Abstract
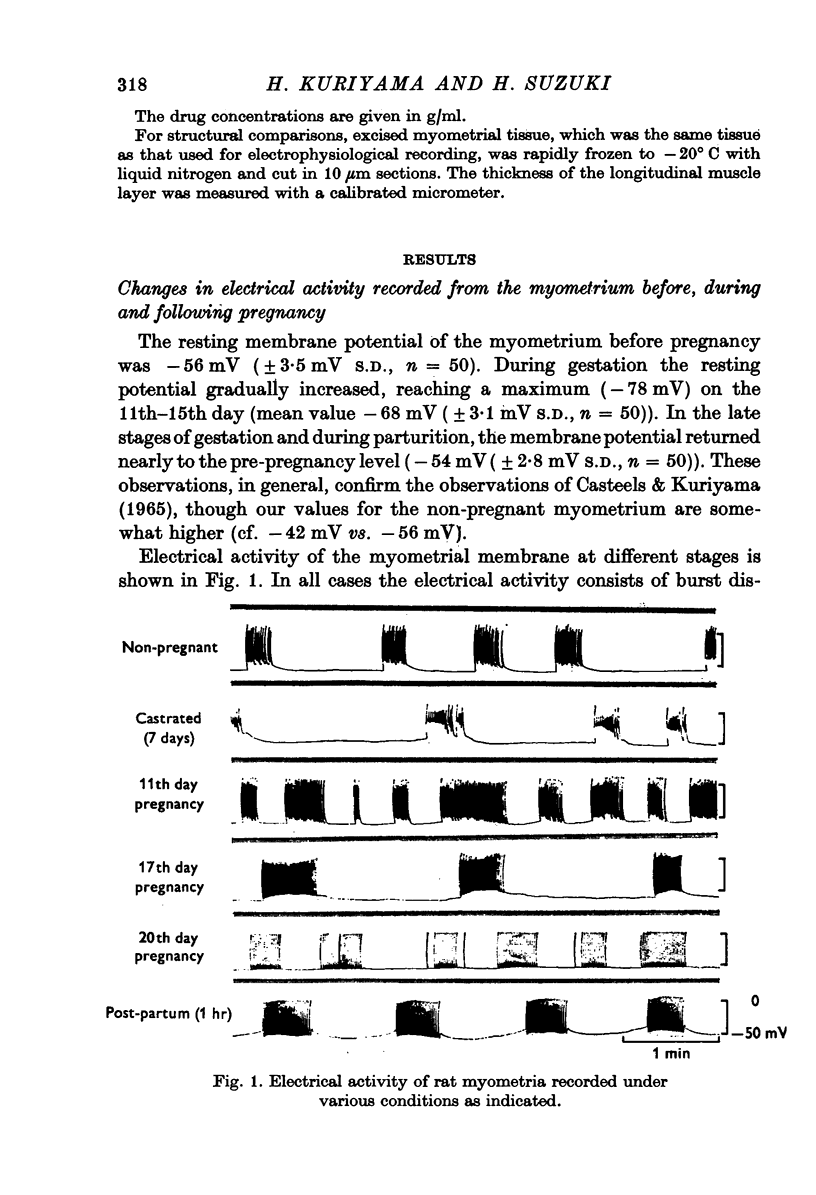
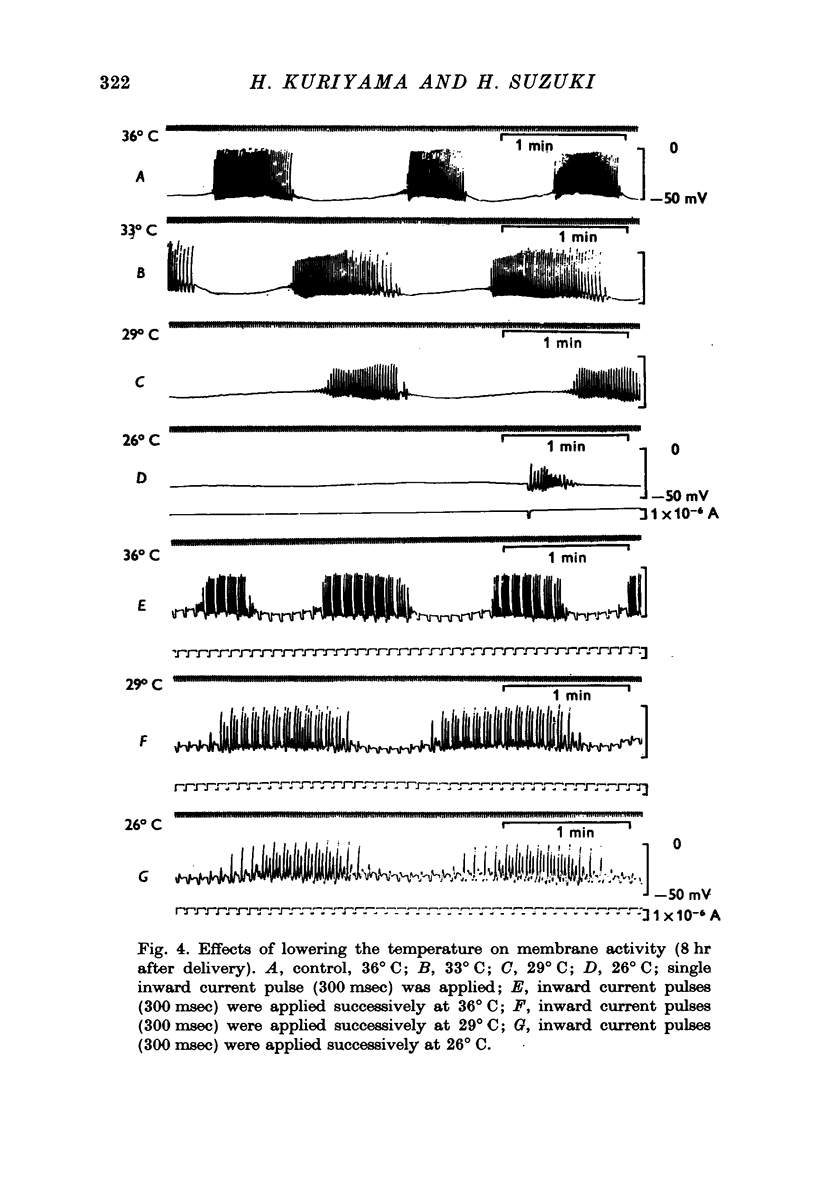
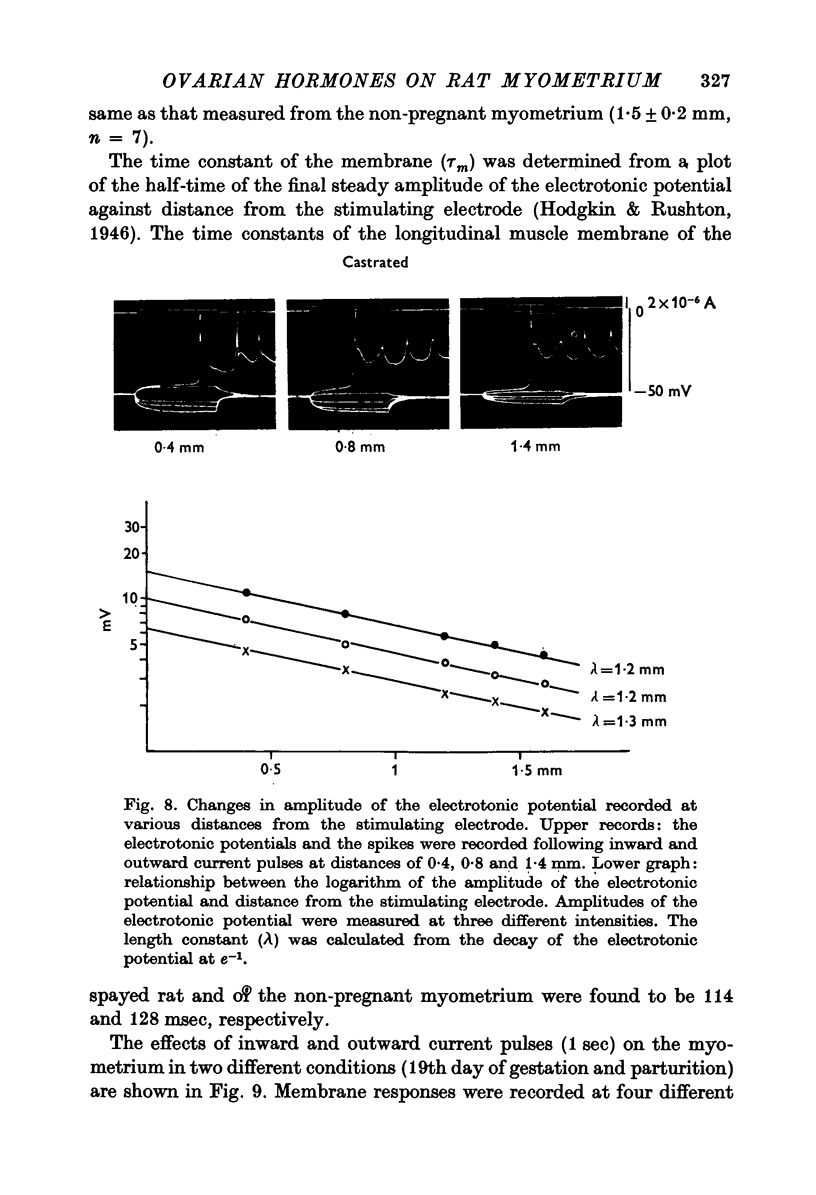
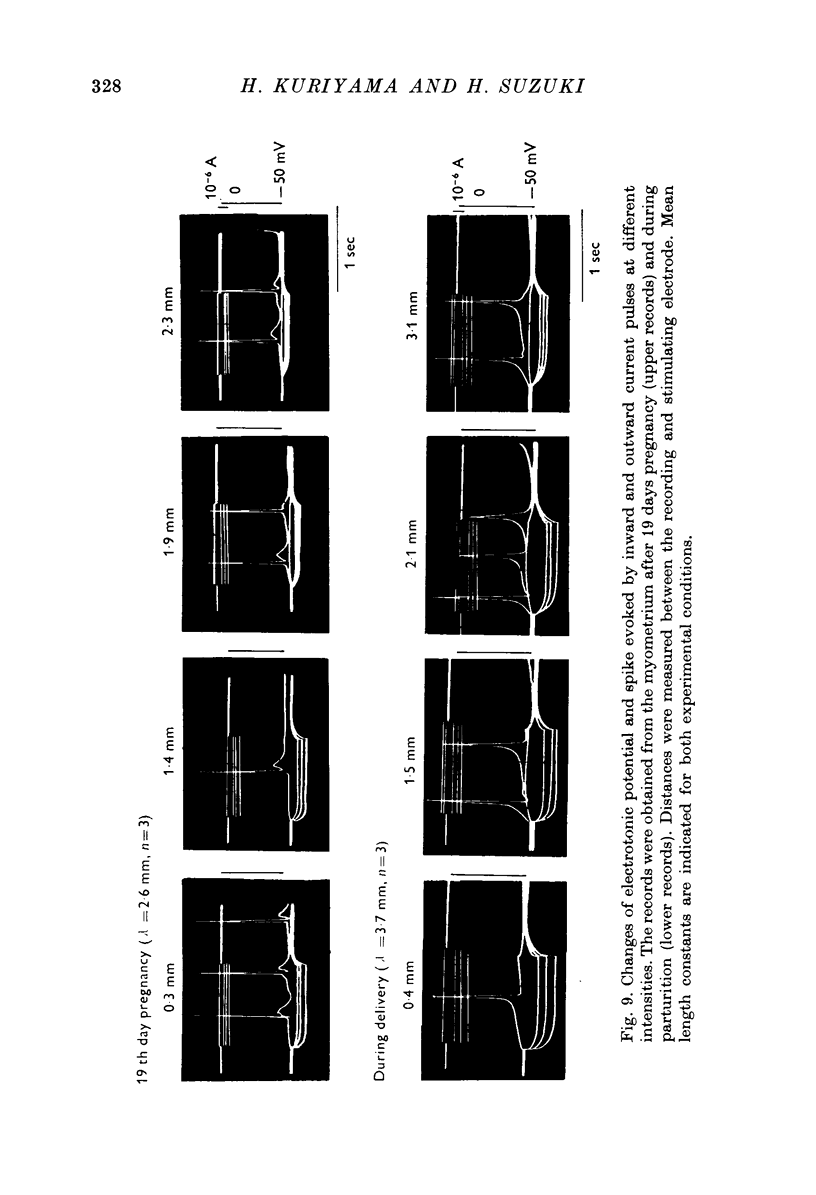
1. The membrane properties of the rat myometrium, during gestation and following ovarian hormone treatment, have been investigated with the micro-electrode technique. 2. Spontaneously generated bursts of electrical activity alternating with silent periods were recorded from non-pregnant, pregnant and post-partum myometria. The membrane potential was highest during the middle stage of gestation, but the spike amplitude within a burst was not uniform. In the final stage of gestation and during parturition, the membrane potential was low and the spikes within a burst were of low frequency and uniform amplitude. 3. During parturition and post-partum, a gradual depolarization of the membrane, accompanied by an increase in membrane resistance, occurred before the generation of a burst. 4. Excitability of the membrane fluctuated from a peak just before the generation of a burst to a low after the cessation of a burst. 