Abstract
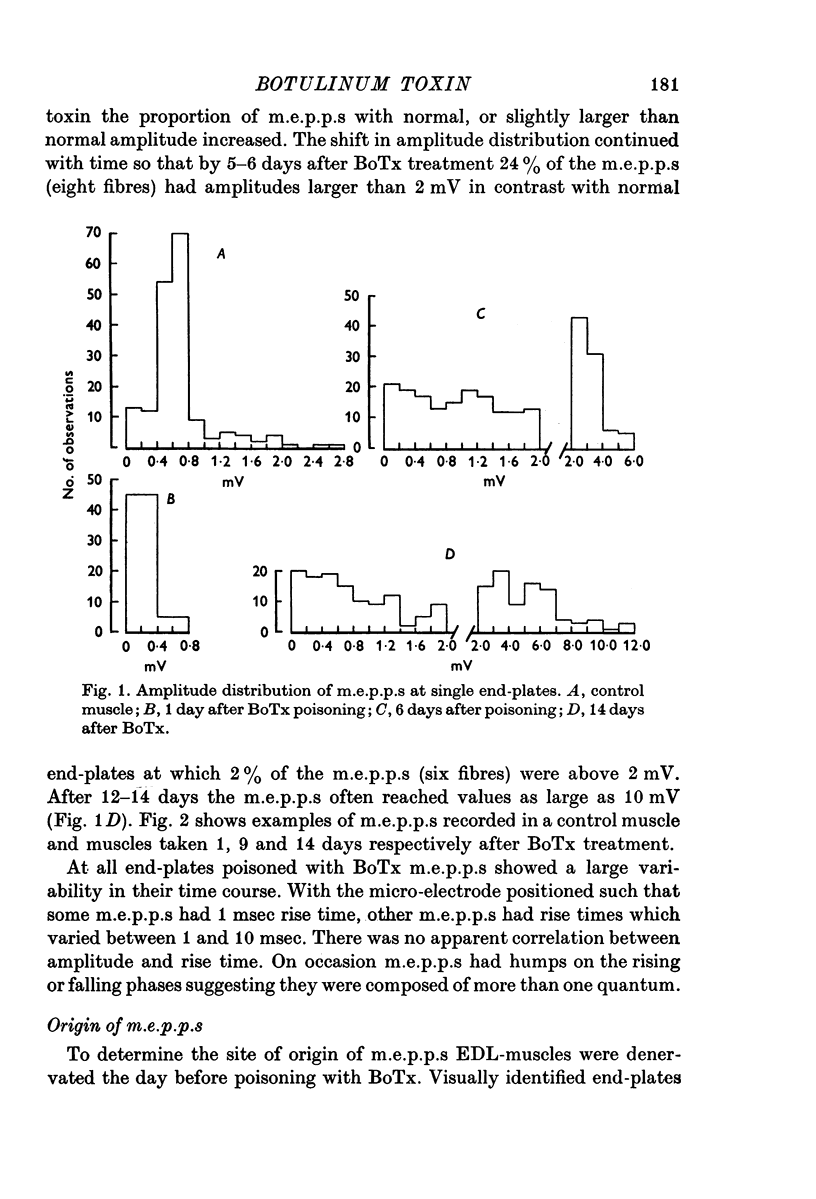
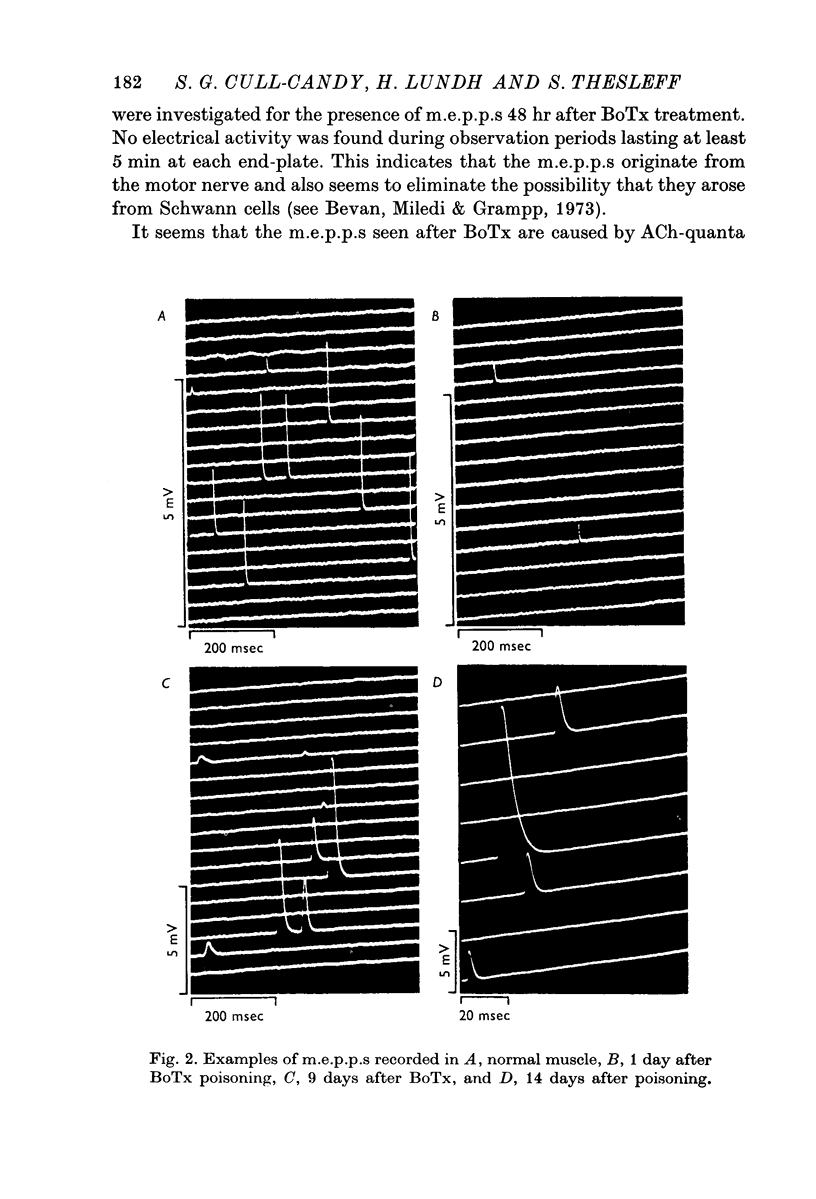
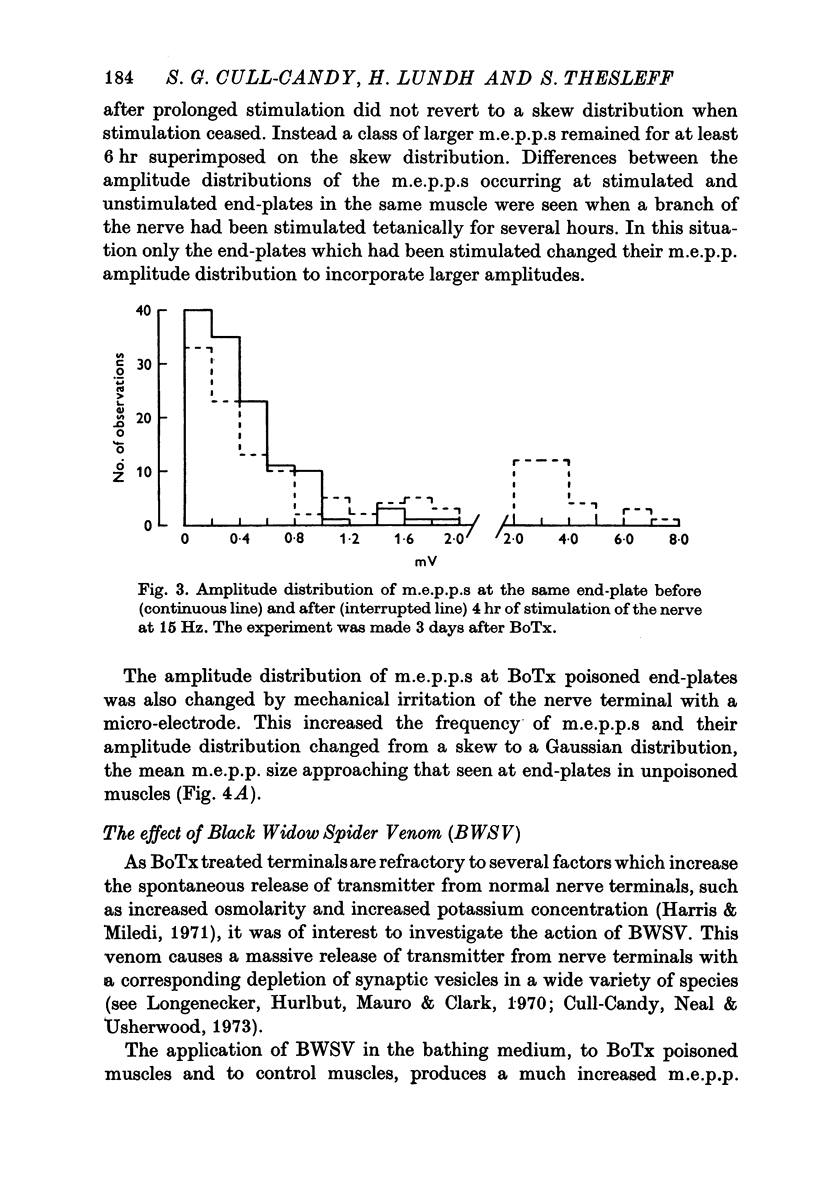
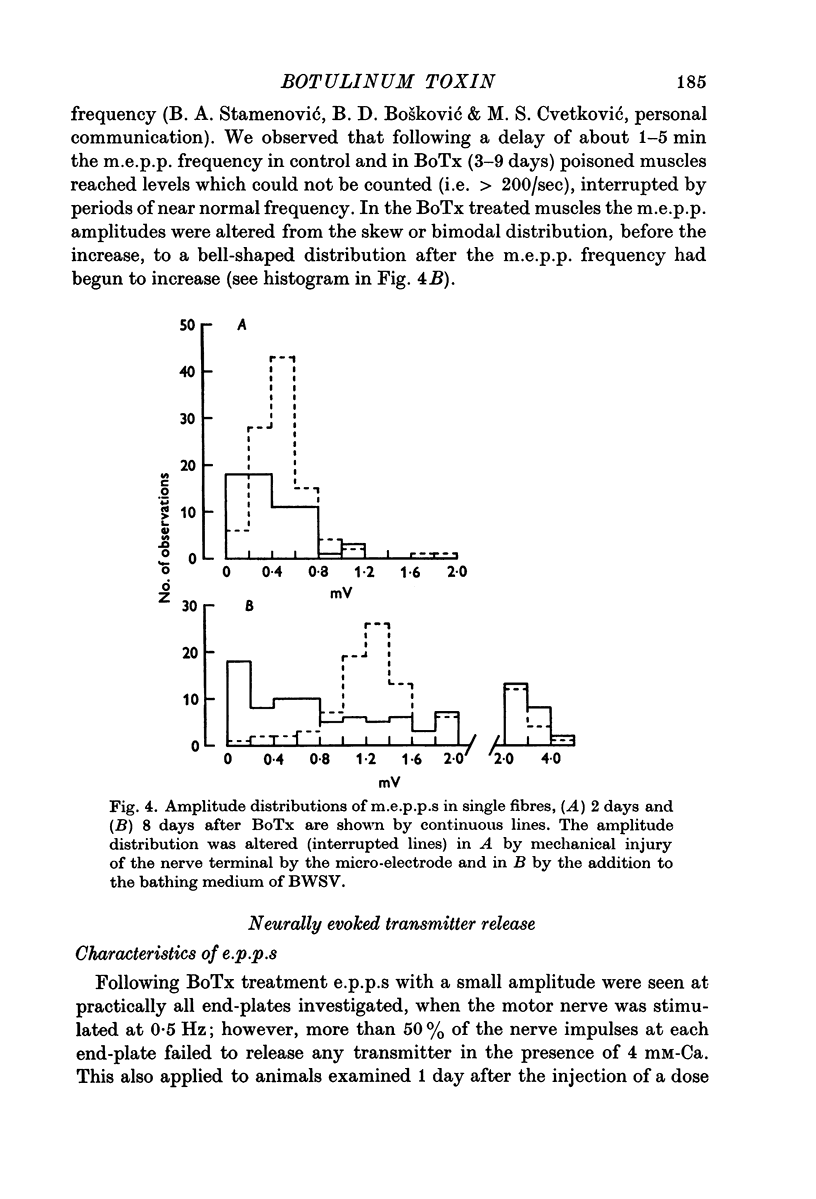
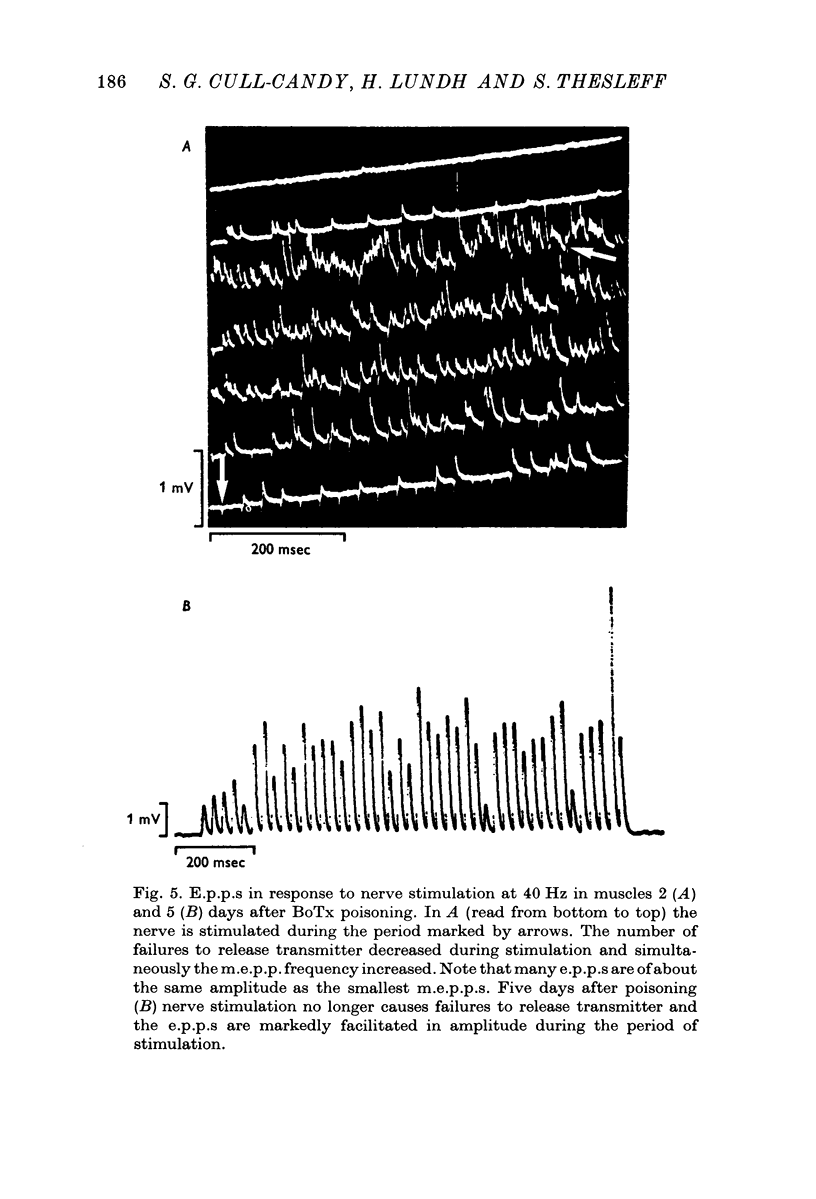
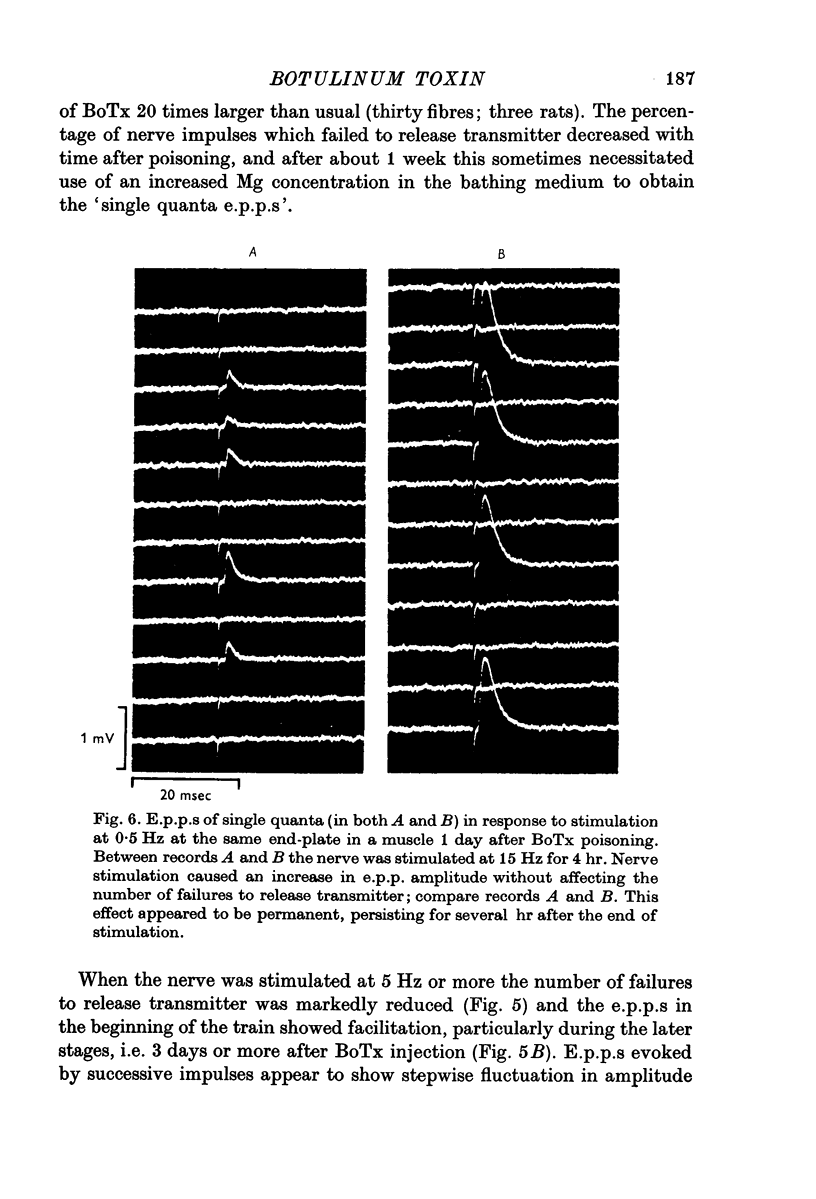
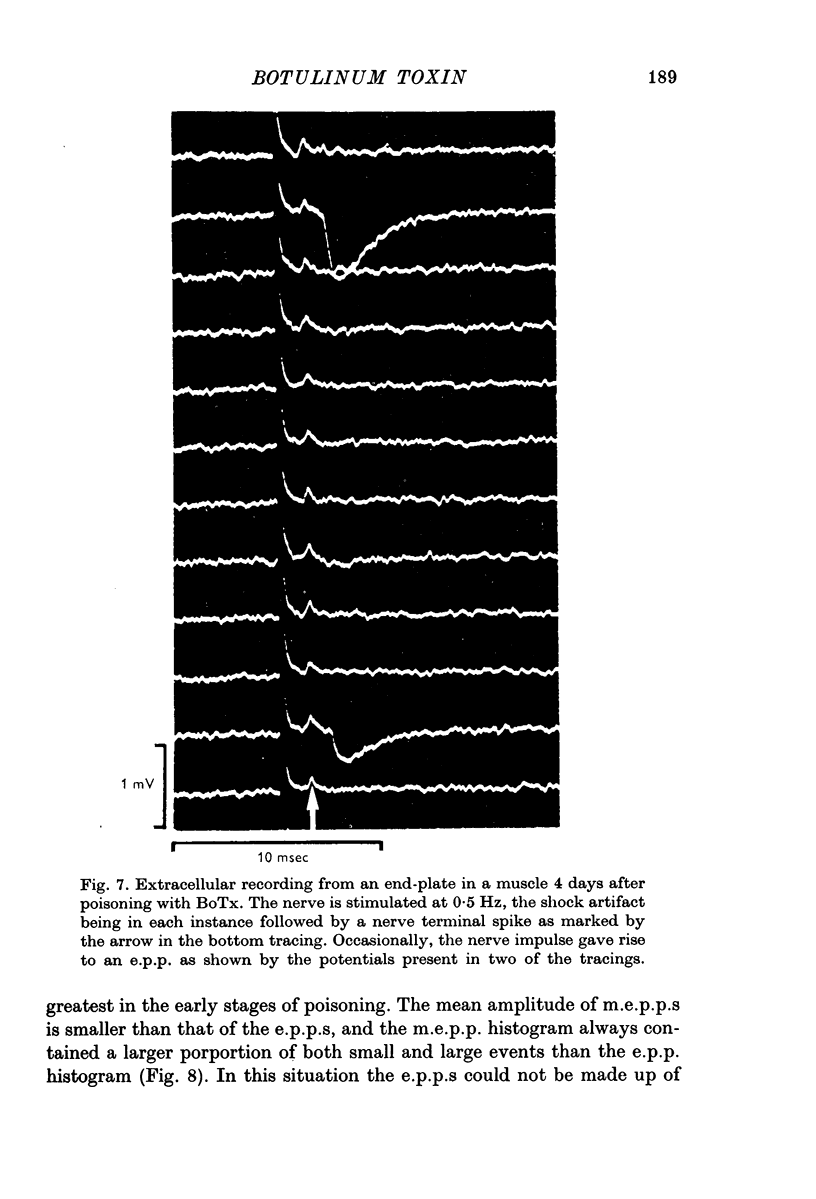
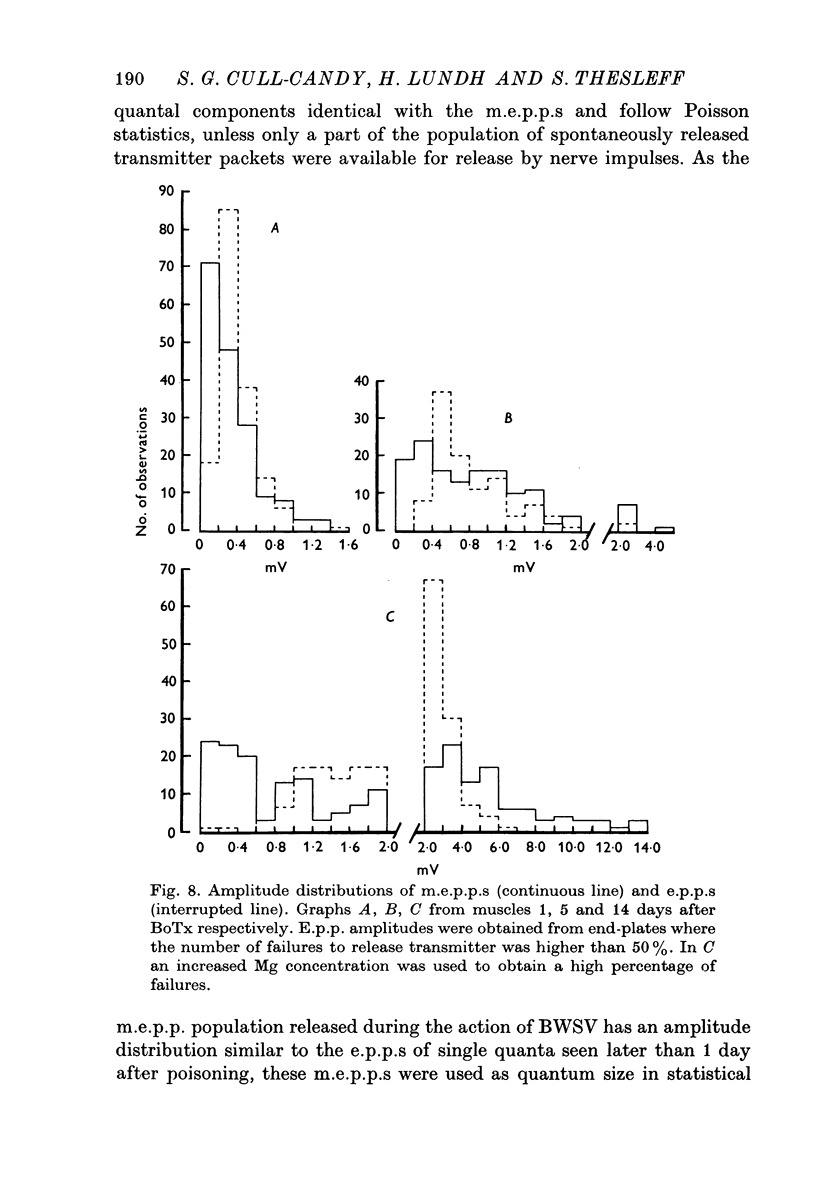
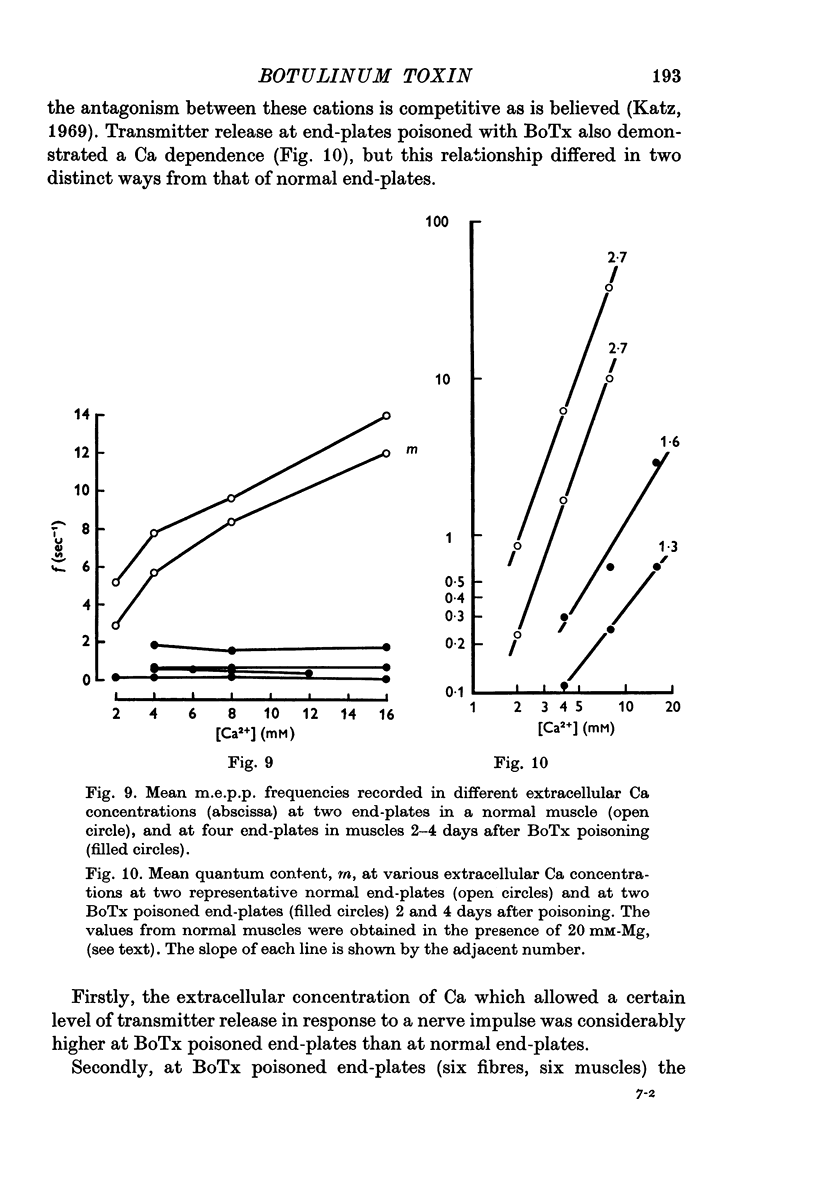
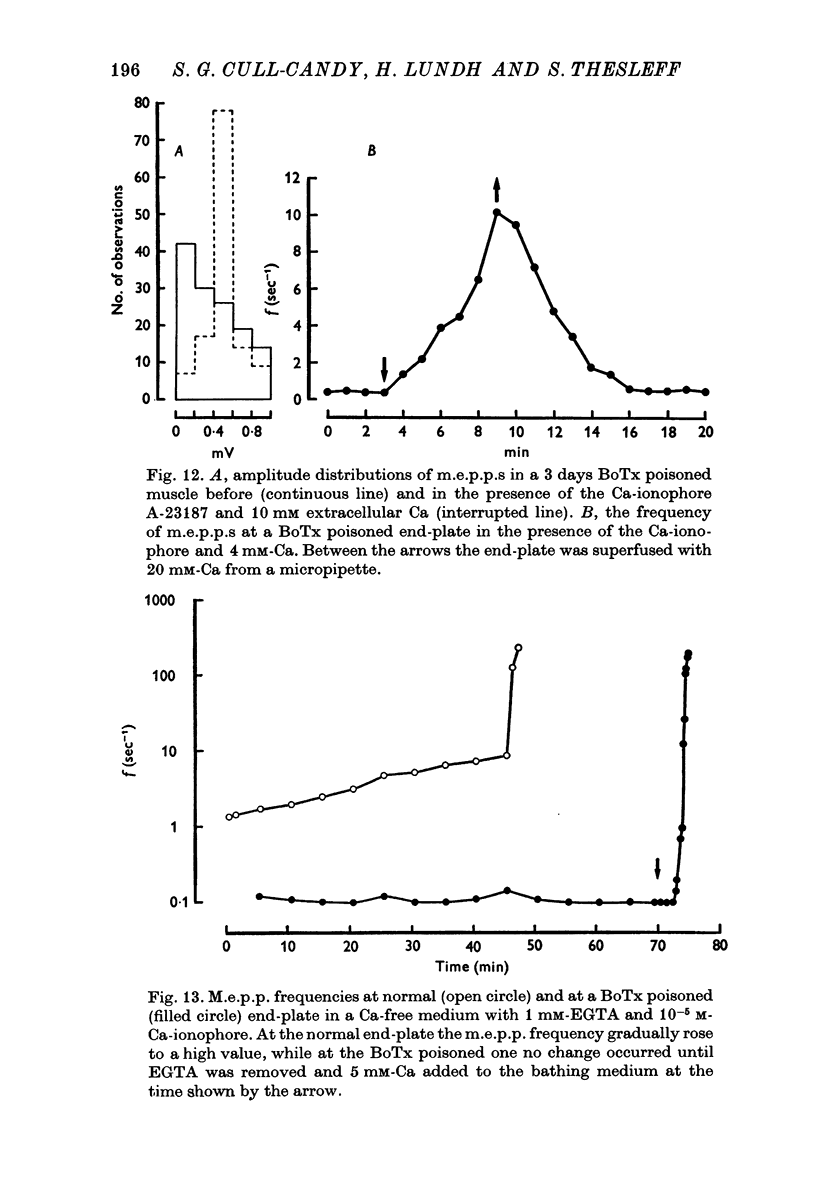
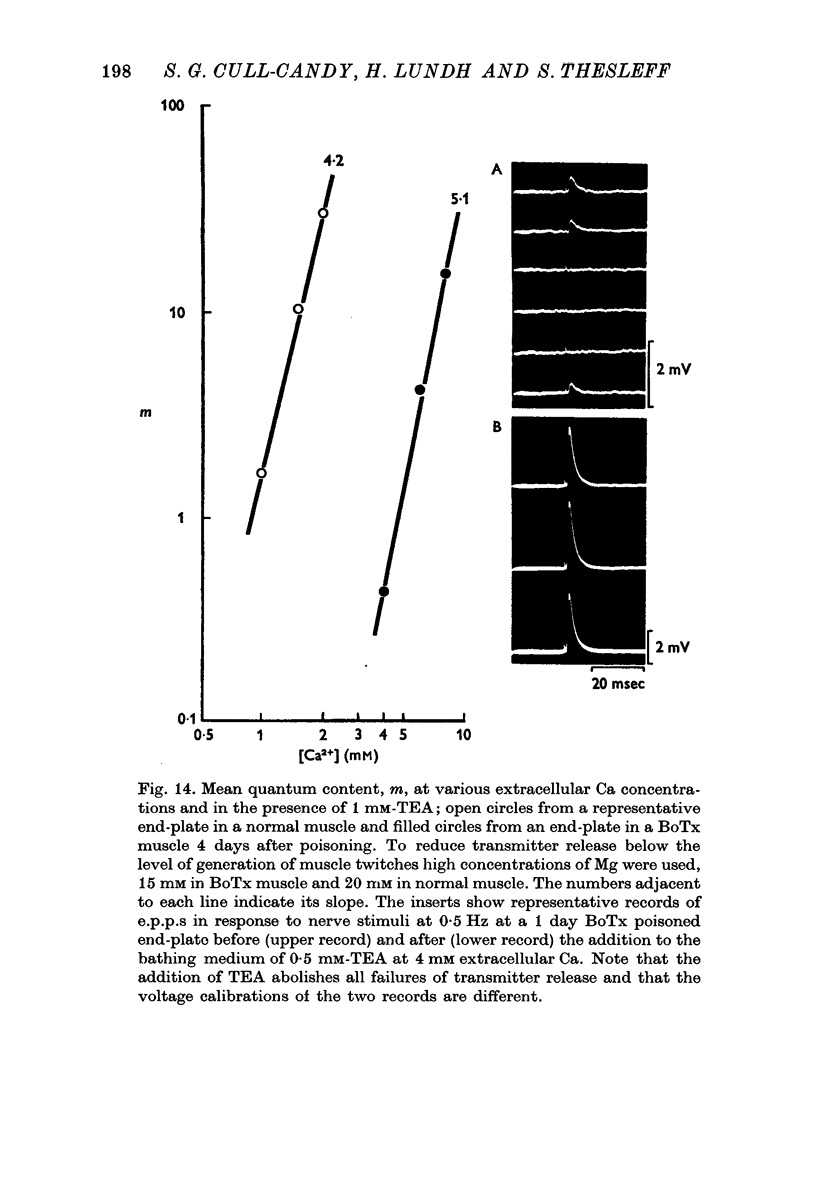
1. Botulinum toxin (BoTx) type A partially blocks spontaneous transmitter release from nerve terminals in the