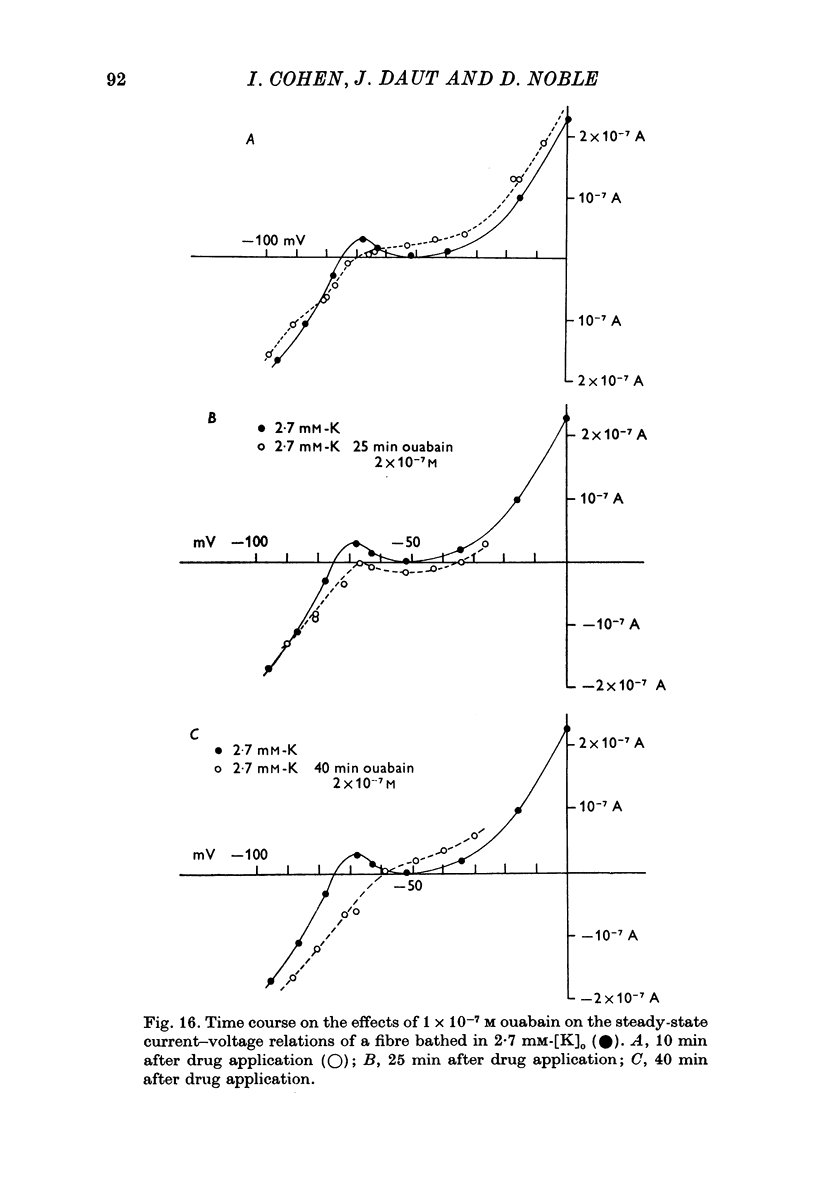
Abstract
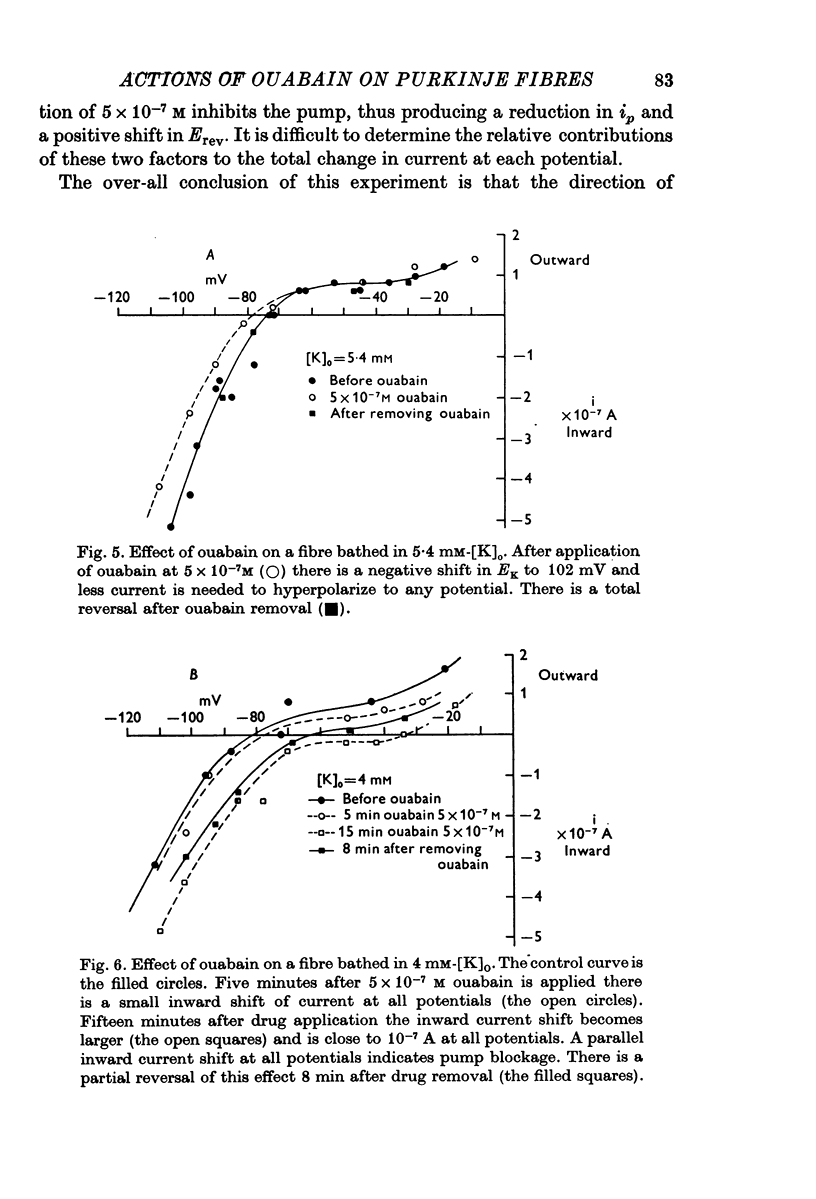
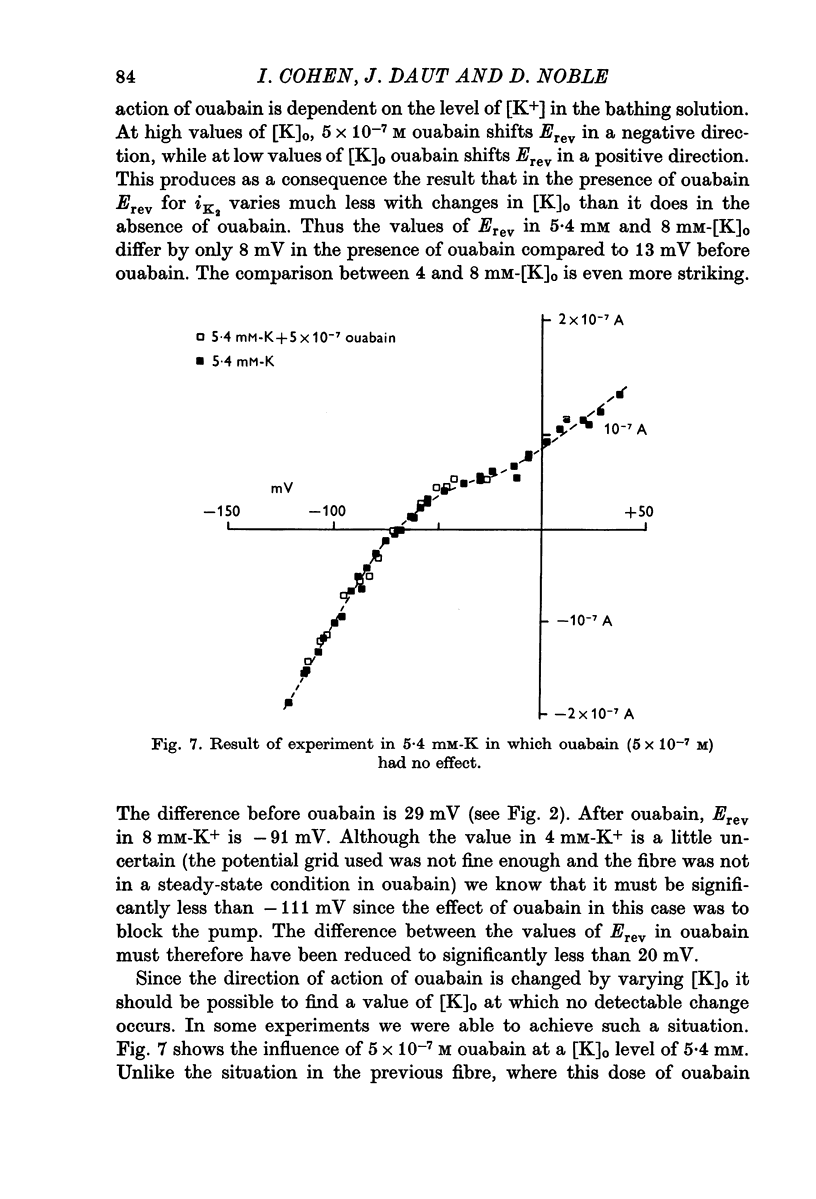
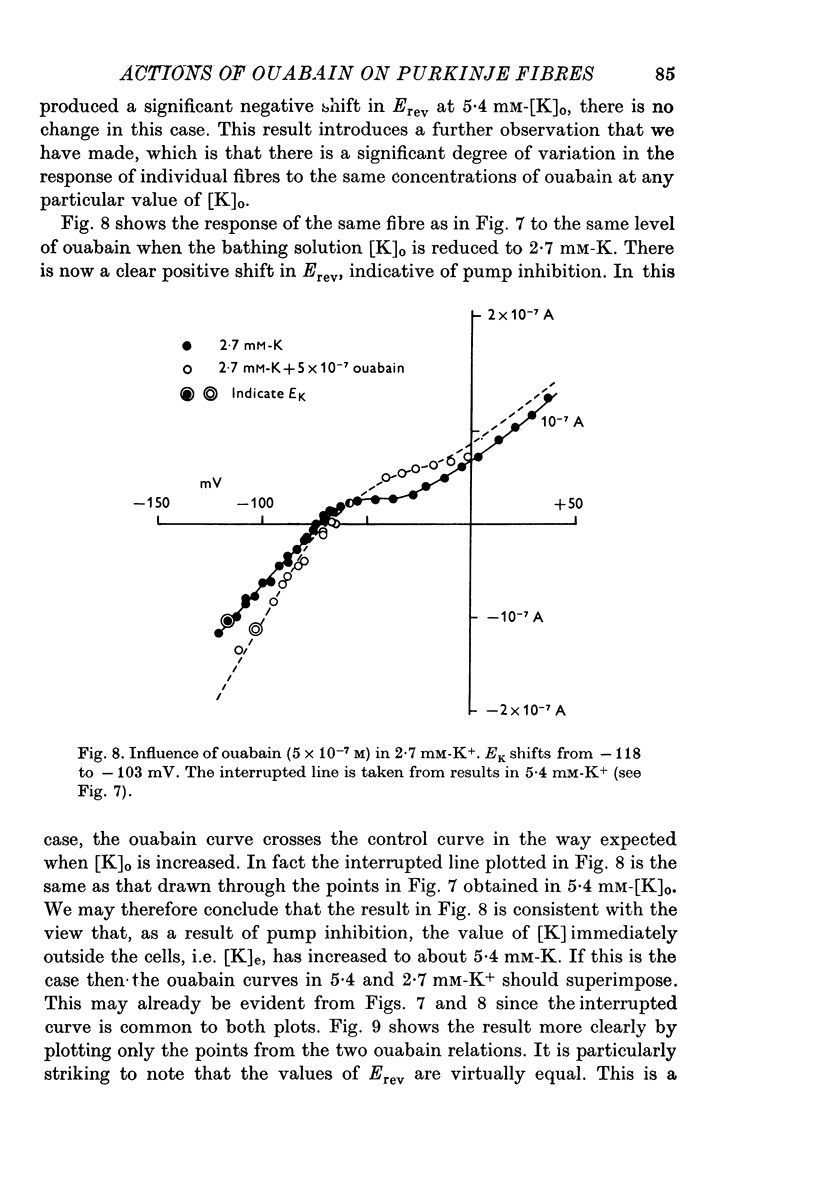
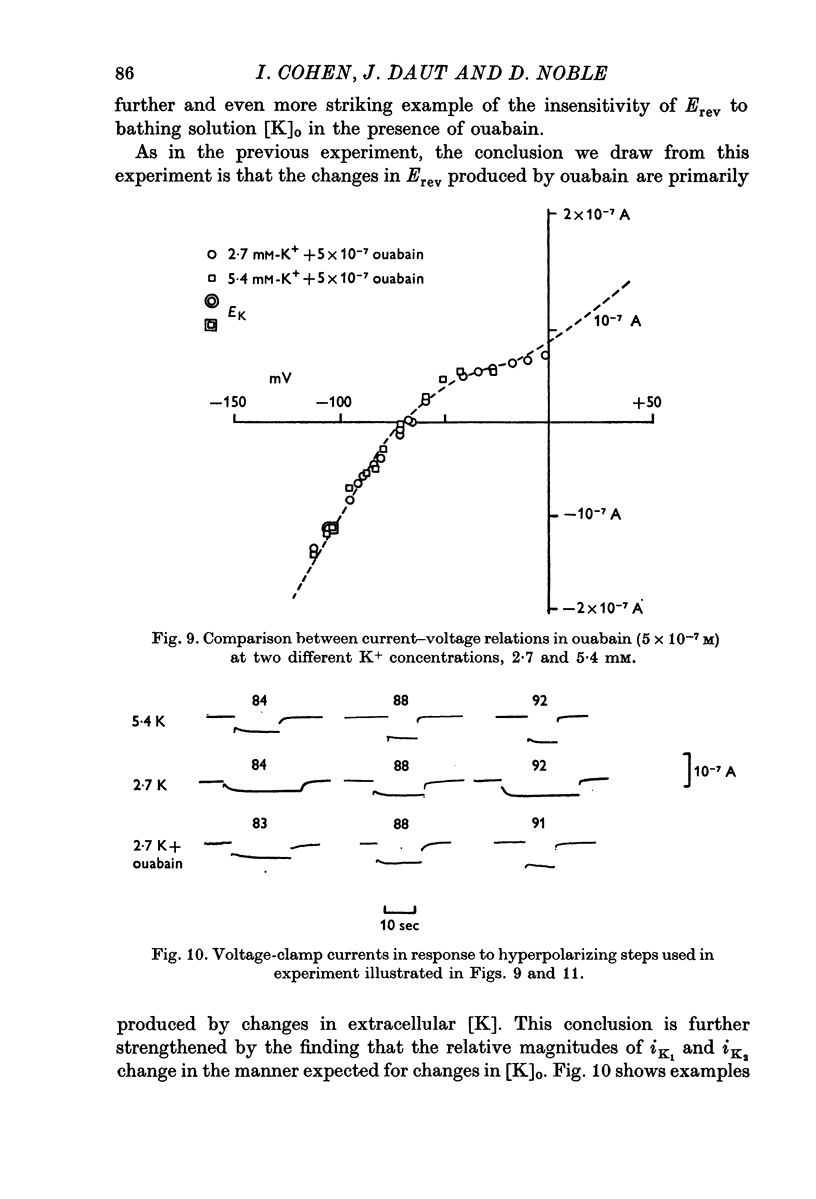
1. The influence of low concentrations (5 X 10(-8) to 5 X 10(-7) M) of ouabain on the K gradient in sheep cardiac Purkinje fibres was observed by measuring changes in the reversal potential for a K specific current iK2, and by measuring total steady-state current-voltage relations. 2. Provided that the bathing solution K concentration, [K]o was not too low, these doses of ouabain were often observed to increase the K gradient, i.e. the reversal potential was shifted in a negative direction. 3. The change in the reversal potential and in the current-voltage relation could be mimicked by reducing the value of [K]o in the absence of ouabain. It is therefore suggested that ouabain may stimulate the Na+-K+ exchange pump and so reduce the K concentration, [K]e, in the clefts of the preparation. 4. At sufficiently low values of [K]o, a dose of ouabain that was stimulatory may become inhibitory. The reversal potential for iK2 then shifts in a positive direction. 