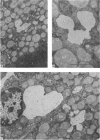
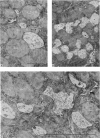
Abstract
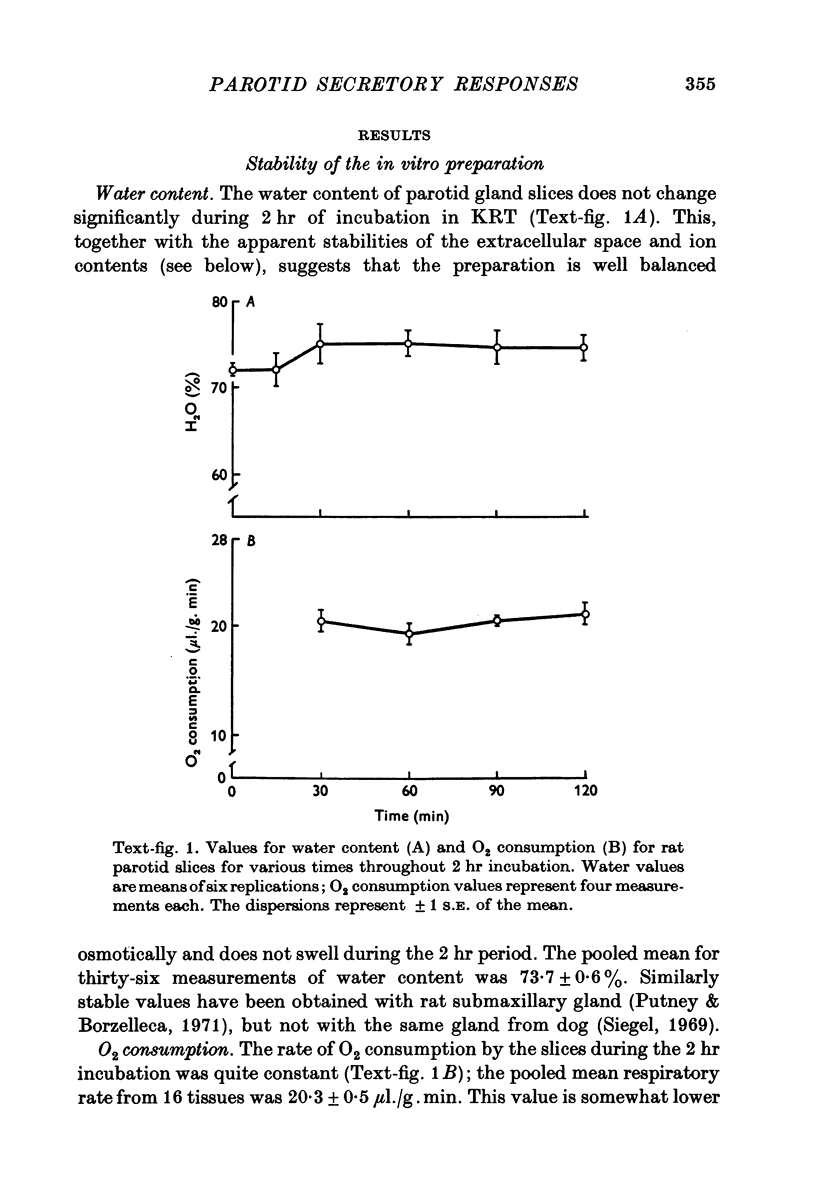
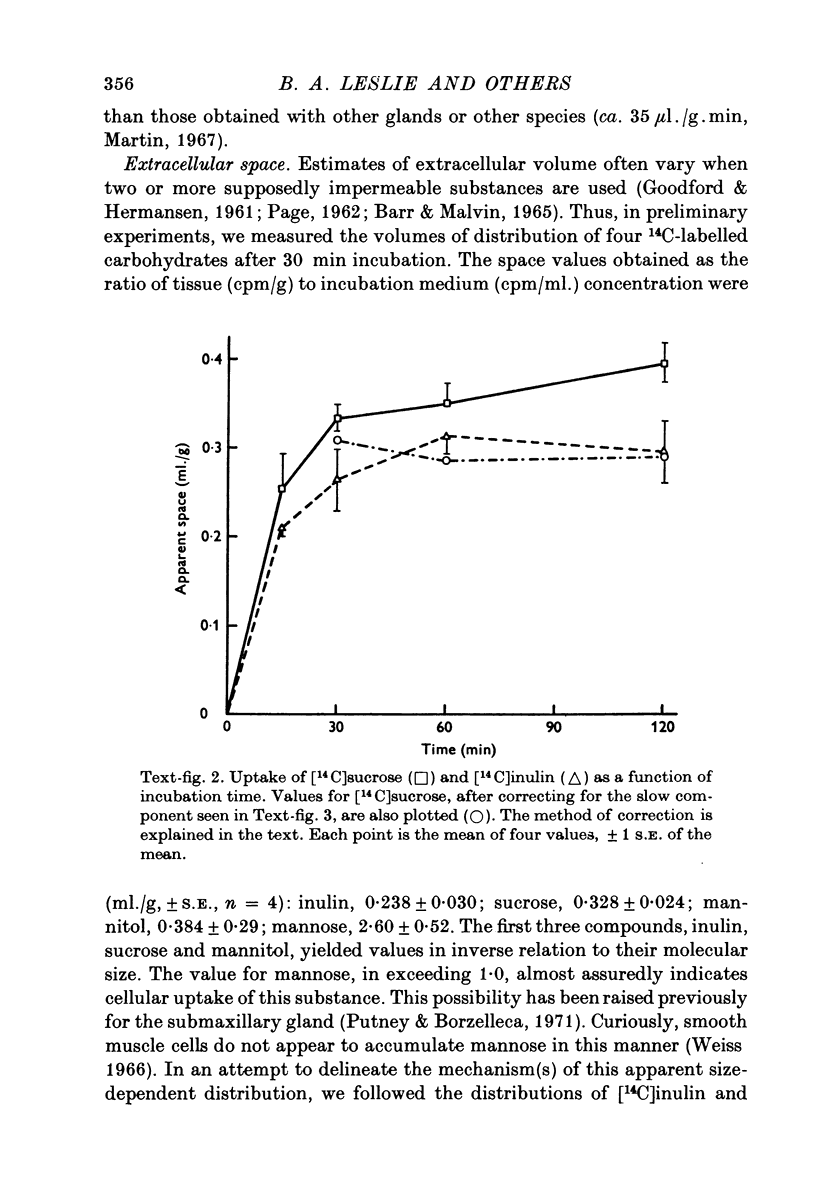
1. Rat parotid gland slices, incubated in a balanced, buffered salt solution, were found to be physiologically stable for up to 2 hr with respect to O2 consumption, water content, extracellular space and cation content. 2. The slices could be stimulated to secrete amylase by activation of alpha-adrenergic, beta-adrenergic or muscarinic cholinergic receptors. 3. The secretion elicited through all three receptors appeared to involve exocytosis as revealed by electron microscopy. 4. The beta-agonist, isoprenaline, increased tissue content of cyclic adenosine 3',5'-monophosphate (cyclic AMP); alpha-adrenergic and cholinergic agents had no effect on the level of this cyclic nucleotide. 5. Secretion via cholinergic or alpha-adrenergic mechanisms required extra-cellular calcium; the beta-adrenergic mechanism did not. 6. It was concluded that stimulation of rat parotid cells activates distinctly separate pathways leading ultimately to exocytosis, one pathway involving cyclic AMP, and the other, external Ca2+ ion.
