Abstract
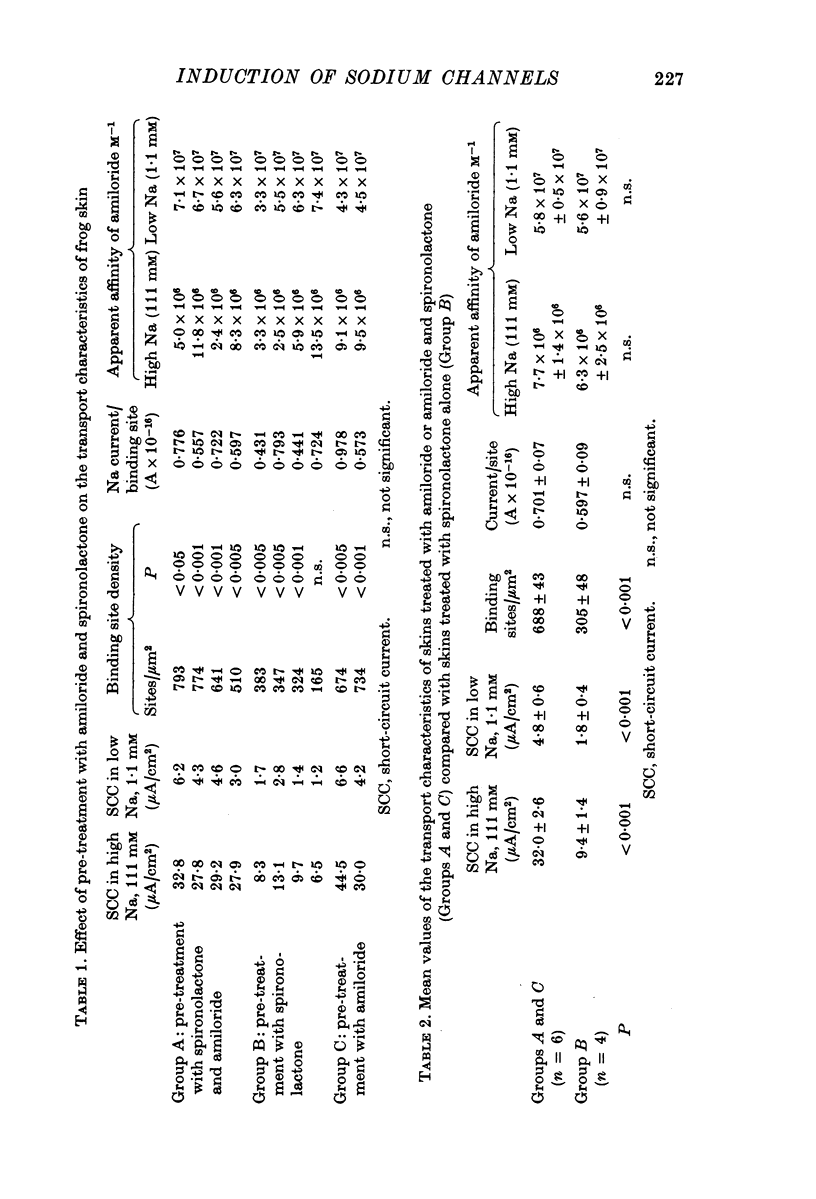
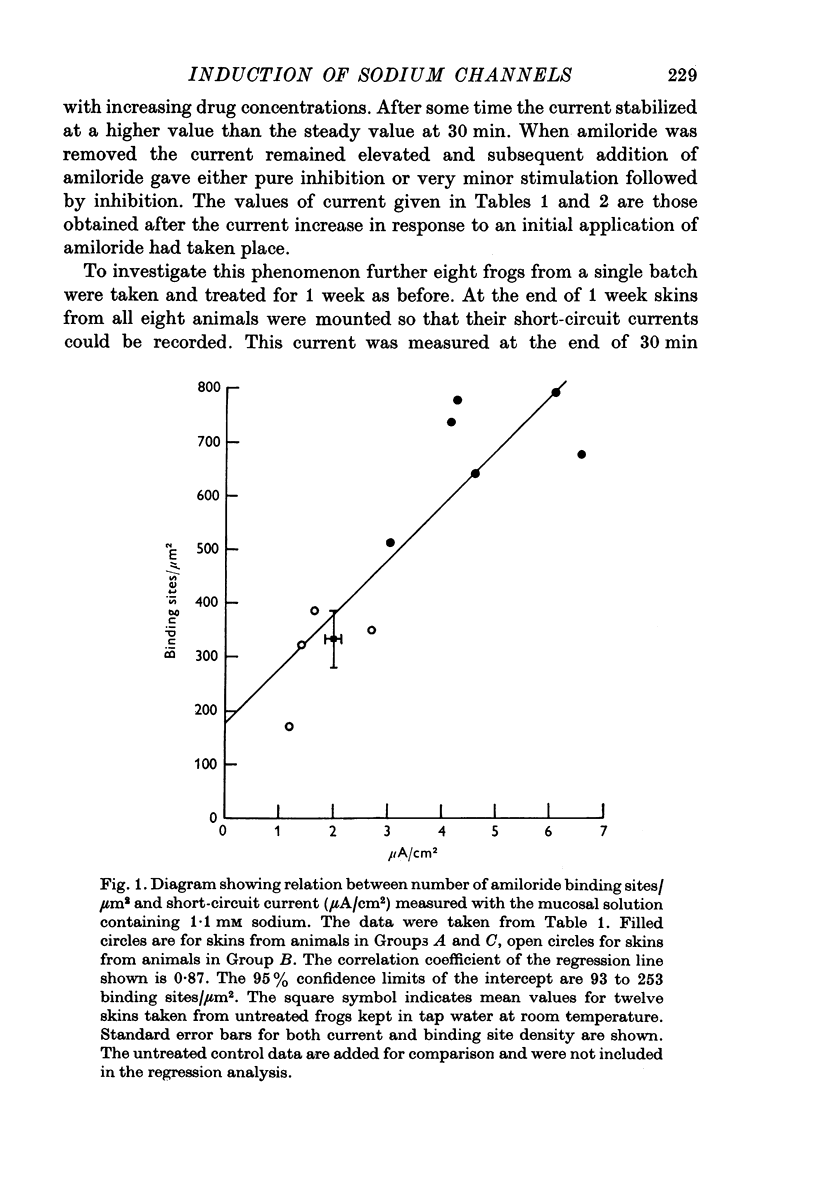
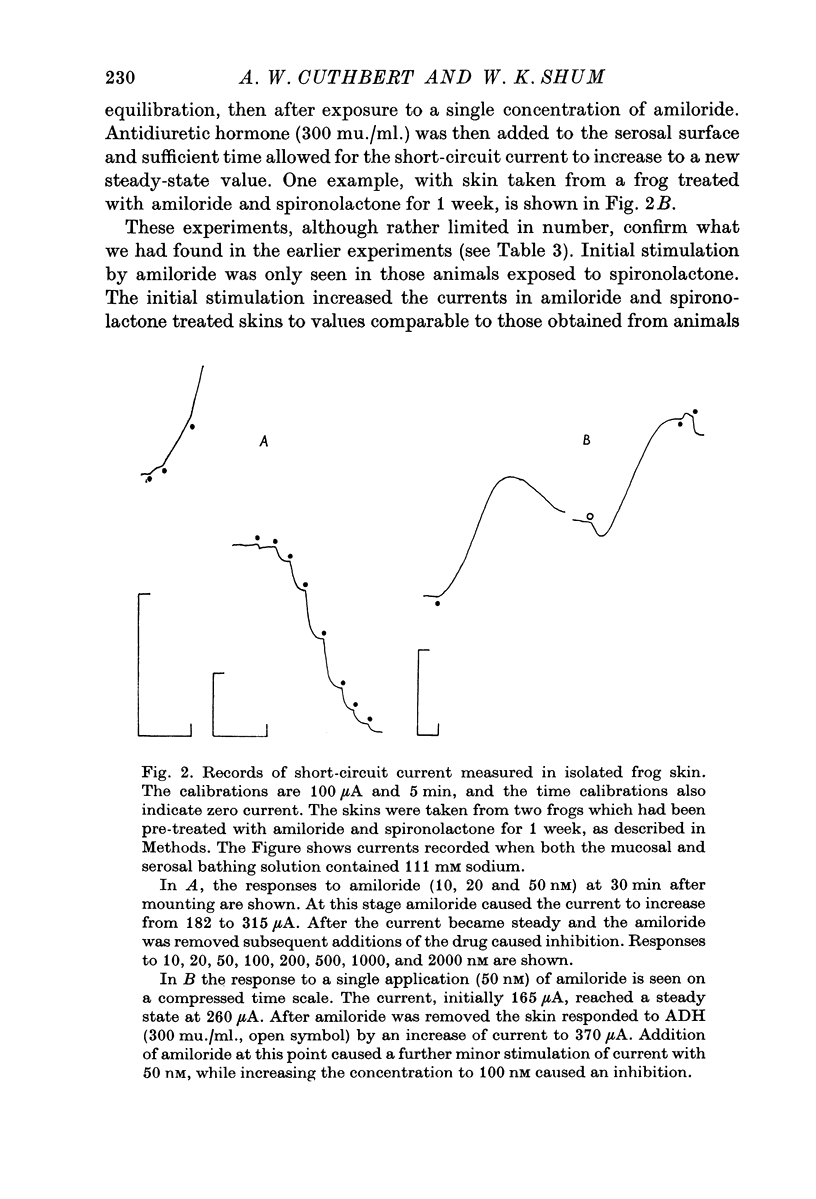
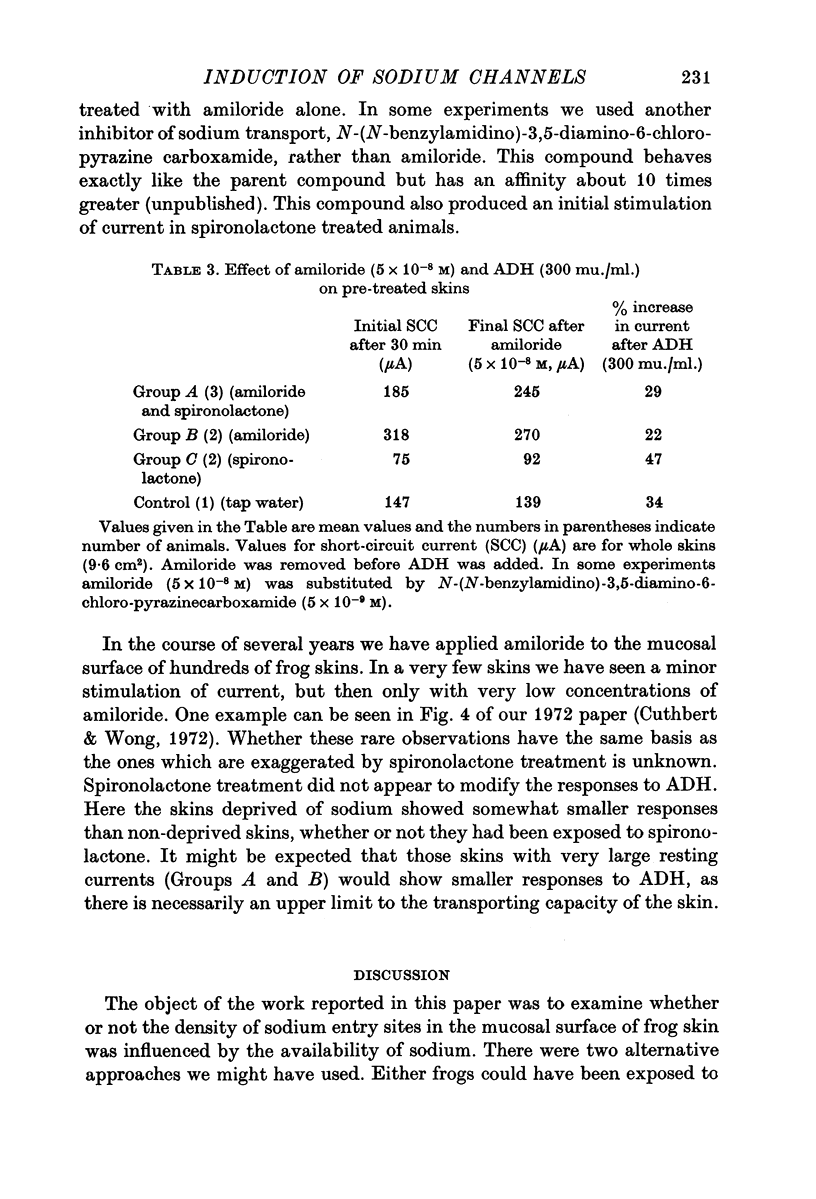
1. Frogs (Rana temporaria) were bathed for 1 week in solutions containing 1-1 mM sodium chloride and either one or both of amiloride (10(-4)M) and spironolactone (10(-5r both of amiloride (10(-4) M) and spironolactone (10(-5) M). This procedure was designed to deplete the sodium transporting compartment of the skin epithelium of sodium, while at the same time antagonizing the effects of endogenous aldosterone. 2. After 1 week the skins were used in vitro to measure the level of sodium transport (short-circuit current) and the density of sodium entry sites in the mucosal surface of the epithelium ([14C]amiloride binding). 3. Sodium deprivation for 1 week caused approximately a doubling of both sodium transport and the density of sodium entry sites in the mucosal surface of the epithelium compared to control skins. 4. When the results for sodium deprived and control skins were pooled there was a highly significant correlation between the density of sodium entry sites and sodium transport. 5. Mechanisms by which sodium deprivation leads to an increase in the density of sodium entry sites are discussed.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AXELSSON J., THESLEFF S. A study of supersensitivity in denervated mammalian skeletal muscle. J Physiol. 