5. During either stimulation or inhibition, the speed of change of reversal potential is consistent with a change in [K]e, which may change fairly rapidly. It is not possible to account for the results solely by changes in intracellular concentration, [K]i. 6. Low concentrations of ouabain were found to have no effect on the activation curve, s infinity (Em), controlling iK2. It is concluded that the changes in iK2 are solely attributable to changes in reversal potential. 7. Since net stimulation of the Na+-K+ exchange pump was observed to occur at doses of ouabain that exert a strong positive inotropic action on Purkinje fibres (Blood, 1975), it is not likely that the inotropic action is causally related to net pump inhibition.
Full text
PDF



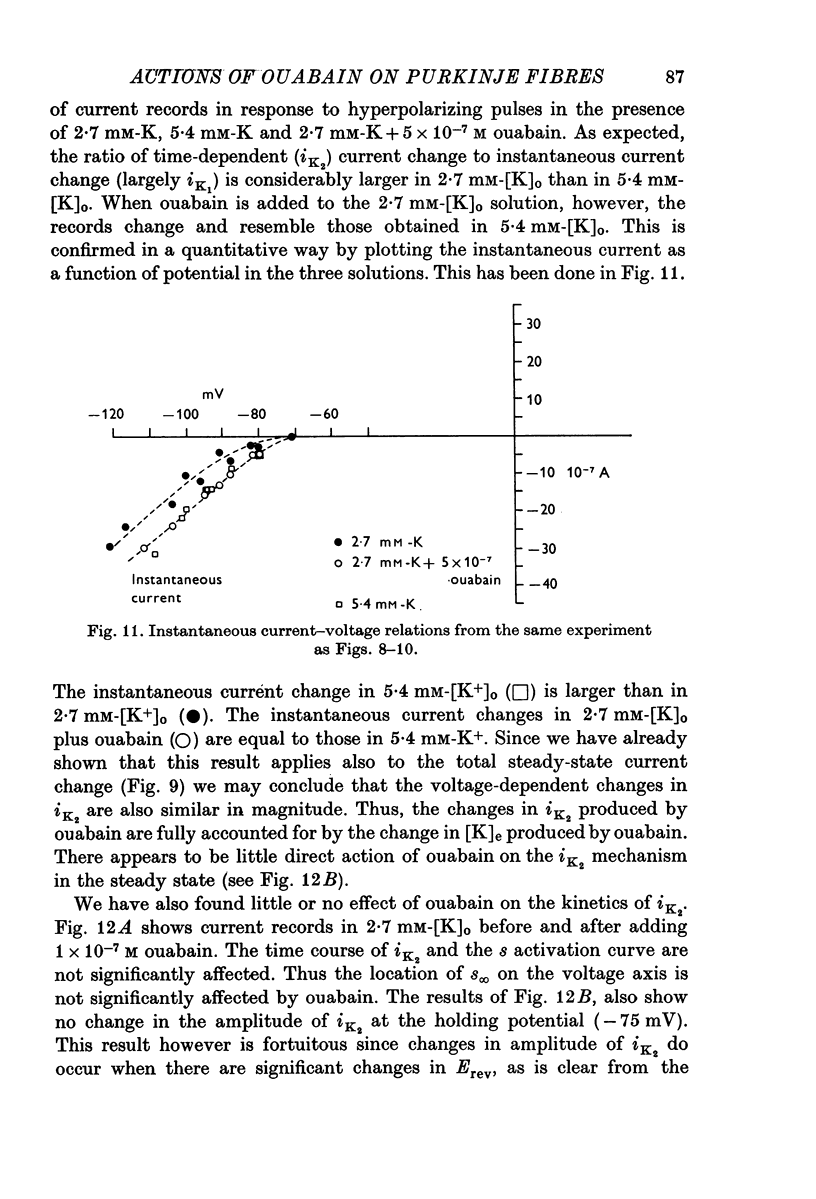
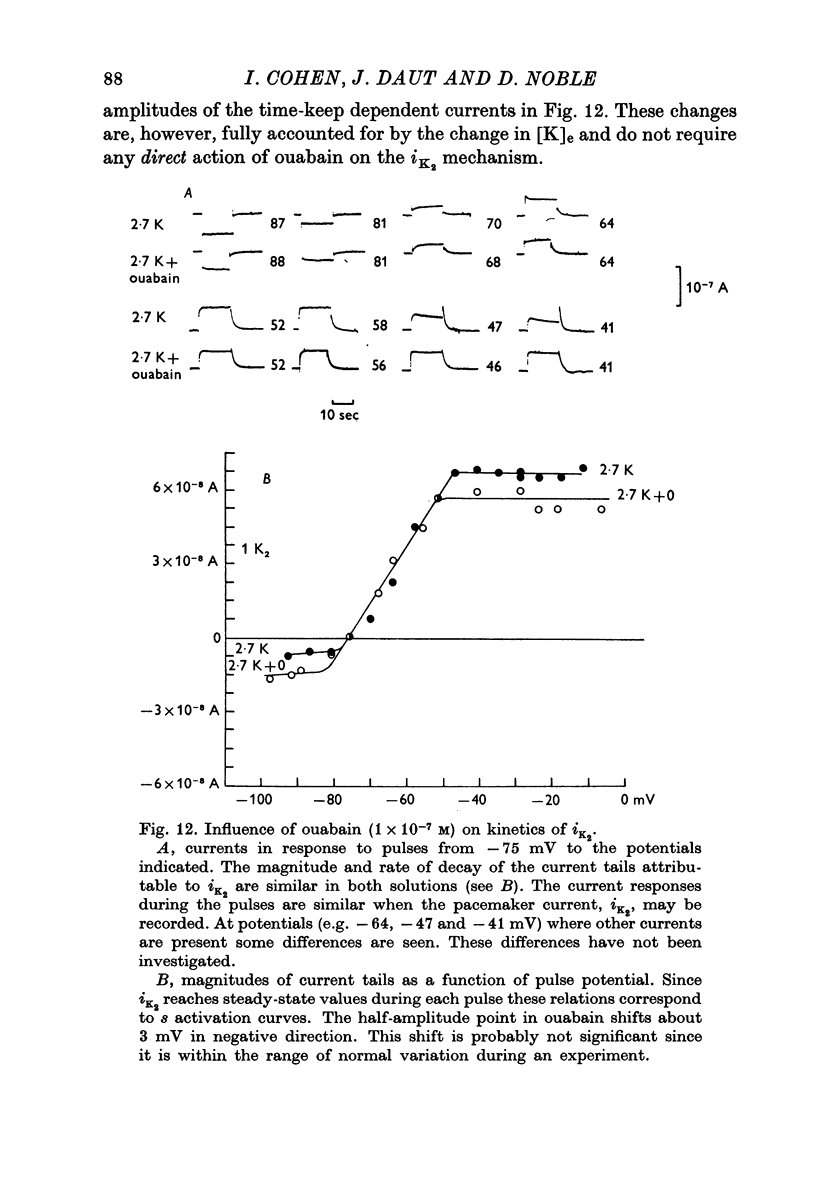
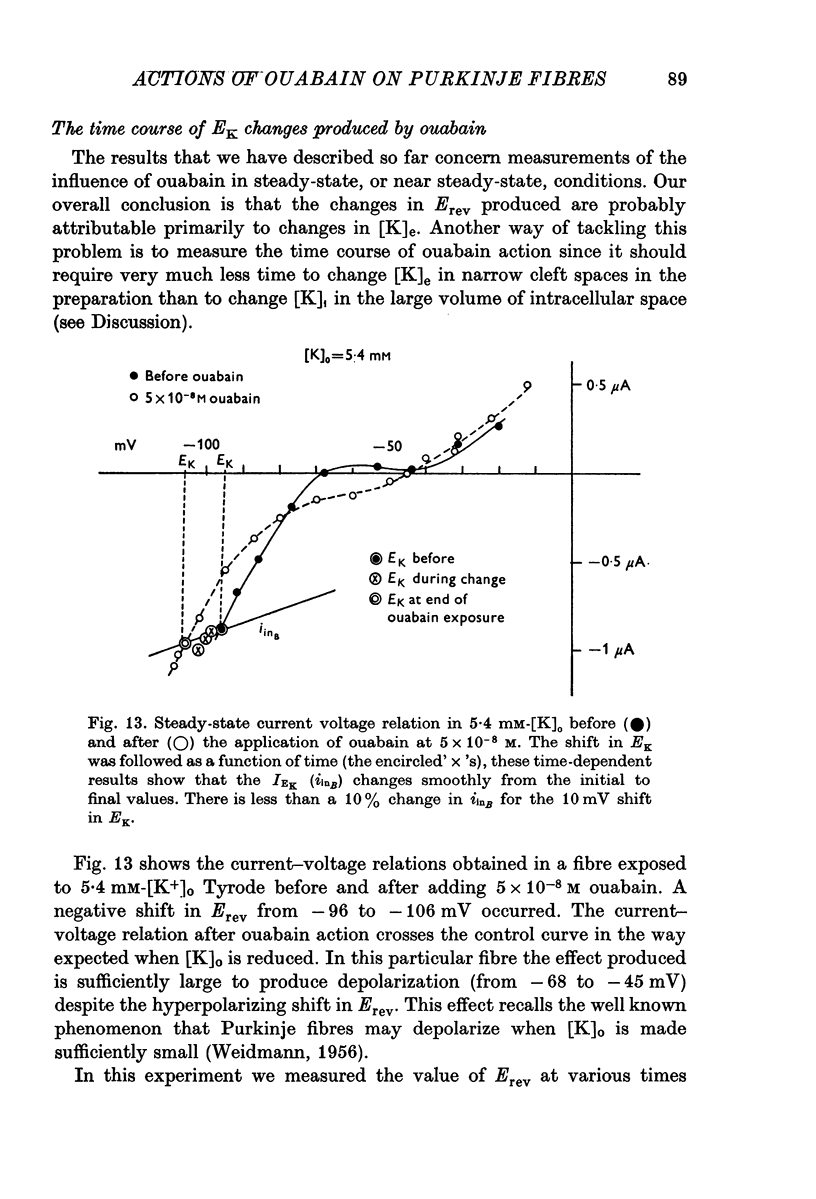
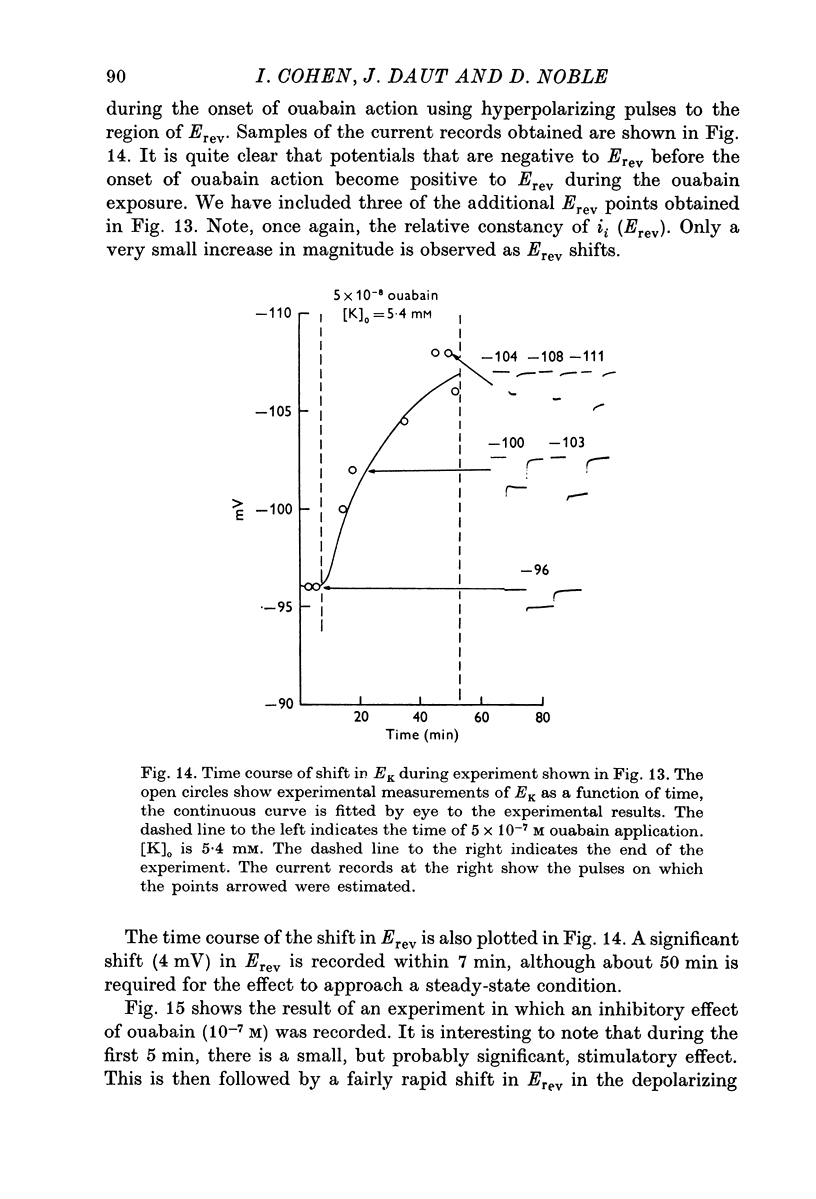











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akera T., Baskin S. I., Tobin T., Brody T. M. Ouabain: temporal relationship between the inotropic effect and the in vitro binding to, and dissociation from, (Na + + K + )- activated ATPase. Naunyn Schmiedebergs Arch Pharmacol. 1973;277(2):151–162. doi: 10.1007/BF00501156. [DOI] [PubMed] [Google Scholar]
- Akera T., Larsen F. S., Brody T. M. Correlation of cardiac sodium- and potassium-activated adenosine triphosphatase activity with ouabain-induced inotropic stimulation. J Pharmacol Exp Ther. 1970 May;173(1):145–151. [PubMed] [Google Scholar]