Full text
PDF













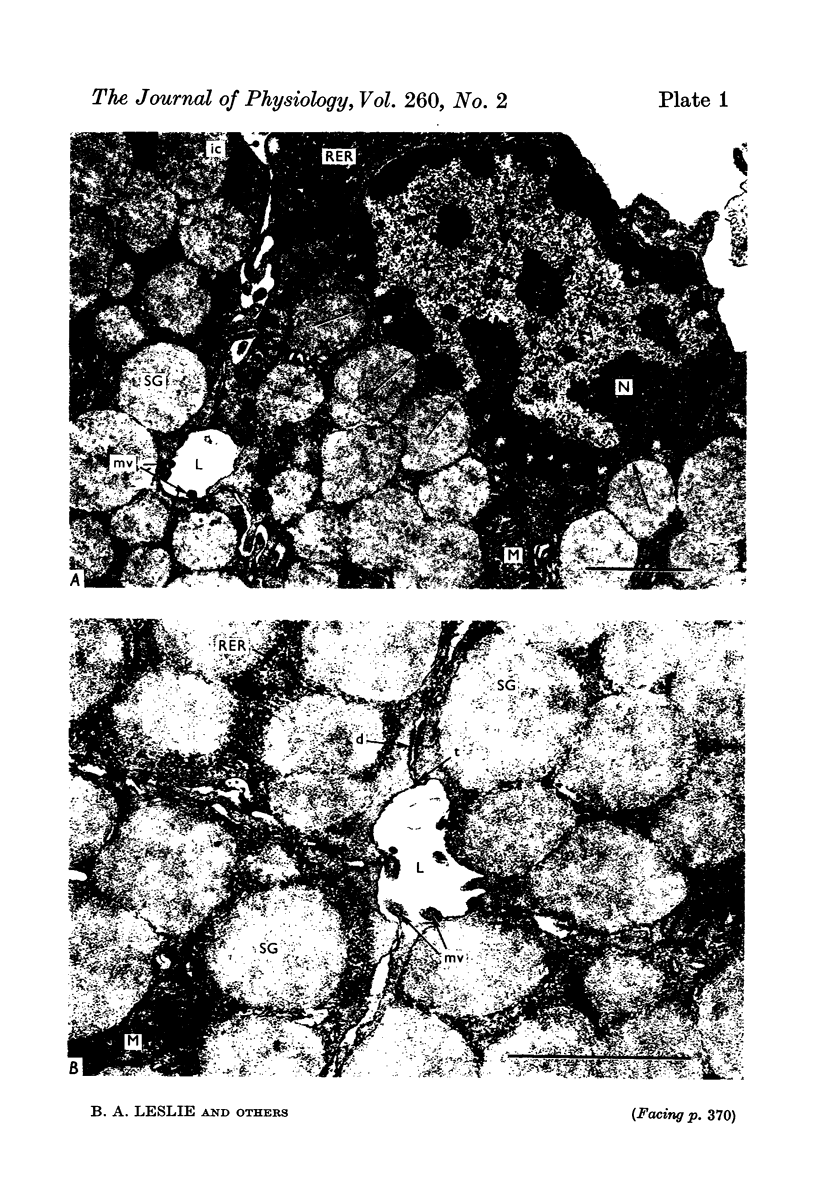
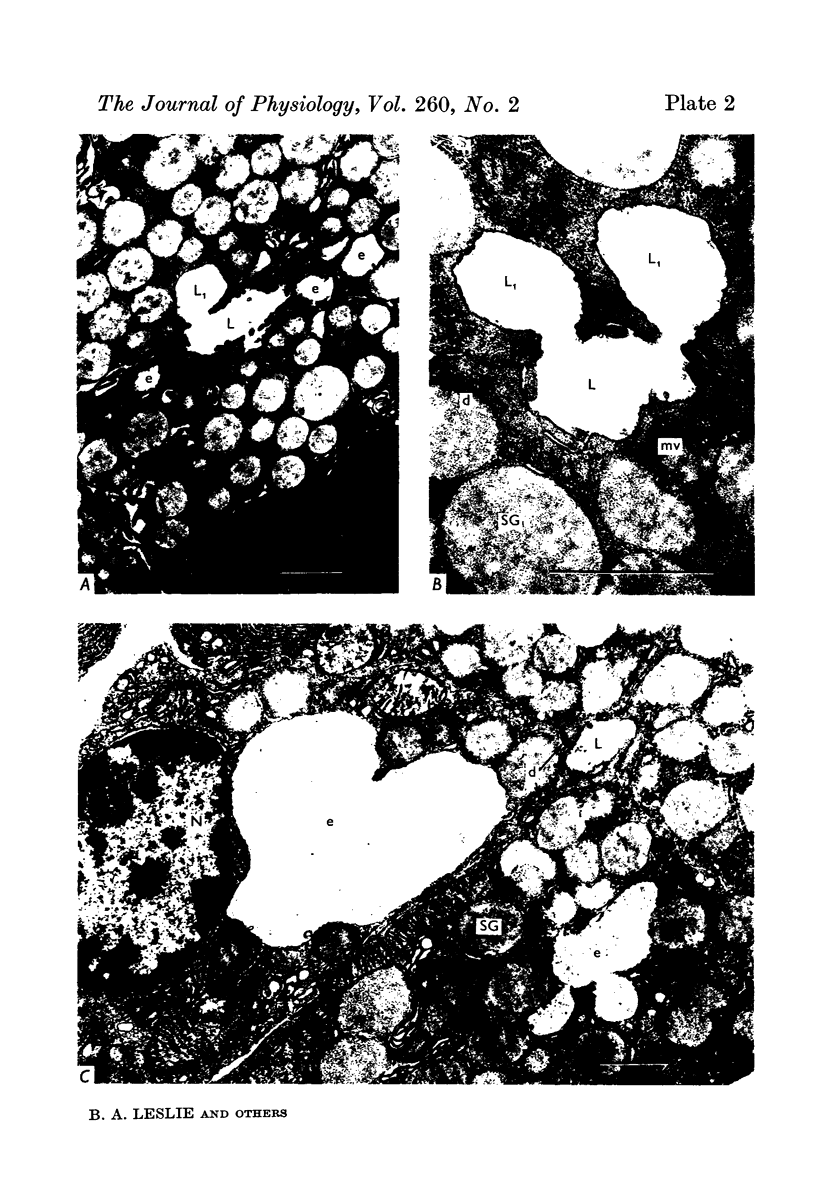
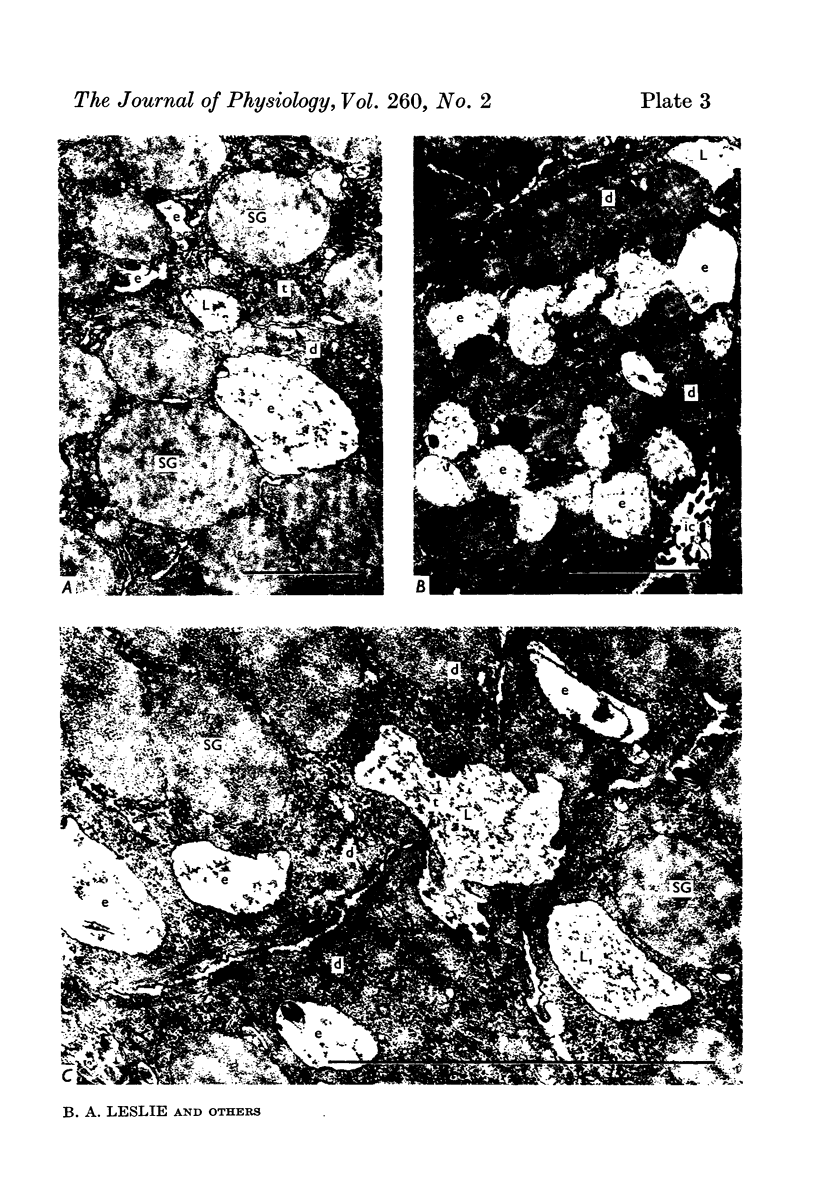
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amsterdam A., Ohad I., Schramm M. Dynamic changes in the ultrastructure of the acinar cell of the rat parotid gland during the secretory cycle. J Cell Biol. 1969 Jun;41(3):753–773. doi: 10.1083/jcb.41.3.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amsterdam A., Schramm M., Ohad I., Salomon Y., Selinger Z. Concomitant synthesis of membrane protein and exportable protein of the secretory granule in rat parotid gland. J Cell Biol. 1971 Jul;50(1):187–200. doi: 10.1083/jcb.50.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARR L., MALVIN R. L. ESTIMATION OF EXTRACELLULAR SPACES OF SMOOTH MUSCLE USING DIFFERENT-SIZED MOLECULES. Am J Physiol. 1965 May;208:1042–1045. doi: 10.1152/ajplegacy.1965.208.5.1042. [DOI] [PubMed] [Google Scholar]
- BDOLAH A., BEN-ZVI R., SCHRAMM M. THE MECHANISM OF ENZYME SECRETION BY THE CELL. II. SECRETION OF AMYLASE AND OTHER PROTEINS BY SLICES OF RAT PAROTID GLAND. Arch Biochem Biophys. 1964 Jan;104:58–66. doi: 10.1016/s0003-9861(64)80034-5. [DOI] [PubMed] [Google Scholar]
- BDOLAH A., SCHRAMM M. THE FUNCTION OF 3'5' CYCLIC AMP IN ENZYME SECRETION. Biochem Biophys Res Commun. 1965 Feb 3;18:452–454. doi: 10.1016/0006-291x(65)90730-8. [DOI] [PubMed] [Google Scholar]
- BIANCHI C. P., SHANES A. M. Calcium influx in skeletal muscle at rest, during activity, and during potassium contracture. J Gen Physiol. 1959 Mar 20;42(4):803–815. doi: 10.1085/jgp.42.4.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babad H., Ben-Zvi R., Bdolah A., Schramm M. The mechanism of enzyme secretion by the cell. 4. Effects of inducers, substrates and inhibitors on amylase secretion by rat parotid slices. Eur J Biochem. 1967 Mar;1(1):96–101. doi: 10.1111/j.1432-1033.1967.tb00049.x. [DOI] [PubMed] [Google Scholar]