rat. Minature end-plate potentials (m.e.p.p.s) are present at all end-plates, initially with a low frequency but increasing with time after posoning. Their amplitude distribution is at first skew with a predominace of very small m.e.p.p.s but, after a few days, larger than normal m.e.p.p.s appear. 2. Tetanic nerve stimulation, Black Widow Spider Venom, the Caionophore A 23187 or mechanical damage to nerve terminals increases the frequency of m.e.p.p.s and alters the amplitude distribution of m.e.p.p.s towards a normal Gaussian one; the m.e.p.p. size approaches that seen at normal end-plates. This was seen at any time after poisoning. 3. Nerve stimulation gives rise to end-plate potentials (e.p.p.s) of low amplitude and high failure rate. Statistical analysis indicates that evoked release is quantal in nature and follows Poisson statistics, quantum size being initially very small, but after a few days approaching normal size. Short-term tetanic nerve stimulation reversibly increases the quantum content of e.p.p.s and during early stages of paralysis long-term (2 hr) stimulation causes an apparently permanent increase in quantum size. 4. Raising the extracellular Ca concentration from 2 to 16 mM increases the frequency of m.e.p.p.s in normal muscle but not in BoTx poisoned ones. K-free medium or ouabain, which are believed to raise the intracellular Ca concentration in nerve terminals, similarly increases m.e.p.p. frequency in normal but not in poisoned muscles. When the Ca-ionophore A 23187 is used together with high extracellular Ca (greater than 4 mM) massive release of transmitter occurs from poisoned terminals. 5. The extracellular Ca concentration which causes a certain level of transmitter release in reponse to nerve impulses is considerably higher at BoTx poisoned end-plates than at normal ones. The slope value for Ca dependence of transmitter release is about 1-5 compared with about 3 at normal end-plates. 6. Tetraethylammonium (TEA) greatly increases the amount of transmitter released by nerve impulses and restores neuromuscular transmission during all stages of poisoning, although it has not effect on spontaneous transmitter release. In the presence of TEA the power relation between Ca concentration and quantum content at the BoTx poisoned end-plate is similar to that seen at normal end-plates. 7. It is suggested that in BoTx poisoning the mechanism for transmitter release has a reduced sensitivity to Ca, and the level for activation by intracellular Ca is elevated. Once the intracellular concentration of Ca is raised to this level, by tetanic nerve stimulation, mechanical injury to nerve terminals, the Ca-ionophore or the prolongation of the nerve action potential with TEA, augmented transmitter release occurs, similar to that which occurs in normal nerve terminals at a lower level of Ca.
