Abstract
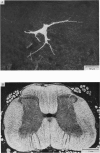
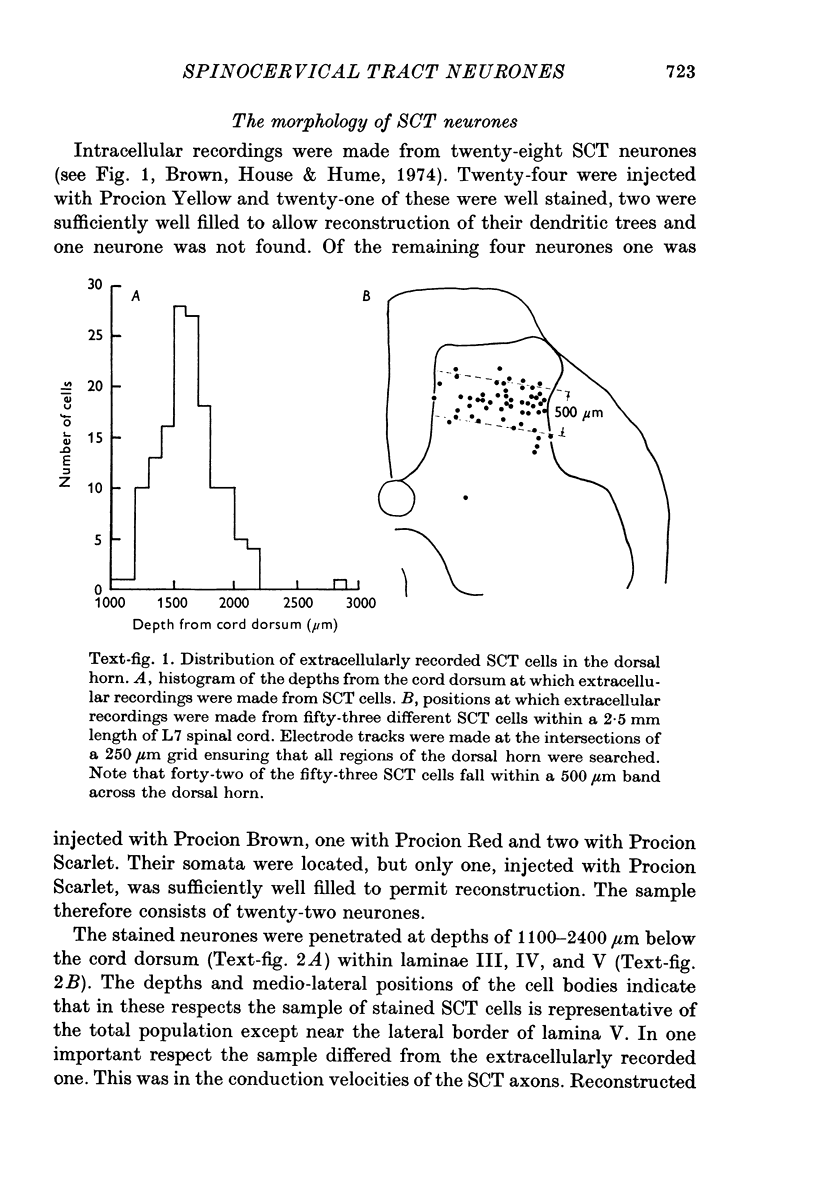