- Baskin S. I., Dutta S., Marks B. H. The effects of diphenylhydantoin and potassium on the biological activity of ouabain in the guinea-pig heart. Br J Pharmacol. 1973 Jan;47(1):85–96. doi: 10.1111/j.1476-5381.1973.tb08161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batzri S., Selinger Z. Enzyme secretion mediated by the epinephrine -receptor in rat parotid slices. Factors governing efficiency of the process. J Biol Chem. 1973 Jan 10;248(1):356–360. [PubMed] [Google Scholar]
- Benz L., Eckstein B., Matthews E. K., Williams J. A. Control of pancreatic amylase release in vitro: effects of ions, cyclic AMP, and colchicine. Br J Pharmacol. 1972 Sep;46(1):66–67. doi: 10.1111/j.1476-5381.1972.tb06849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J. The interaction of cyclic nucleotides and calcium in the control of cellular activity. Adv Cyclic Nucleotide Res. 1975;6:1–98. [PubMed] [Google Scholar]
- Butcher F. R., Goldman J. A., Nemerovski Effect of adrenergic agents on alpha-amylase release and adenosine 3',5'-monophosphate accumulation in rat parotid tissue slices. Biochim Biophys Acta. 1975 May 5;392(1):82–94. doi: 10.1016/0304-4165(75)90168-3. [DOI] [PubMed] [Google Scholar]
- Butcher F. R. The role of calcium and cyclic nucleotides in alpha-amylase release from slices of rat parotid: studies with the divalent cation ionophore A-23187. Metabolism. 1975 Mar;24(3):409–418. doi: 10.1016/0026-0495(75)90120-1. [DOI] [PubMed] [Google Scholar]
- Cope G. H., Williams M. A. Quantitative analyses of the constituent membranes of parotid acinar cells and of the changes evident after induced exocytosis. Z Zellforsch Mikrosk Anat. 1973 Dec 6;145(3):311–330. doi: 10.1007/BF00307161. [DOI] [PubMed] [Google Scholar]