1959 Jun 23;147(1):178–193. doi: 10.1113/jphysiol.1959.sp006233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley P. J. Amiloride: a potent inhibitor of sodium transport across the toad bladder. J Physiol. 1968 Mar;195(2):317–330. doi: 10.1113/jphysiol.1968.sp008460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley P. J. Role of the skin in amphibian sodium metabolism. Science. 1973 Aug 17;181(4100):686–687. doi: 10.1126/science.181.4100.686. [DOI] [PubMed] [Google Scholar]
- Biber T. U., Curran P. F. Direct measurement of uptake of sodium at the outer surface of the frog skin. J Gen Physiol. 1970 Jul;56(1):83–99. doi: 10.1085/jgp.56.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biber T. U. Effect of changes in transepithelial transport on the uptake of sodium across the outer surface of the frog skin. J Gen Physiol. 1971 Aug;58(2):131–144. doi: 10.1085/jgp.58.2.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boardman L., Huett M., Lamb J. F., Newton J. P., Polson J. M. Evidence for the genetic control of the sodium pump density in HeLa cells. J Physiol. 1974 Sep;241(3):771–794. doi: 10.1113/jphysiol.1974.sp010684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert A. W. An upper limit to the number of sodium channels in frog skin epithelium. J Physiol. 1973 Feb;228(3):681–692. doi: 10.1113/jphysiol.1973.sp010106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert A. W. Neurohypophyseal hormones and sodium transport. Philos Trans R Soc Lond B Biol Sci. 1971 Aug 20;262(842):103–109. doi: 10.1098/rstb.1971.0081. [DOI] [PubMed] [Google Scholar]
