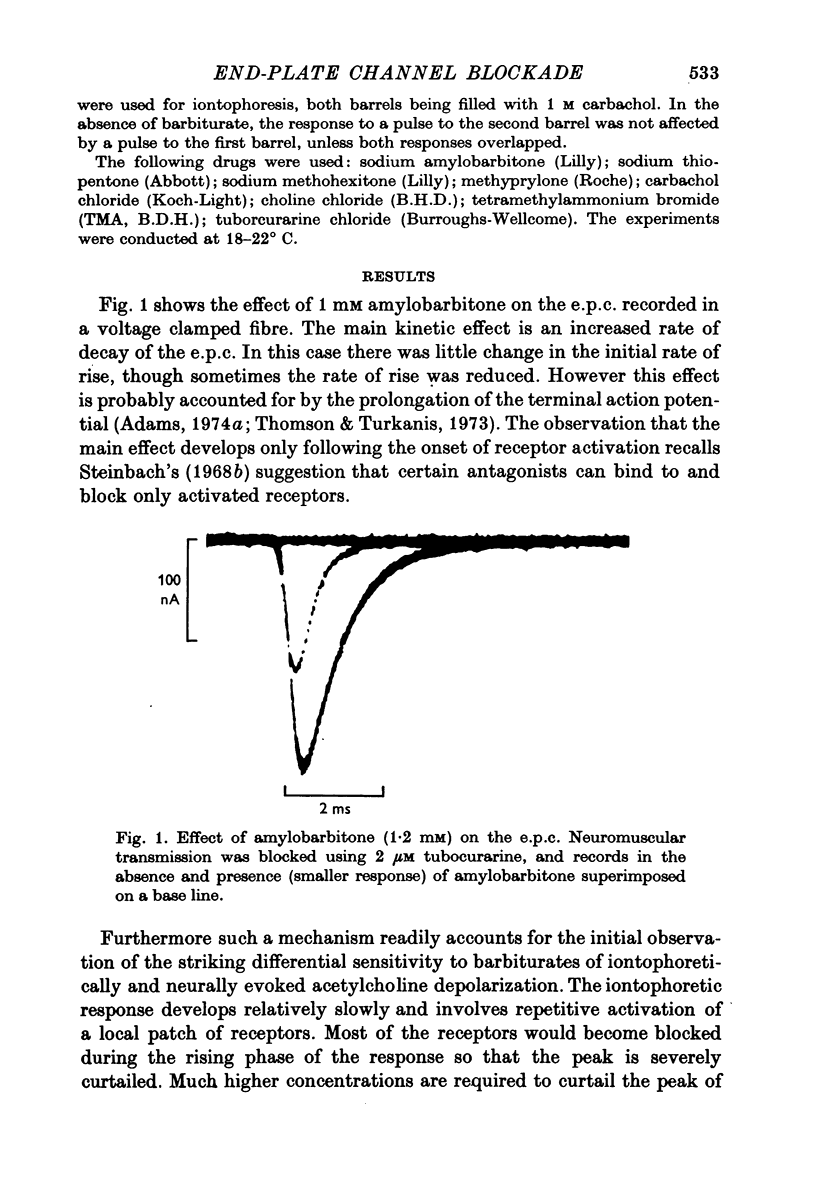
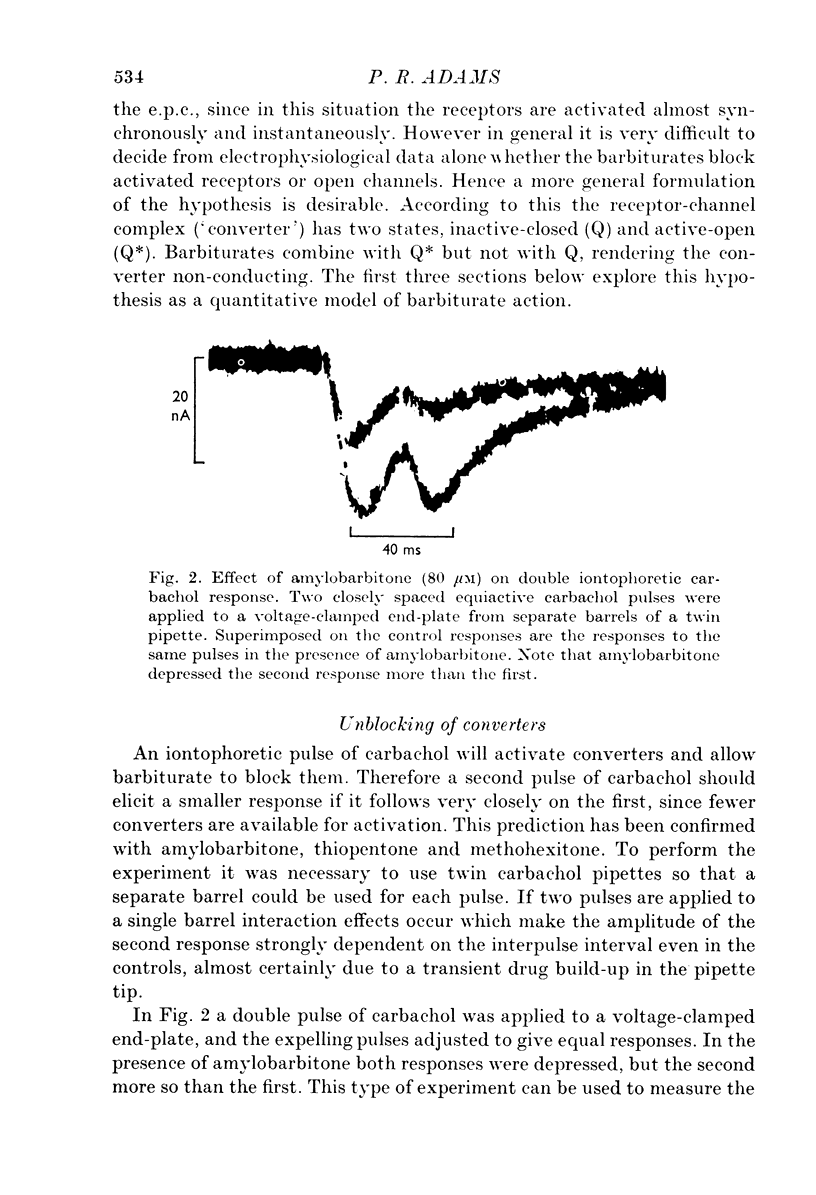
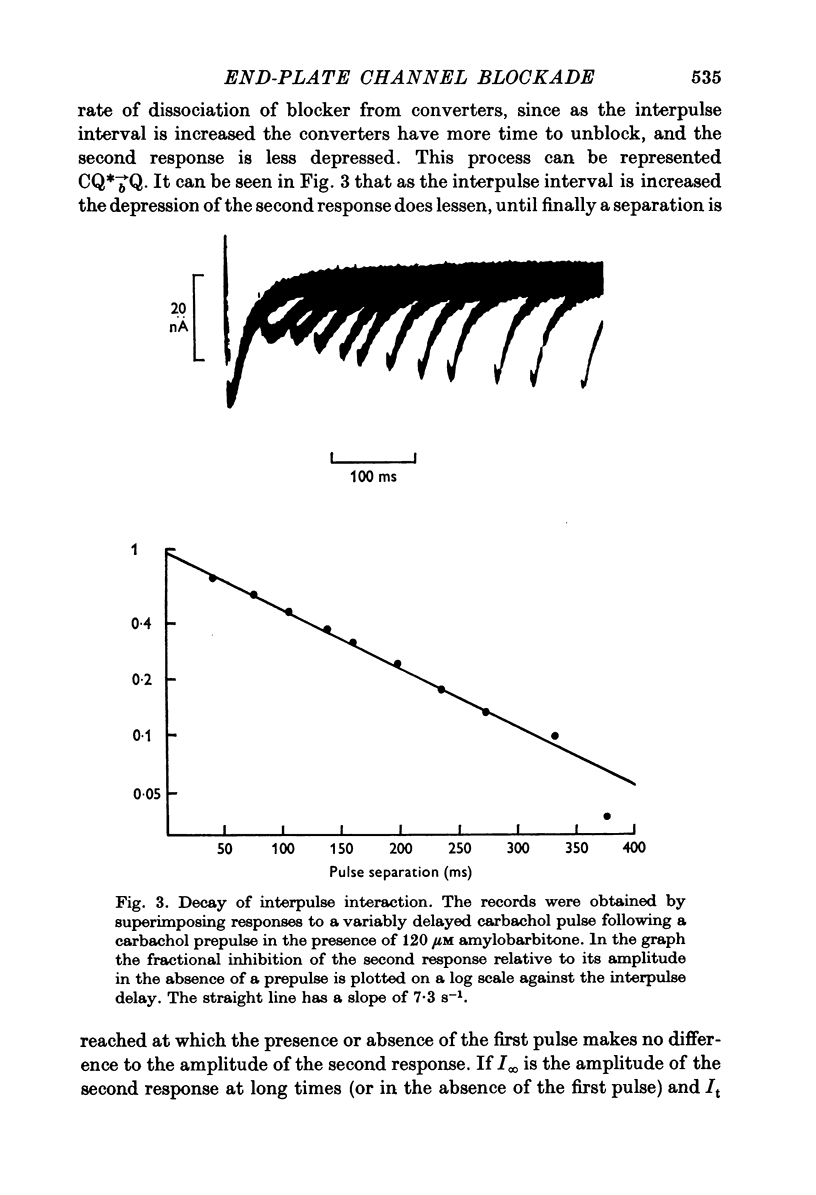
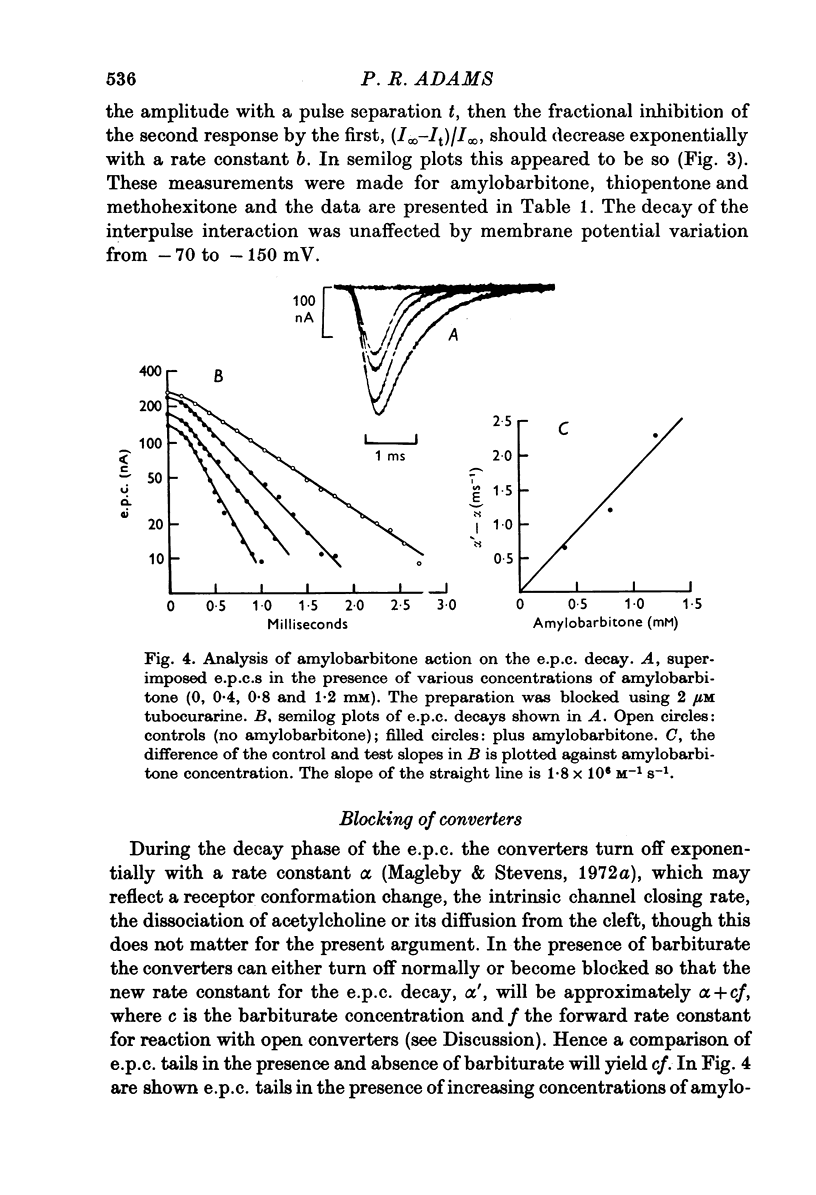
Abstract
1. The actions of amylobarbitone, thiopentone, methohexitone and methyprylone at voltage-clamped frog end-plates were studied. 2. In the presence of barbiturates the conductance change evoked by an iontophoretic carbachol application was reduced by a prepulse of carbachol. The extra inhibition evoked by a prepulse disappeared exponentially with a time constant of 150-200 ms. 3. Barbiturates produce an increased rate of decay of nerve evoked endplate currents. Tne concentration and voltage dependence of the barbtiruate e.p.c. decay rates tally with the hypothesis that the increased rate of decay is due to block of active receptor-channel complexes by barbiturates with a rate constant of 10(6) M-1S-1. 4. Conductance changes produced by bath applied agonists were depressed by thiopentone, the effect becoming greater the higher the agonist concentration. This effect, and also the observation that the concentration of thiopentone required to depress the bath agonist response is much greater than the apparent dissociation constant for binding to active receptor-channel complexes calculated from kinetic measurements, suggest that the selectivity for binding to open receptor-channel complexes is very high. 5. Methyprylone, which is structurally similar to the barbiturates, is only a weak antagonist and shows no interpulse interaction. It was predicted that methyprylone should produce fast and slow components in the e.p.c. decay, and this prediction was verified. 6. In the presence of barbiturates large iontophoretic carbachol applications produce conductance changes which show fast and slow components. Under these conditions the effects of carbachol prepulses become complex. However the effects are qualitatively consistent with the notion that different components of the response are contributed by channels located at various distances from the iontophoretic pipette tip. 7. All the data agree with a model in which the channel has three stages: closed, open and blocked. Only open channels can block, and blocked channels can only open.