- Aronson R. S., Gelles J. M., Hoffman B. F. Effect of ouabain on the current underlying spontaneous diastolic depolarization in cardiac Purkinje fibers. Nat New Biol. 1973 Sep 26;245(143):118–120. doi: 10.1038/newbio245118a0. [DOI] [PubMed] [Google Scholar]
- BROWN T. E., ACHESON G. H., GRUPP G. The saturated-lactone glycoside dihydro-ouabain: effects on potassium balance of the dog heart. J Pharmacol Exp Ther. 1962 Apr;136:107–113. [PubMed] [Google Scholar]
- Baker P. F., Blaustein M. P., Hodgkin A. L., Steinhardt R. A. The influence of calcium on sodium efflux in squid axons. J Physiol. 1969 Feb;200(2):431–458. doi: 10.1113/jphysiol.1969.sp008702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Willis J. S. Inhibition of the sodium pump in squid giant axons by cardiac glycosides: dependence on extracellular ions and metabolism. J Physiol. 1972 Jul;224(2):463–475. doi: 10.1113/jphysiol.1972.sp009905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besch H. R., Jr, Allen J. C., Glick G., Schwartz A. Correlation between the inotropic action of ouabain and its effects on subcellular enzyme systems from canine myocardium. J Pharmacol Exp Ther. 1970 Jan;171(1):1–12. [PubMed] [Google Scholar]