Full text
PDF













Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambache N. The peripheral action of Cl. botulinum toxin. J Physiol. 1949 Mar 15;108(2):127–141. [PMC free article] [PubMed] [Google Scholar]
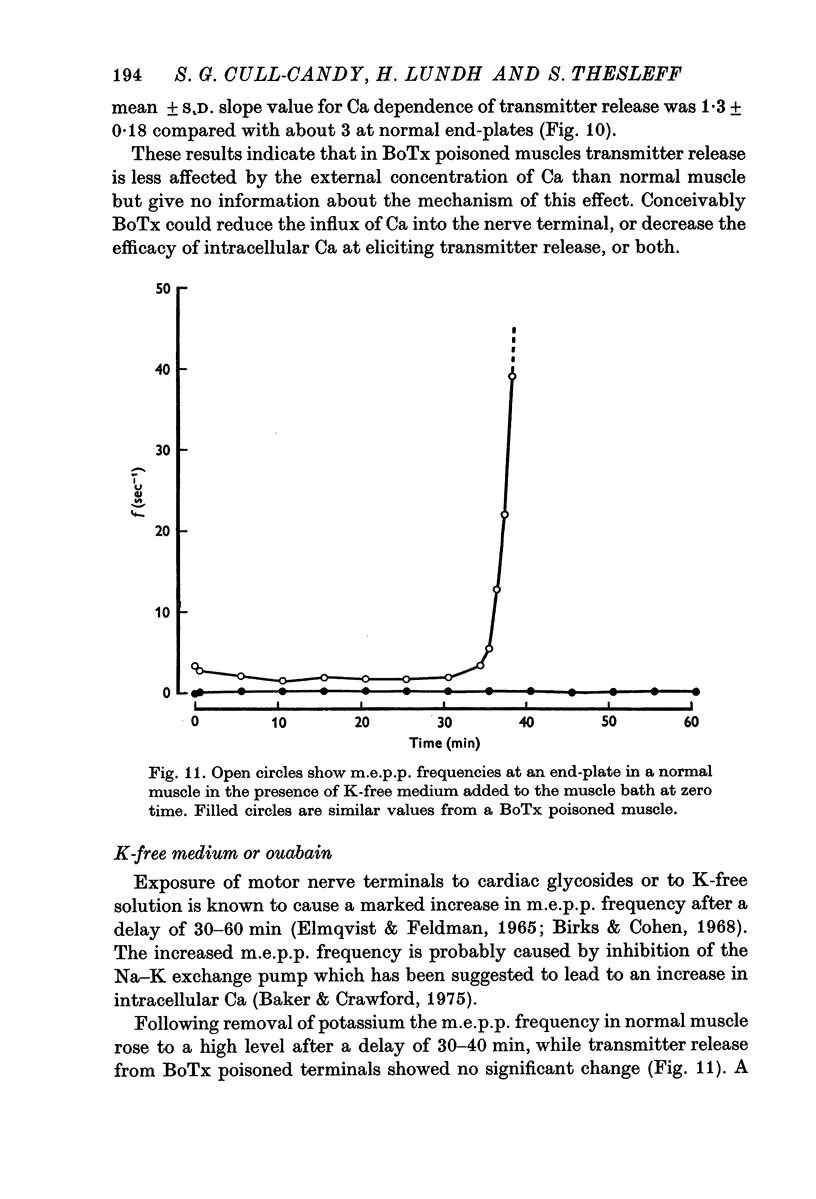
- Baker P. F., Crawford A. C. A note of the mechanism by which inhibitors of the sodium pump accelerate spontaneous release of transmitter from motor nerve terminals. J Physiol. 1975 May;247(1):209–226. doi: 10.1113/jphysiol.1975.sp010928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., McLachlan E. M., Taylor R. S. The formation of synapses in reinnervated mammalian striated muscle. J Physiol. 1973 Sep;233(3):481–500. doi: 10.1113/jphysiol.1973.sp010319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan S., Miledi R., Grampp W. Induced transmitter release from Schwann cells and its suppression by actinomycin D. Nat New Biol. 1973 Jan 17;241(107):85–86. doi: 10.1038/newbio241085a0. [DOI] [PubMed] [Google Scholar]
- Birks R. I., Cohen M. W. The action of sodium pump inhibitors on neuromuscular transmission. Proc R Soc Lond B Biol Sci. 1968 Jul 9;170(1021):381–399. doi: 10.1098/rspb.1968.0046. [DOI] [PubMed] [Google Scholar]
- Boroff D. A., del Castillo J., Evoy W. H., Steinhardt R. A. Observations on the action of type A botulinum toxin on frog neuromuscular junctions. J Physiol. 1974 Jul;240(2):227–253. doi: 10.1113/jphysiol.1974.sp010608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke J. D., Okamoto K., Quastel D. M. The role of calcium in depolarization-secretion coupling at the motor nerve terminal. J Physiol. 1973 Jan;228(2):459–497. doi: 10.1113/jphysiol.1973.sp010095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke J. D., Quastel D. M. Transmitter release by mammalian motor nerve terminals in response to focal polarization. J Physiol. 1973 Jan;228(2):377–405. doi: 10.1113/jphysiol.1973.sp010092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cull-Candy S. G., Neal H., Usherwood P. N. Action of black widow spider venom on an aminergic synapse. Nature. 1973 Feb 2;241(5388):353–354. doi: 10.1038/241353a0. [DOI] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Quantal components of the end-plate potential. J Physiol. 1954 Jun 28;124(3):560–573. doi: 10.1113/jphysiol.1954.sp005129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis M. J., Miledi R. Characteristics of transmitter release at regenerating frog neuromuscular junctions. J Physiol. 1974 Jun;239(3):571–594. doi: 10.1113/jphysiol.1974.sp010583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis M. J., Miledi R. Non-transmitting neuromuscular junctions during an early stage of end-plate reinnervation. J Physiol. 1974 Jun;239(3):553–570. doi: 10.1113/jphysiol.1974.sp010582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge F. A., Jr, Rahamimoff R. Co-operative action a calcium ions in transmitter release at the neuromuscular junction. J Physiol. 1967 Nov;193(2):419–432. doi: 10.1113/jphysiol.1967.sp008367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchen L. W. An electron microscopic study of the changes induced by botulinum toxin in the motor end-plates of slow and fast skeletal muscle fibres of the mouse. J Neurol Sci. 1971 Sep;14(1):47–60. doi: 10.1016/0022-510x(71)90129-8. [DOI] [PubMed] [Google Scholar]
- Duchen L. W. Changes in motor innervation and cholinesterase localization induced by botulinum toxin in skeletal muscle of the mouse: differences between fast and slow muscles. J Neurol Neurosurg Psychiatry. 1970 Feb;33(1):40–54. doi: 10.1136/jnnp.33.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis K. O., Bryant S. H. Excitation-contraction uncoupling in skeletal muscle by dantrolene sodium. Naunyn Schmiedebergs Arch Pharmacol. 1972;274(1):107–109. doi: 10.1007/BF00501011. [DOI] [PubMed] [Google Scholar]
- Elmqvist D., Feldman D. S. Effects of sodium pump inhibitors on spontaneous acetylcholine release at the neuromuscular junction. J Physiol. 1965 Dec;181(3):498–505. doi: 10.1113/jphysiol.1965.sp007778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FATT P., KATZ B. Spontaneous subthreshold activity at motor nerve endings. J Physiol. 1952 May;117(1):109–128. [PMC free article] [PubMed] [Google Scholar]
- Gage P. W. The effect of methyl, ethyl and n-propyl alcohol on neuromuscular transmission in the rat. J Pharmacol Exp Ther. 1965 Nov;150(2):236–243. [PubMed] [Google Scholar]