Full text
PDF


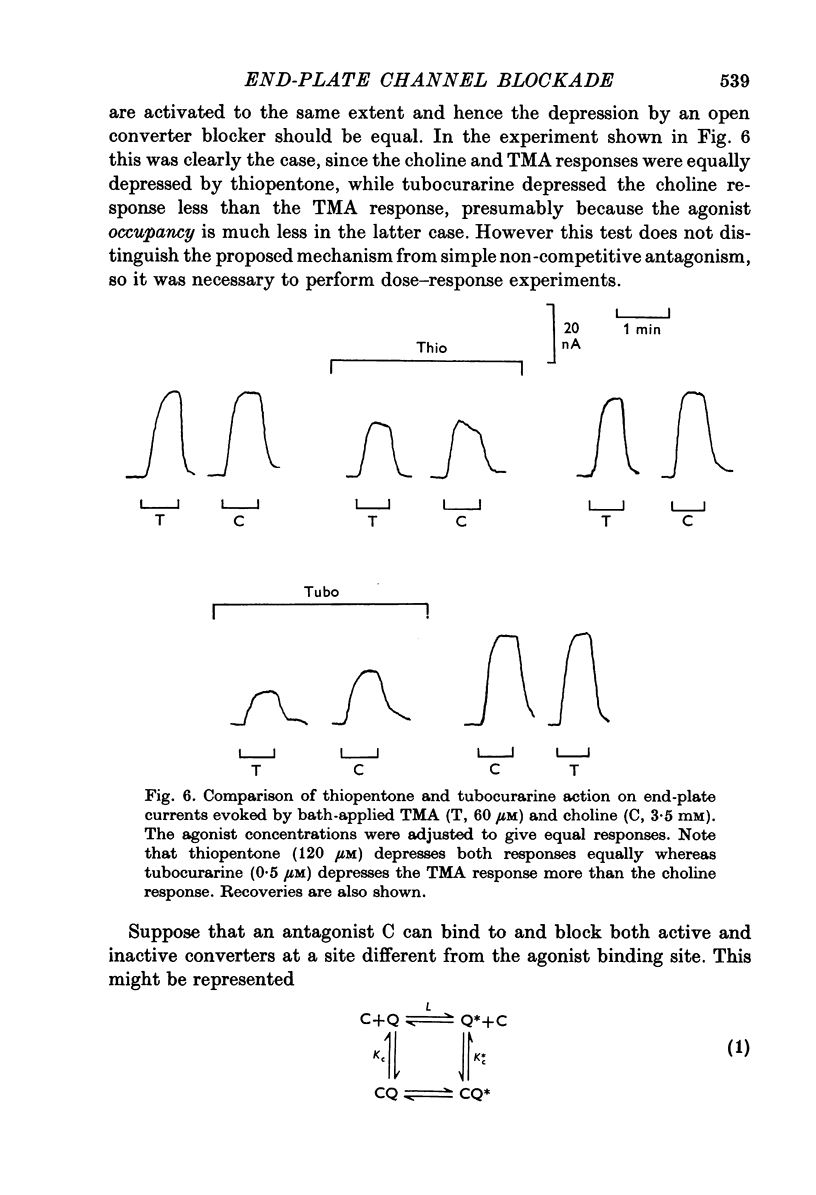
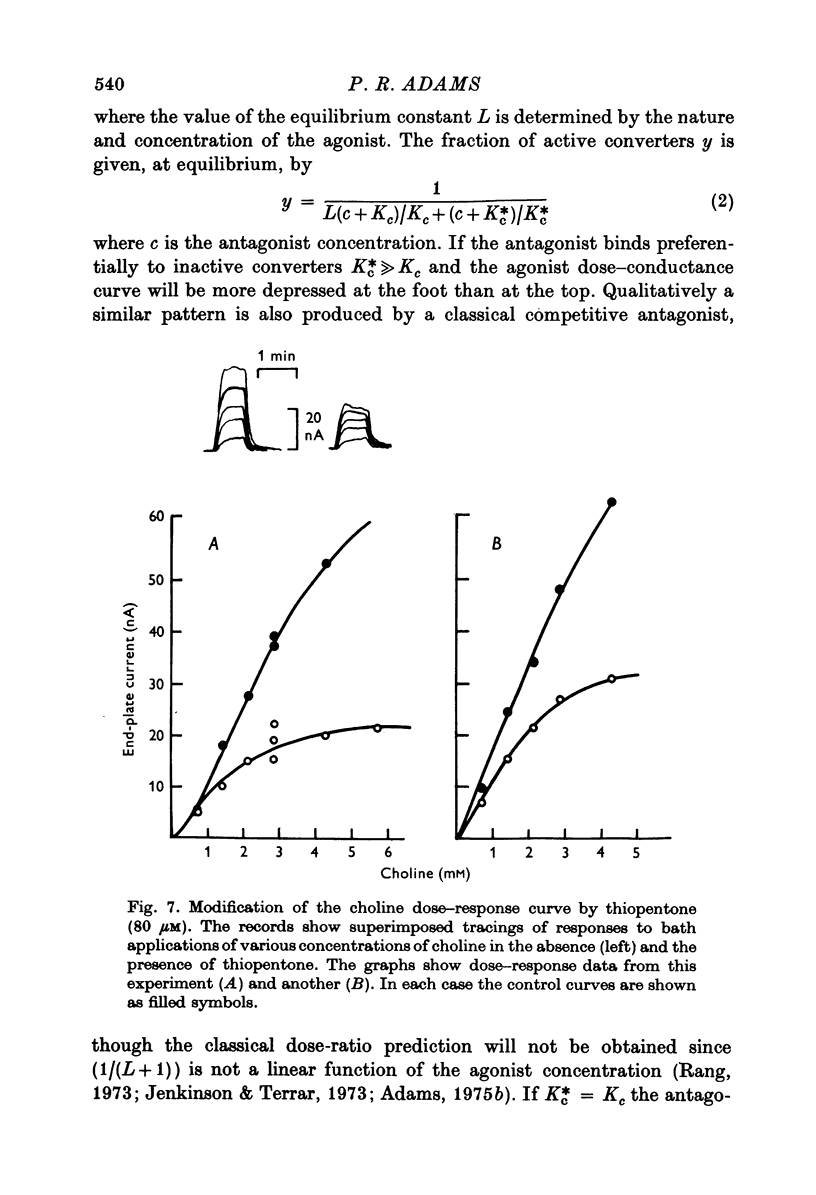

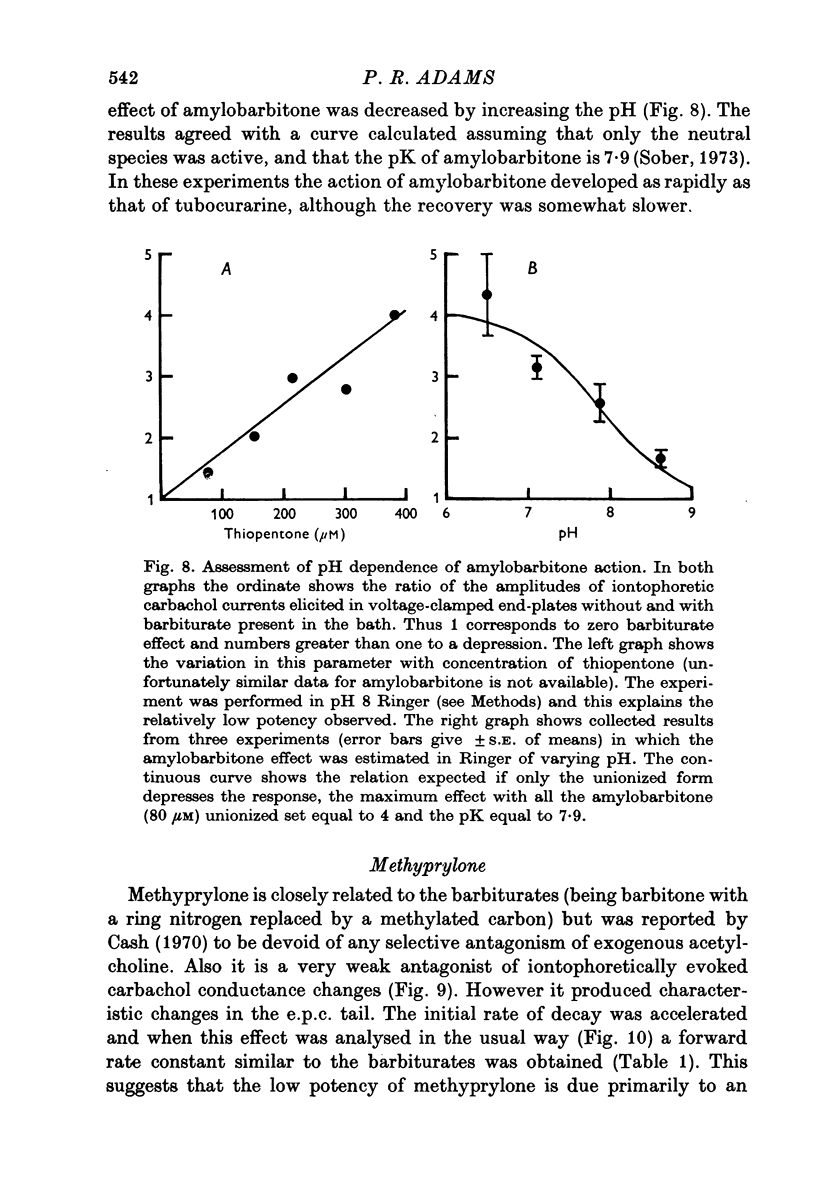
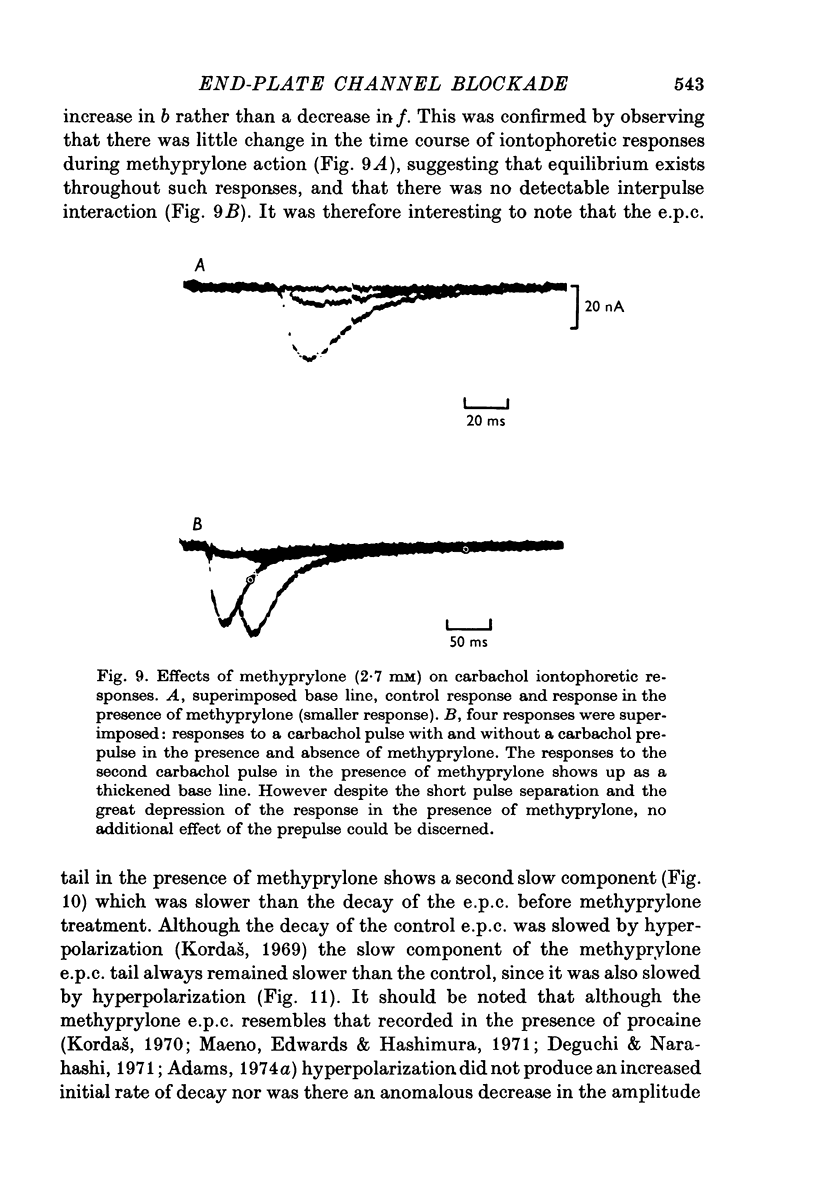
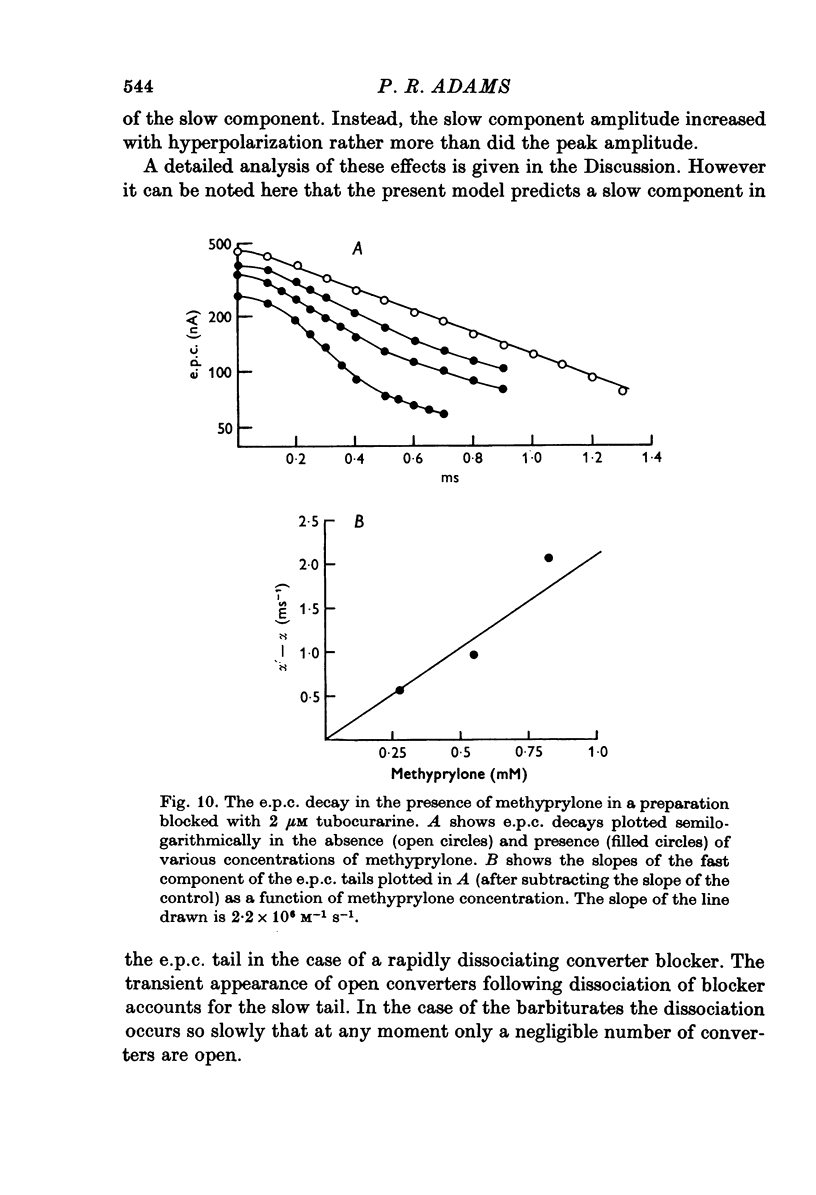
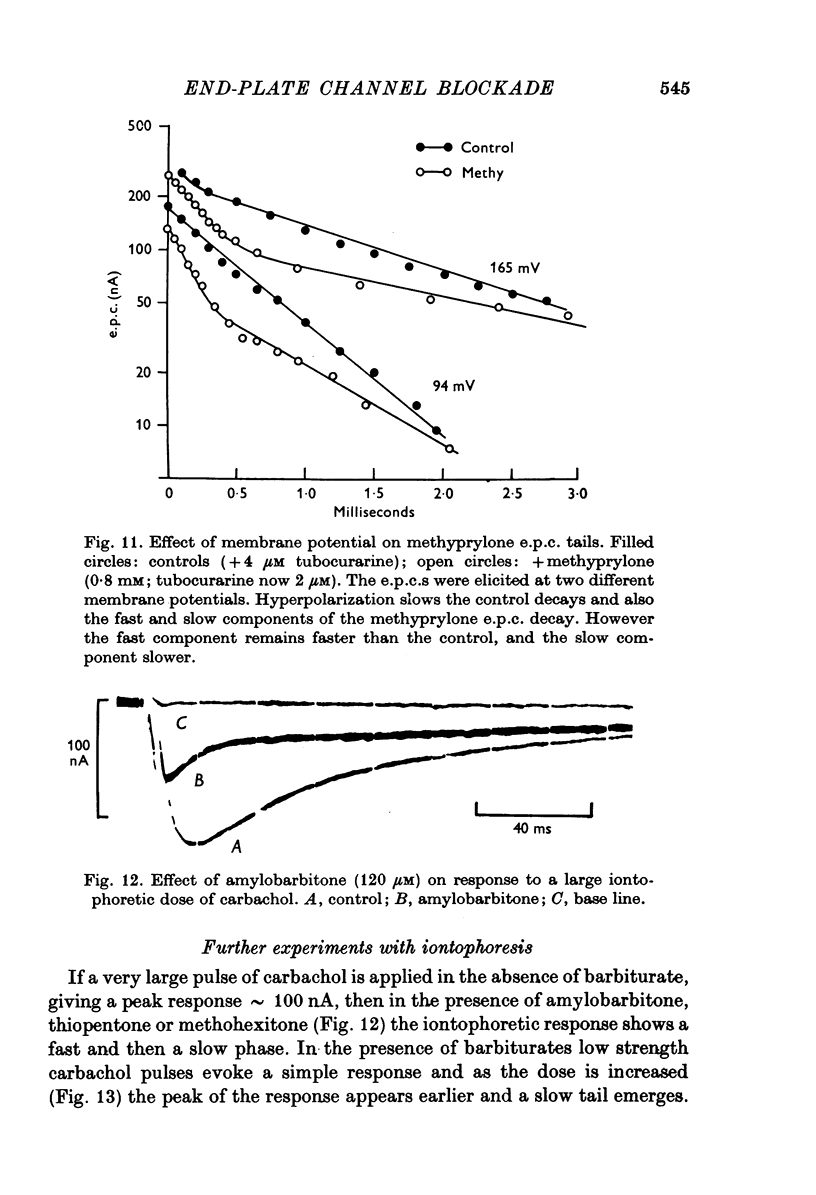
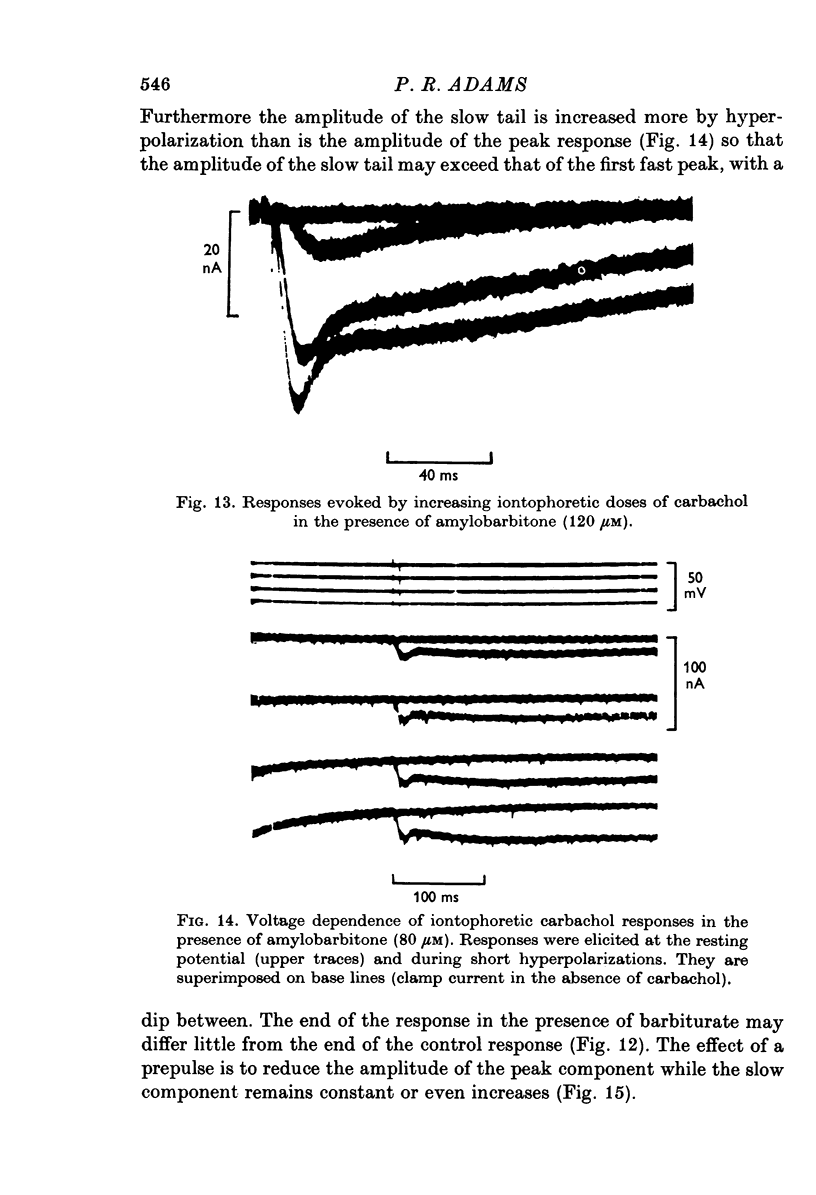
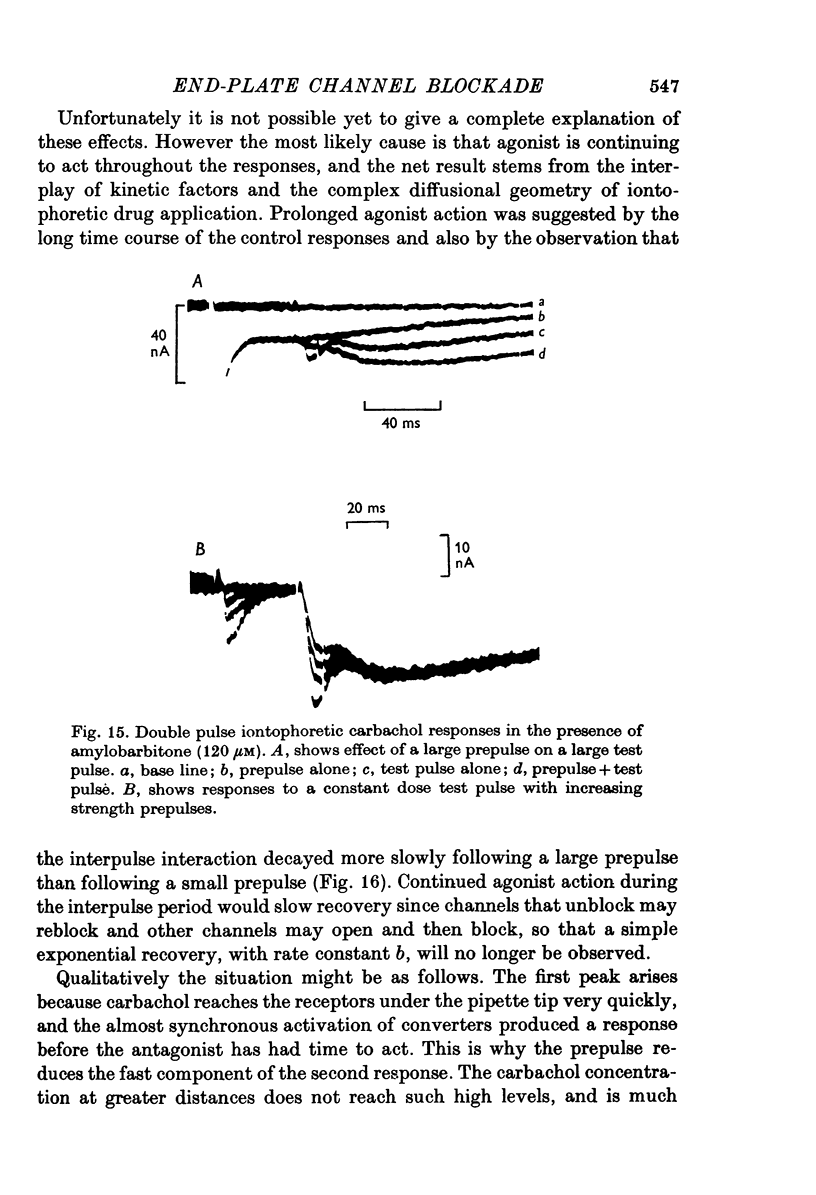




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. R. A model for the procaine end-plate current. J Physiol. 1975 Mar;246(2):61P–63P. [PubMed] [Google Scholar]
- Adams P. R. An analysis of the dose-response curve at voltage-clamped frog-endplates. Pflugers Arch. 1975 Oct 28;360(2):145–153. doi: 10.1007/BF00580537. [DOI] [PubMed] [Google Scholar]
- Adams P. R., Cash H. C., Quilliam J. P. Extrinsic and intrinsic acetylcholine and barbiturate effects on frog skeletal muscle. Br J Pharmacol. 1970 Nov;40(3):552P–553P. [PMC free article] [PubMed] [Google Scholar]
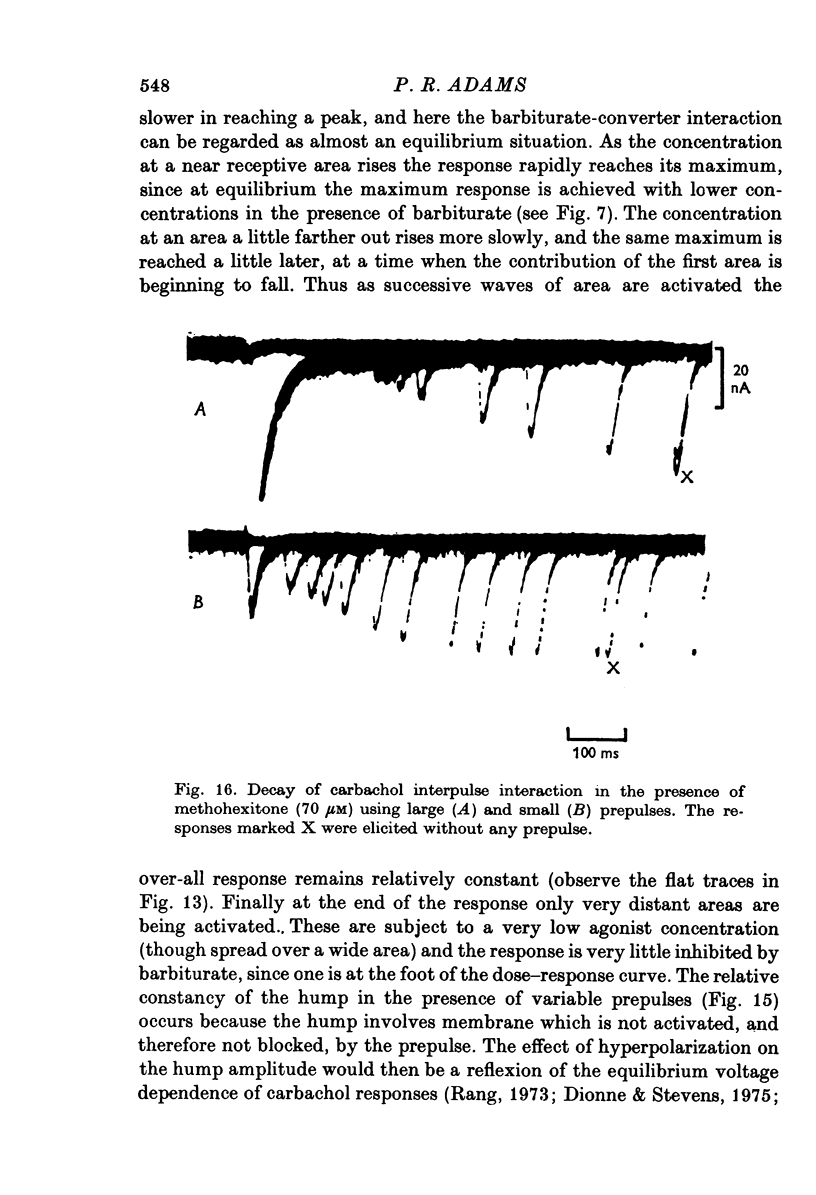
- Adams P. R. Drug interactions at the motor endplate. Pflugers Arch. 1975 Oct 28;360(2):155–164. doi: 10.1007/BF00580538. [DOI] [PubMed] [Google Scholar]