- CARMELIET E. E. Chloride ions and the membrane potential of Purkinje fibres. J Physiol. 1961 Apr;156:375–388. doi: 10.1113/jphysiol.1961.sp006682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen I., Daut J., Noble D. Proceedings: The influence of extracellular potassium ions on the action of ouabain on membrane currents in sheep Purkinje fibres. J Physiol. 1975 Jul;249(1):42P–43P. [PubMed] [Google Scholar]
- Cohen I., Daut J., Noble D. The effects of potassium and temperature on the pace-maker current, iK2, in Purkinje fibres. J Physiol. 1976 Aug;260(1):55–74. doi: 10.1113/jphysiol.1976.sp011504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUDEL J., TRAUTWEIN W. Elektrophysiologiche Messungen zur Strophanthinwirkung am Herzmuskel. Naunyn Schmiedebergs Arch Exp Pathol Pharmakol. 1958;232(2):393–407. [PubMed] [Google Scholar]
- Dudel J., Peper K., Rüdel R., Trautwein W. The potassium component of membrane current in Purkinje fibers. Pflugers Arch Gesamte Physiol Menschen Tiere. 1967;296(4):308–327. doi: 10.1007/BF00362531. [DOI] [PubMed] [Google Scholar]
- GLYNN I. M. The action of cardiac glycosides on sodium and potassium movements in human red cells. J Physiol. 1957 Apr 3;136(1):148–173. doi: 10.1113/jphysiol.1957.sp005749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfraind T. The therapeutic mode of action of cardiac glycosides. Arch Int Pharmacodyn Ther. 1973 Dec;206(2):384–388. [PubMed] [Google Scholar]