- Dormer R. L., Ashcroft S. J. Studies on the role of calcium ions in the stimulation by adrenaline of amylase release from rat parotid. Biochem J. 1974 Dec;144(3):543–550. doi: 10.1042/bj1440543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durham J. P., Baserga R., Butcher F. R. The effect of isoproterenol and its analogs upon adenosine 3',5'-monophosphate and guanosine 3',5'-monophosphate levels in mouse parotid gland in vivo. Relationship to the stimulation of DNA synthesis. Biochim Biophys Acta. 1974 Nov 4;372(1):196–217. doi: 10.1016/0304-4165(74)90087-7. [DOI] [PubMed] [Google Scholar]
- Foreman J. C., Mongar J. L., Gomperts B. D. Calcium ionophores and movement of calcium ions following the physiological stimulus to a secretory process. Nature. 1973 Oct 5;245(5423):249–251. doi: 10.1038/245249a0. [DOI] [PubMed] [Google Scholar]
- GOODFORD P. J., HERMANSEN K. Sodium and potassium movements in the unstriated muscle of the guinea-pig taenia coli. J Physiol. 1961 Oct;158:426–448. doi: 10.1113/jphysiol.1961.sp006778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett J. R., Thulin A. Changes in parotid acinar cells accompanying salivary secretion in rats on sympathetic or parasympathetic nerve stimulation. Cell Tissue Res. 1975 Jun 9;159(2):179–193. doi: 10.1007/BF00219154. [DOI] [PubMed] [Google Scholar]
- Hand A. R. The effects of acute starvation on parotid acinar cells. Ultrastructural and cytochemical observations on ad libitum-fed and starved rats. Am J Anat. 1972 Sep;135(1):71–92. doi: 10.1002/aja.1001350107. [DOI] [PubMed] [Google Scholar]
- Ishida H., Miki N., Yoshida H. Role of Ca2+ in the secretion of amylase from the parotid gland. Jpn J Pharmacol. 1971 Apr;21(2):227–238. doi: 10.1254/jjp.21.227. [DOI] [PubMed] [Google Scholar]
- Jaanus S. D., Rubin R. P. Analysis of the role of cyclic adenosine 3',5'-monophosphate in catecholamine release. J Physiol. 1974 Mar;237(2):465–476. doi: 10.1113/jphysiol.1974.sp010492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- PAGE E. Cat heart muscle in vitro. III. The extracellular space. J Gen Physiol. 1962 Nov;46:201–213. doi: 10.1085/jgp.46.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARKS H. F. On the fine structure of the parotid gland of mouse and rat. Am J Anat. 1961 May;108:303–329. doi: 10.1002/aja.1001080306. [DOI] [PubMed] [Google Scholar]