- Adams P. R. Voltage dependence of agonist responses at voltage-clamped frog endplates. Pflugers Arch. 1976 Jan 30;361(2):145–151. doi: 10.1007/BF00583458. [DOI] [PubMed] [Google Scholar]
- Anderson C. R., Stevens C. F. Voltage clamp analysis of acetylcholine produced end-plate current fluctuations at frog neuromuscular junction. J Physiol. 1973 Dec;235(3):655–691. doi: 10.1113/jphysiol.1973.sp010410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong C. M. Interaction of tetraethylammonium ion derivatives with the potassium channels of giant axons. J Gen Physiol. 1971 Oct;58(4):413–437. doi: 10.1085/jgp.58.4.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker J. L. CNS depressants: effects on post-synaptic pharmacology. Brain Res. 1975 Jul 4;92(1):35–55. doi: 10.1016/0006-8993(75)90526-0. [DOI] [PubMed] [Google Scholar]
- Blaustein M. P. Barbiturates block sodium and potassium conductance increases in voltage-clamped lobster axons. J Gen Physiol. 1968 Mar;51(3):293–307. doi: 10.1085/jgp.51.3.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deguchi T., Narahashi T. Effects of procaine on ionic conductances of end-plate membranes. J Pharmacol Exp Ther. 1971 Feb;176(2):423–433. [PubMed] [Google Scholar]
- Dionne V. E., Stevens C. F. Voltage dependence of agonist effectiveness at the frog neuromuscular junction: resolution of a paradox. J Physiol. 1975 Oct;251(2):245–270. doi: 10.1113/jphysiol.1975.sp011090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage P. W., McBurney R. N. Effects of membrane potential, temperature and neostigmine on the conductance change caused by a quantum or acetylcholine at the toad neuromuscular junction. J Physiol. 1975 Jan;244(2):385–407. doi: 10.1113/jphysiol.1975.sp010805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage P. W., McBurney R. N., Schneider G. T. Effects of some aliphatic alcohols on the conductance change caused by a quantum of acetylcholine at the toad end-plate. J Physiol. 1975 Jan;244(2):409–429. doi: 10.1113/jphysiol.1975.sp010806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson D. H., Terrar D. A. Influence of chloride ions on changes in membrane potential during prolonged application of carbachol to frog skeletal muscle. Br J Pharmacol. 