- HOLLAND W. C., GREIG M. E., DUNN C. E. Factors affecting the action of lanatoside C on the potassium content of isolated perfused guinea pig hearts. Am J Physiol. 1954 Feb;176(2):227–231. doi: 10.1152/ajplegacy.1954.176.2.227. [DOI] [PubMed] [Google Scholar]
- Hansen O., Skou J. C. A study on the influence of the concentration of Mg 2+ , P i , K + , Na + , and Tris on (Mg 2+ + P i )-supported g-strophanthin binding to (Na + = K + )activated ATPase from ox brain. Biochim Biophys Acta. 1973 Jun 7;311(1):51–66. doi: 10.1016/0005-2736(73)90254-x. [DOI] [PubMed] [Google Scholar]
- Hansen O. The influence of monovalent cations and Ca2+ on G-strophanthin binding to (Na+ plus K+)-activated ATPase. Ann N Y Acad Sci. 1974;242(0):635–645. doi: 10.1111/j.1749-6632.1974.tb19122.x. [DOI] [PubMed] [Google Scholar]
- Isenberg G., Trautwein W. The effect of dihydro-ouabain and lithium-ions on the outward current in cardiac Purkinje fibers. Evidence for electrogenicity of active transport. Pflugers Arch. 1974;350(1):41–54. doi: 10.1007/BF00586737. [DOI] [PubMed] [Google Scholar]
- Langer G. A., Serena S. D. Effects of strophanthidin upon contraction and ionic exchange in rabbit ventricular myocardium: relation to control of active state. J Mol Cell Cardiol. 1970 Mar;1(1):65–90. doi: 10.1016/0022-2828(70)90029-5. [DOI] [PubMed] [Google Scholar]