- Petersen O. H., Pedersen G. L. Membrane effects mediated by alpha-and beta-adrenoceptors in mouse parotid acinar cells. J Membr Biol. 1974;16(4):353–362. doi: 10.1007/BF01872423. [DOI] [PubMed] [Google Scholar]
- Poisner A. M. Direct stimulant effect of aminophylline on catecholamine release from the adrenal medulla. Biochem Pharmacol. 1973 Feb 15;22(4):469–476. doi: 10.1016/0006-2952(73)90288-8. [DOI] [PubMed] [Google Scholar]
- Putney J. W., Jr, Borzelleca J. F. On the mechanisms of 14 C-salicylic acid distribution in rat submaxillary gland in vitro. J Pharmacol Exp Ther. 1971 Apr;177(1):263–275. [PubMed] [Google Scholar]
- Reed P. W., Lardy H. A. A23187: a divalent cation ionophore. J Biol Chem. 1972 Nov 10;247(21):6970–6977. [PubMed] [Google Scholar]
- Ridderstap A. S., Bonting S. L. Cyclic AMP and enzyme secretion by the isolated rabbit pancreas. Pflugers Arch. 1969;313(1):62–70. doi: 10.1007/BF00586329. [DOI] [PubMed] [Google Scholar]
- Rossignol B., Herman G., Chambaut A. M., Keryer G. The calcium ionophore A 23 187 as a probe for studying the role of Ca2+ ions in the mediation of carbachol effects on rat salivary glands: protein secretion and metabolism of phospholipids and glycogen. FEBS Lett. 1974 Jul 15;43(2):241–246. doi: 10.1016/0014-5793(74)81010-0. [DOI] [PubMed] [Google Scholar]
- Rubin R. P. The role of calcium in the release of neurotransmitter substances and hormones. Pharmacol Rev. 1970 Sep;22(3):389–428. [PubMed] [Google Scholar]
- SCHNEYER L. H., SCHNEYER C. A. Electrolyte and inulin spaces of rat salivary glands and pancreas. Am J Physiol. 1960 Oct;199:649–652. doi: 10.1152/ajplegacy.1960.199.4.649. [DOI] [PubMed] [Google Scholar]