1973 Feb;47(2):363–376. doi: 10.1111/j.1476-5381.1973.tb08334.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
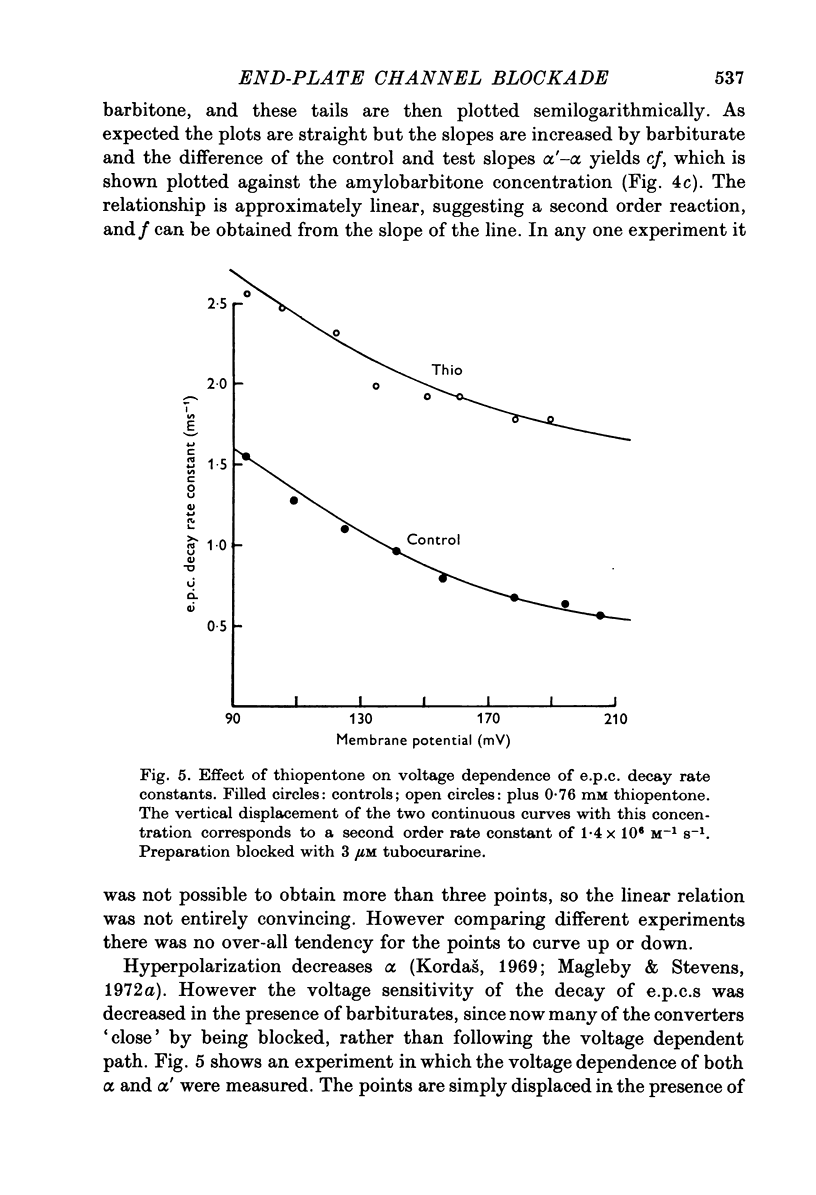
- Kordas M. The effect of membrane polarization on the time course of the end-plate current in frog sartorius muscle. J Physiol. 1969 Oct;204(2):493–502. doi: 10.1113/jphysiol.1969.sp008926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeno T., Edwards C., Hashimura S. Difference in effects of end-plate potentials between procaine and lidocaine as revealed by voltage-clamp experiments. J Neurophysiol. 1971 Jan;34(1):32–46. doi: 10.1152/jn.1971.34.1.32. [DOI] [PubMed] [Google Scholar]
- Magazanik L. G. Vliianie nekotorykh membrannykh stabilizatorov na funktsiiu nervno-myshechnogo sinapsa. Fiziol Zh SSSR Im I M Sechenova. 1971 Sep;57(9):1313–1321. [PubMed] [Google Scholar]