- Langer G. A. Sodium exchange in dog ventricular muscle. Relation to frequency of contraction and its possible role in the control of myocardial contractility. J Gen Physiol. 1967 May;50(5):1221–1239. doi: 10.1085/jgp.50.5.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederer W. J., Tsien R. W. Proceedings: Transient inward current underlying strophanthidin's enhancement of pace-maker activity in Purkinje fibres. J Physiol. 1975 Jul;249(1):40P–41P. [PubMed] [Google Scholar]
- Lee K. S., Klaus W. The subcellular basis for the mechanism of inotropic action of cardiac glycosides. Pharmacol Rev. 1971 Sep;23(3):193–261. [PubMed] [Google Scholar]
- MUELLER P. OUABAIN EFFECTS ON CARDIAC CONTRACTION, ACTION POTENTIAL, AND CELLULAR POTASSIUM. Circ Res. 1965 Jul;17:46–56. doi: 10.1161/01.res.17.1.46. [DOI] [PubMed] [Google Scholar]
- Mobley B. A., Page E. The surface area of sheep cardiac Purkinje fibres. J Physiol. 1972 Feb;220(3):547–563. doi: 10.1113/jphysiol.1972.sp009722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble D., Tsien R. W. Outward membrane currents activated in the plateau range of potentials in cardiac Purkinje fibres. J Physiol. 1969 Jan;200(1):205–231. doi: 10.1113/jphysiol.1969.sp008689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble D., Tsien R. W. The kinetics and rectifier properties of the slow potassium current in cardiac Purkinje fibres. J Physiol. 