- SCHNEYER L. H., SCHNEYER C. A. Electrolyte and water transport by salivary gland slices. Am J Physiol. 1962 Sep;203:567–571. doi: 10.1152/ajplegacy.1962.203.3.567. [DOI] [PubMed] [Google Scholar]
- SCOTT B. L., PEASE D. C. Electron microscopy of the salivary and lacrimal glands of the rat. Am J Anat. 1959 Jan;104:115–161. doi: 10.1002/aja.1001040106. [DOI] [PubMed] [Google Scholar]
- SHANES A. M., BIANCHI C. P. Radiocalcium release by stimulated and potassium-treated sartorius muscles of the frog. J Gen Physiol. 1960 Jan;43:481–493. doi: 10.1085/jgp.43.3.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHANES A. M., BIANCHI C. P. The distribution and kinetics of release of radiocalcium in tendon and skeletal muscle. J Gen Physiol. 1959 May 20;42(5):1123–1137. doi: 10.1085/jgp.42.5.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneyer C. A., Hall H. D. Comparison of rat salivas evoked by auriculo-temporal and pilocarpine stimulation. Am J Physiol. 1965 Sep;209(3):484–488. doi: 10.1152/ajplegacy.1965.209.3.484. [DOI] [PubMed] [Google Scholar]
- Schramm M. Amylase secretion in rat parotid slices by apparent activation of endogenous catecholamine. Biochim Biophys Acta. 1968 Oct 15;165(3):546–549. doi: 10.1016/0304-4165(68)90238-9. [DOI] [PubMed] [Google Scholar]
- Schramm M., Selinger Z., Salomon Y., Eytan E., Batzri S. Pseudopodia formation by secretory granules. Nat New Biol. 1972 Dec 13;240(102):203–205. doi: 10.1038/newbio240203a0. [DOI] [PubMed] [Google Scholar]
- Schramm M., Selinger Z. The function of alpha- and beta-adrenergic receptors and a cholinergic receptor in the secretory cell of rat parotid gland. Adv Cytopharmacol. 1974;2:29–32. [PubMed] [Google Scholar]
- Schramm M., Selinger Z. The functions of cyclic AMP and calcium as alternative second messengers in parotid gland and pancreas. J Cyclic Nucleotide Res. 1975;1(4):181–192. [PubMed] [Google Scholar]