- Magleby K. L., Stevens C. F. A quantitative description of end-plate currents. J Physiol. 1972 May;223(1):173–197. doi: 10.1113/jphysiol.1972.sp009840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magleby K. L., Stevens C. F. The effect of voltage on the time course of end-plate currents. J Physiol. 1972 May;223(1):151–171. doi: 10.1113/jphysiol.1972.sp009839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rang H. P. Allosteric mechanisms at neuromuscular junctions. Neurosci Res Program Bull. 1973 Jun;11(3):220–224. [PubMed] [Google Scholar]
- Schwarz J. R., Ulbricht W., Wagner H. H. The rate of action of tetrodotoxin on myelinated nerve fibres of Xenopus laevis and Rana esculenta. J Physiol. 1973 Aug;233(1):167–194. doi: 10.1113/jphysiol.1973.sp010304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyama I., Narahashi T. Mechanism of blockade of neuromuscular transmission by pentobarbital. J Pharmacol Exp Ther. 1975 Jan;192(1):95–104. [PubMed] [Google Scholar]
- Steinbach A. B. A kinetic model for the action of xylocaine on receptors for acetylcholine. J Gen Physiol. 1968 Jul;52(1):162–180. doi: 10.1085/jgp.52.1.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbach A. B. Alteration by xylocaine (lidocaine) and its derivatives of the time course of the end plate potential. J Gen Physiol. 1968 Jul;52(1):144–161. doi: 10.1085/jgp.52.1.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strichartz G. R. The inhibition of sodium currents in myelinated nerve by quaternary derivatives of lidocaine. J Gen Physiol. 1973 Jul;62(1):37–57. doi: 10.1085/jgp.62.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THESLEFF S. The effect of anesthetic agents on skeletal muscle membrane. Acta Physiol Scand. 1956 Nov 5;37(4):335–349. doi: 10.1111/j.1748-1716.1956.tb01369.x. [DOI] [PubMed] [Google Scholar]
- Thomson T. D., Turkanis S. A. Barbiturate-induced transmitter release at a frog neuromuscular junction. Br J Pharmacol. 1973 May;48(1):48–58. doi: 10.1111/j.1476-5381.1973.tb08221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodhull A. M. Ionic blockage of sodium channels in nerve. J Gen Physiol. 1973 Jun;61(6):687–708. doi: 10.1085/jgp.61.6.687. [DOI] [PMC free article] [PubMed] [Google Scholar]