- Harris A. J., Miledi R. The effect of type D botulinum toxin on frog neuromuscular junctions. J Physiol. 1971 Sep;217(2):497–515. doi: 10.1113/jphysiol.1971.sp009582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard J. I., Jones S. F., Landau E. M. On the mechanism by which calcium and magnesium affect the spontaneous release of transmitter from mammalian motor nerve terminals. J Physiol. 1968 Feb;194(2):355–380. doi: 10.1113/jphysiol.1968.sp008413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOSEFSSON J. O., THESLEFF S. Electromyographic findings in experimental botulinum intoxication. Acta Physiol Scand. 1961 Feb-Mar;51:163–168. doi: 10.1111/j.1748-1716.1961.tb02124.x. [DOI] [PubMed] [Google Scholar]
- KATZ B., THESLEFF S. On the factors which determine the amplitude of the miniature end-plate potential. J Physiol. 1957 Jul 11;137(2):267–278. doi: 10.1113/jphysiol.1957.sp005811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao C. Y. Tetrodotoxin, saxitoxin and their significance in the study of excitation phenomena. Pharmacol Rev. 1966 Jun;18(2):997–1049. [PubMed] [Google Scholar]
- Katz B., Miledi R. A study of synaptic transmission in the absence of nerve impulses. J Physiol. 1967 Sep;192(2):407–436. doi: 10.1113/jphysiol.1967.sp008307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. Further study of the role of calcium in synaptic transmission. J Physiol. 1970 May;207(3):789–801. doi: 10.1113/jphysiol.1970.sp009095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. The role of calcium in neuromuscular facilitation. J Physiol. 1968 Mar;195(2):481–492. doi: 10.1113/jphysiol.1968.sp008469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriebel M. E., Gross C. E. Multimodal distribution of frog miniature endplate potentials in adult denervated and tadpole leg muscle. J Gen Physiol. 1974 Jul;64(1):85–103. doi: 10.1085/jgp.64.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusano K., Livengood D. R., Werman R. Correlation of transmitter release with membrane properties of the presynaptic fiber of the squid giant synapse. J Gen Physiol. 1967 Dec;50(11):2579–2601. doi: 10.1085/jgp.50.11.2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longenecker H. E., Jr, Hurlbut W. P., Mauro A., Clark A. W. Effects of black widow spider venom on the frog neuromuscular junction. Effects on end-plate potential, miniature end-plate potential and nerve terminal spike. Nature. 1970 Feb 21;225(5234):701–703. doi: 10.1038/225701a0. [DOI] [PubMed] [Google Scholar]
- Miledi R., Spitzer N. C. Absence of action potentials in frog slow muscle fibres paralysed by botulinum toxin. J Physiol. 1974 Aug;241(1):183–199. doi: 10.1113/jphysiol.1974.sp010648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R., Thies R. Tetanic and post-tetanic rise in frequency of miniature end-plate potentials in low-calcium solutions. J Physiol. 1971 Jan;212(1):245–257. doi: 10.1113/jphysiol.1971.sp009320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahamimoff R. A dual effect of calcium ions on neuromuscular facilitation. J Physiol. 1968 Mar;195(2):471–480. doi: 10.1113/jphysiol.1968.sp008468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell J. T., Hansen E. L., Thorn N. A. Calcium and stimulus-secretion coupling in the neurohypophysis. III. Ca2+ ionophore (A-23187)-induced release of vasopressin from isolated rat neurophypophyses. Acta Endocrinol (Copenh) 1974 Nov;77(3):443–450. [PubMed] [Google Scholar]
- Spitzer N. Miniature end-plate potentials at mammalian neuromuscular junctions poisoned by botulinum toxin. Nat New Biol. 1972 May 3;237(70):26–27. doi: 10.1038/newbio237026a0. [DOI] [PubMed] [Google Scholar]
- THESLEFF S. Supersensitivity of skeletal muscle produced by botulinum toxin. J Physiol. 1960 Jun;151:598–607. doi: 10.1113/jphysiol.1960.sp006463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thesleff S. Acetylcholine utulization in myasthenia gravis. Ann N Y Acad Sci. 1966 Jan 26;135(1):195–208. doi: 10.1111/j.1749-6632.1966.tb45473.x. [DOI] [PubMed] [Google Scholar]
- Tonge D. A. Chronic effects of botulinum toxin on neuromuscular transmission and sensitivity to acetylcholine in slow and fast skeletal muscle of the mouse. J Physiol. 1974 Aug;241(1):127–139. doi: 10.1113/jphysiol.1974.sp010644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong D. T., Wilkinson J. R., Hamill R. L., Horng J. S. Effects of antibiotic ionophore, A23187, on oxidative phosphorylation and calcium transport of liver mitochondria. Arch Biochem Biophys. 1973 Jun;156(2):578–585. doi: 10.1016/0003-9861(73)90308-1. [DOI] [PubMed] [Google Scholar]