1968 Mar;195(1):185–214. doi: 10.1113/jphysiol.1968.sp008454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okita G. T., Richardson F., Roth-Schechter B. F. Dissociation of the positive inotropic action of digitalis from inhibition of sodium- and potassium-activated adenosine triphosphate. J Pharmacol Exp Ther. 1973 Apr;185(1):1–11. [PubMed] [Google Scholar]
- Peper K., Trautwein W. A note on the pacemaker current in Purkinje fibers. Pflugers Arch. 1969 Jun 19;309(4):356–361. doi: 10.1007/BF00587758. [DOI] [PubMed] [Google Scholar]
- Peters T., Raben R. H., Wassermann O. Evidence for a dissociation between positive inotropic effect and inhibition of the Na+-K+-ATPase by ouabain, cassaine and their alkylating derivatives. Eur J Pharmacol. 1974 May;26(2):166–174. doi: 10.1016/0014-2999(74)90223-4. [DOI] [PubMed] [Google Scholar]
- SANYAL P. N., SAUNDERS P. R. Effect of therapeutic and toxic concentrations of ouabain upon potassium content of myocardium. Proc Soc Exp Biol Med. 1961 Mar;106:639–641. doi: 10.3181/00379727-106-26427. [DOI] [PubMed] [Google Scholar]
- SLEATOR W., Jr, FURCHGOTT R. F., DE GUBAREFF T., KRESPI V. ACTION POTENTIALS OF GUINEA PIG ATRIA UNDER CONDITIONS WHICH ALTER CONTRACTION. Am J Physiol. 1964 Feb;206:270–282. doi: 10.1152/ajplegacy.1964.206.2.270. [DOI] [PubMed] [Google Scholar]