5. Displacement of the membrane potential by electrical current or by lowering the temperature modified the slope spontaneous depolarization, but the fluctuations of excitability persisted. The Q10 value for the frequency of spontaneous bursts, measured between 36 and 30 degrees C, was 3-8. 6. Hyperpolarization of the membrane increased the maximum rate of rise of the spike, but beyond -70 mV, the rate of rise was reduced. Half-inactivation of spike generation of spike generation occurred at a membrane potential less negative than the interburst potential, indicating that the current carrying system was not fully activated during parturition. 7. In both normal and spayed rats, oestradiol hyperpolarized the membrane and the burst of spikes was generated hyperpolarized the membrane and the burst of spikes was generated on a sustained depolarization. Progesterone slightly hyperpolarized the membrane and burst discharges occurred without a sustained depolarization. Simultaneous treatment with progesterone and oestradiol produced a plateau potential of long duration during burst discharges. 8. The thickness of the muscle layer, length constant of the tissue and time constant of the membrane were measured during gestation and from spayed rats under various hormonal conditions. The length constant of the tissue was increased by oestradiol and was further increased by simultaneous treatment withoestradiol and progesterone. The increase in tissue thickness appeared to have the most marked influence on the length constant. 9. The resting and active membrane properties of the progresterone treated myometrium were similar to those observed during the middle stages of gestation. The oestradiol-treated myometrium did not resemble that during the last stages of gestation and parturition, which was simulated by combination of the two hormones, oestradiol preceding progesterone.
Full text
PDF













Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe Y. Effects of changing the ionic environment on passive and active membrane properties of pregnant rat uterus. J Physiol. 1971 Apr;214(1):173–190. doi: 10.1113/jphysiol.1971.sp009426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abe Y., Tomita T. Cable properties of smooth muscle. J Physiol. 1968 May;196(1):87–100. doi: 10.1113/jphysiol.1968.sp008496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bülbring E., Casteels R., Kuriyama H. Membrane potential and ion content in cat and guinea-pig myometrium and the response to adrenaline and noradrenaline. Br J Pharmacol. 