- Cuthbert A. W., Okpako D., Shwm W. K. Proceedings: Aldosterone, moulting and the number of sodium channels in frog skin. Br J Pharmacol. 1974 May;51(1):128P–129P. [PMC free article] [PubMed] [Google Scholar]
- Cuthbert A. W., Shum W. K. Amiloride and the sodium channel. Naunyn Schmiedebergs Arch Pharmacol. 1974;281(3):261–269. doi: 10.1007/BF00500595. [DOI] [PubMed] [Google Scholar]
- Cuthbert A. W., Shum W. K. Characteristics of the entry process for sodium in transporting epithelia as revealed with amiloride. J Physiol. 1976 Mar;255(3):587–604. doi: 10.1113/jphysiol.1976.sp011297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert A. W., Shum W. K. Effects of vasopressin and aldosterone on amiloride binding in toad bladder epithelial cells. Proc R Soc Lond B Biol Sci. 1975 Jun 17;189(1097):543–575. doi: 10.1098/rspb.1975.0072. [DOI] [PubMed] [Google Scholar]
- Cuthbert A. W., Shum W. K. Estimation of the lifespan of amiloride binding sites in the membranes of toad bladder epithelial cells. J Physiol. 1976 Mar;255(3):605–618. doi: 10.1113/jphysiol.1976.sp011298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert A. W., Wong P. Y. The role of calcium ions in the interaction of amiloride with membrane receptors. Mol Pharmacol. 1972 Mar;8(2):222–229. [PubMed] [Google Scholar]