- Selinger Z., Eimerl S., Schramm M. A calcium ionophore simulating the action of epinephrine on the alpha-adrenergic receptor. Proc Natl Acad Sci U S A. 1974 Jan;71(1):128–131. doi: 10.1073/pnas.71.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selinger Z., Naim E. The effect of calcium on amylase secretion by rat parotid slices. Biochim Biophys Acta. 1970 Apr 21;203(2):335–337. doi: 10.1016/0005-2736(70)90148-3. [DOI] [PubMed] [Google Scholar]
- Selinger Z., Sharoni Y., Schramm M. Modification of the secretory granule during secretion in the rat parotid gland. Adv Cytopharmacol. 1974;2:23–28. [PubMed] [Google Scholar]
- Siegel I. A. Effects of metabolic inhibitors on potassium transport in submaxillary glands. Am J Physiol. 1969 Apr;216(4):728–733. doi: 10.1152/ajplegacy.1969.216.4.728. [DOI] [PubMed] [Google Scholar]
- Simson J. V. Discharge and restitution of secretory material in the rat parotid gland in response to isoproterenol. Z Zellforsch Mikrosk Anat. 1969;101(2):175–191. doi: 10.1007/BF00335726. [DOI] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- Steiner A. L., Kipnis D. M., Utiger R., Parker C. Radioimmunoassay for the measurement of adenosine 3',5'-cyclic phosphate. Proc Natl Acad Sci U S A. 1969 Sep;64(1):367–373. doi: 10.1073/pnas.64.1.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TUTTLE R. S., WITT P. N., FARAH A. The influence of ouabain on intracellular sodium and potassium concentrations in the rabbit myocardium. J Pharmacol Exp Ther. 1961 Sep;133:281–287. [PubMed] [Google Scholar]
- Wallach D., Schramm M. Calcium and the exportable protein in rat parotid gland. Parallel subcellular distribution and concomitant secretion. Eur J Biochem. 1971 Aug 16;21(3):433–437. doi: 10.1111/j.1432-1033.1971.tb01489.x. [DOI] [PubMed] [Google Scholar]
- Weiss G. B. Homogeneity of extracellular space measurement in smooth muscle. Am J Physiol. 1966 Apr;210(4):771–776. doi: 10.1152/ajplegacy.1966.210.4.771. [DOI] [PubMed] [Google Scholar]