1968 Oct;34(2):388–407. doi: 10.1111/j.1476-5381.1968.tb07060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bülbring E., Kuriyama H. The action of catecholamines on guinea-pig taenia coli. Philos Trans R Soc Lond B Biol Sci. 1973 Mar 15;265(867):115–121. doi: 10.1098/rstb.1973.0014. [DOI] [PubMed] [Google Scholar]
- CASTEELS R., KURIYAMA H. MEMBRANE POTENTIAL AND IONIC CONTENT IN PREGNANT AND NON-PREGNANT RAT MYOMETRIUM. J Physiol. 1965 Mar;177:263–287. doi: 10.1113/jphysiol.1965.sp007591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CSAPO A. Progesterone block. Am J Anat. 1956 Mar;98(2):273–291. doi: 10.1002/aja.1000980206. [DOI] [PubMed] [Google Scholar]
- MARSHALL J. M. Regulation of activity in uterine smooth muscle. Physiol Rev Suppl. 1962 Jul;5:213–227. [PubMed] [Google Scholar]
- Marshall J. M. Adrenergic innervation of the female reproductive tract: anatomy, physiology and pharmacology. Ergeb Physiol. 1970;62:6–67. doi: 10.1007/BFb0111421. [DOI] [PubMed] [Google Scholar]
- Tomita T., Watanabe H. Factors controlling myogenic activity in smooth muscle. Philos Trans R Soc Lond B Biol Sci. 1973 Mar 15;265(867):73–85. doi: 10.1098/rstb.1973.0010. [DOI] [PubMed] [Google Scholar]