- DENTON D. A. EVOLUTIONARY ASPECTS OF THE EMERGENCE OF ALDOSTERONE SECRETION AND SALT APPETITE. Physiol Rev. 1965 Apr;45:245–295. doi: 10.1152/physrev.1965.45.2.245. [DOI] [PubMed] [Google Scholar]
- Dörge A., Nagel W. Effect of amiloride on sodium transport in frog skin. II. Sodium transport pool and unidirectional fluxes. Pflugers Arch. 1970;321(2):91–101. doi: 10.1007/BF00586365. [DOI] [PubMed] [Google Scholar]
- EMMELIN N. Supersensitivity following "pharmacological denervation". Pharmacol Rev. 1961 Mar;13:17–37. [PubMed] [Google Scholar]
- Ehrlich E. N., Crabbé J. The mechanism of action of amipramizide. Pflugers Arch. 1968;302(1):79–96. doi: 10.1007/BF00586783. [DOI] [PubMed] [Google Scholar]
- Funder J. W., Feldman D., Highland E., Edelman I. S. Molecular modifications of anti-aldosterone compounds: effects on affinity of spirolactones for renal aldosterone receptors. Biochem Pharmacol. 1974 May 15;23(10):1493–1501. doi: 10.1016/0006-2952(74)90386-4. [DOI] [PubMed] [Google Scholar]
- Katz U. Salt-induced changes in sodium transport across the skin of the euryhaline toad, Bufo viridis. J Physiol. 1975 Jun;247(3):537–550. doi: 10.1113/jphysiol.1975.sp010946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kebabian J. W., Zatz M., Romero J. A., Axelrod J. Rapid changes in rat pineal beta-adrenergic receptor: alterations in l-(3H)alprenolol binding and adenylate cyclase. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3735–3739. doi: 10.1073/pnas.72.9.3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R., Potter L. T. Acetylcholine receptors in muscle fibres. Nature. 1971 Oct 29;233(5322):599–603. doi: 10.1038/233599a0. [DOI] [PubMed] [Google Scholar]
- Porter G. A. In vitro inhibition of aldosterone-stimulated sodium transport by steroidal spirolactones. Mol Pharmacol. 1968 May;4(3):224–237. [PubMed] [Google Scholar]
- Salako L. A., Smith A. J. Effects of amiloride on active sodium transport by the isolated frog skin: evidence concerning site of action. Br J Pharmacol. 1970 Apr;38(4):702–718. doi: 10.1111/j.1476-5381.1970.tb09878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp G. W., Leaf A. Mechanism of action of aldosterone. Physiol Rev. 1966 Oct;46(4):593–633. doi: 10.1152/physrev.1966.46.4.593. [DOI] [PubMed] [Google Scholar]
- Soli A. H., Kahn C. R., Neville D. M., Jr, Roth J. Insulin receptor deficiency in genetic and acquired obesity. J Clin Invest. 1975 Oct;56(4):769–780. doi: 10.1172/JCI108155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaneck G. E., Chu L. L., Edelman I. S. Stereospecific binding of aldosterone to renal chromatin. J Biol Chem. 1970 Oct 25;245(20):5382–5389. [PubMed] [Google Scholar]
- TRENDELENBURG U. Supersensitivity and subsensitivity to sympathomimetic amines. Pharmacol Rev. 1963 Jun;15:225–276. [PubMed] [Google Scholar]