- Schwartz A., Lindenmayer G. E., Allen J. C., McCans J. L. The nature of the cardiac glycoside enzyme complex: mechanism and kinetics of binding and dissociation using a high-activity heart Na+, K+-ATPase. Ann N Y Acad Sci. 1974;242(0):577–597. doi: 10.1111/j.1749-6632.1974.tb19119.x. [DOI] [PubMed] [Google Scholar]
- Schwartz A., Matsui H., Laughter A. H. Tritiated digoxin binding to (Na+ + K+)-activated adenosine triphosphatase: possible allosteric site. Science. 1968 Apr 19;160(3825):323–325. doi: 10.1126/science.160.3825.323. [DOI] [PubMed] [Google Scholar]
- TUTTLE R. S., WITT P. N., FARAH A. The influence of ouabain on intracellular sodium and potassium concentrations in the rabbit myocardium. J Pharmacol Exp Ther. 1961 Sep;133:281–287. [PubMed] [Google Scholar]
- TUTTLE R. S., WITT P. N., FARAH A. Therapeutic and toxic effects of ouabain on K ion fluxes in rabbit aorta. J Pharmacol Exp Ther. 1962 Jul;137:24–30. [PubMed] [Google Scholar]
- Toda N., West T. C. The action of ouabain on the function of the atrioventricular node in rabbits. J Pharmacol Exp Ther. 1969 Oct;169(2):287–297. [PubMed] [Google Scholar]
- ten Eick R. E., Bassett A. L., Okita G. T. Dissociation of electrophysiological and inotropic actions of strophanthidin-3-bromoacetate: possible role of adenosine triphosphatase in the maintenance of the myocardial transmembrane Na + and K + gradients. J Pharmacol Exp Ther. 1973 Apr;185(1):12–23. [PubMed] [Google Scholar]