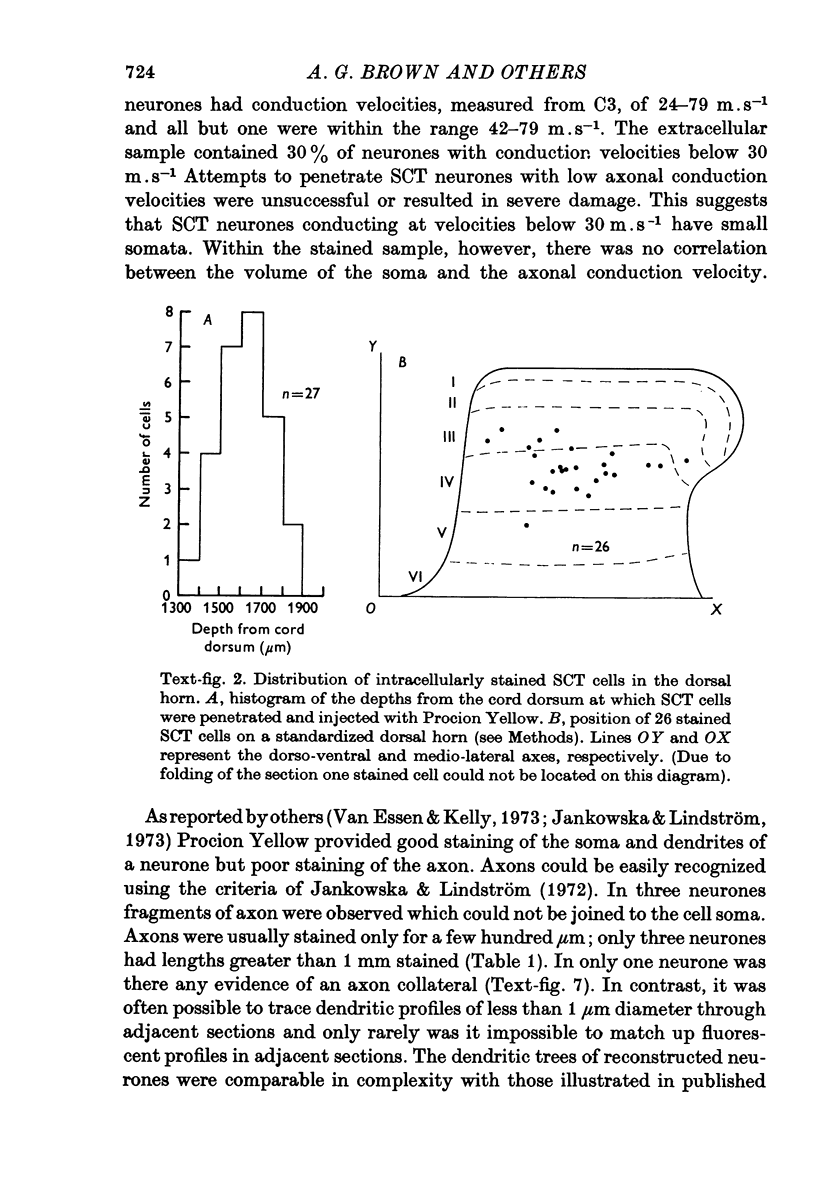
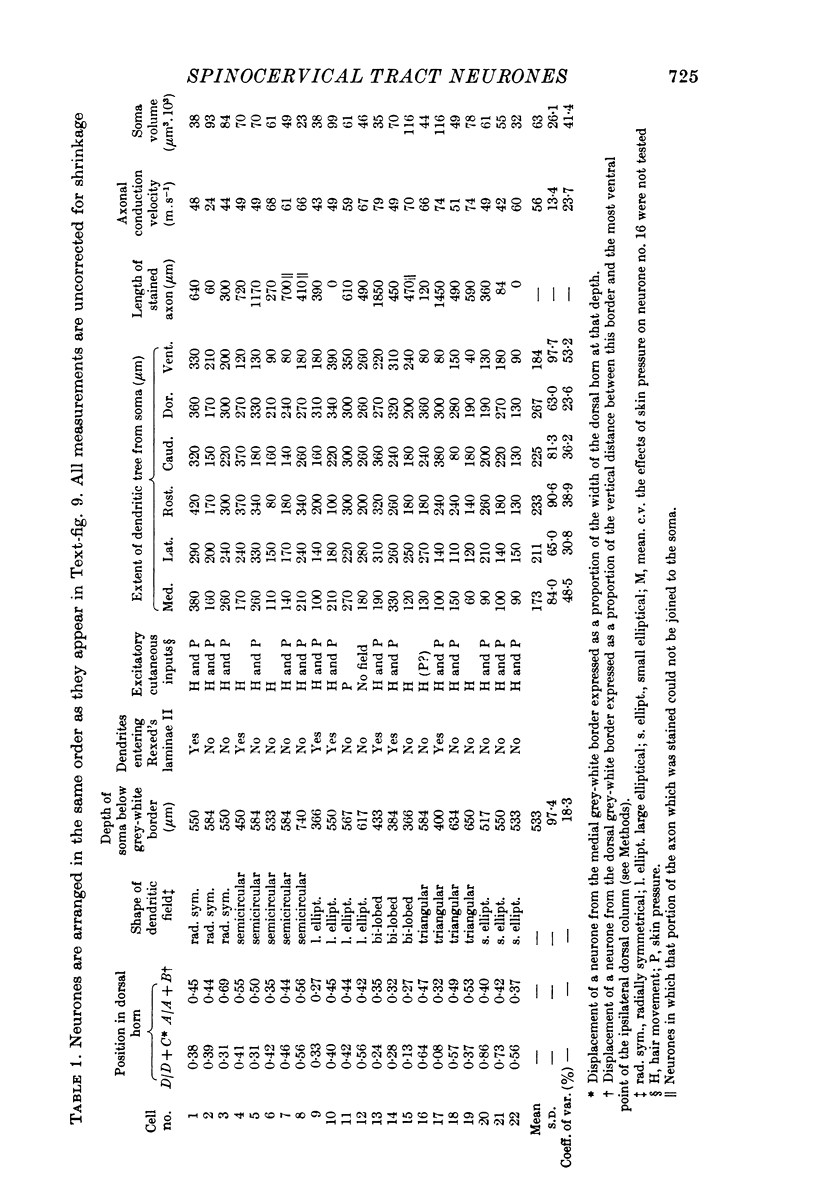
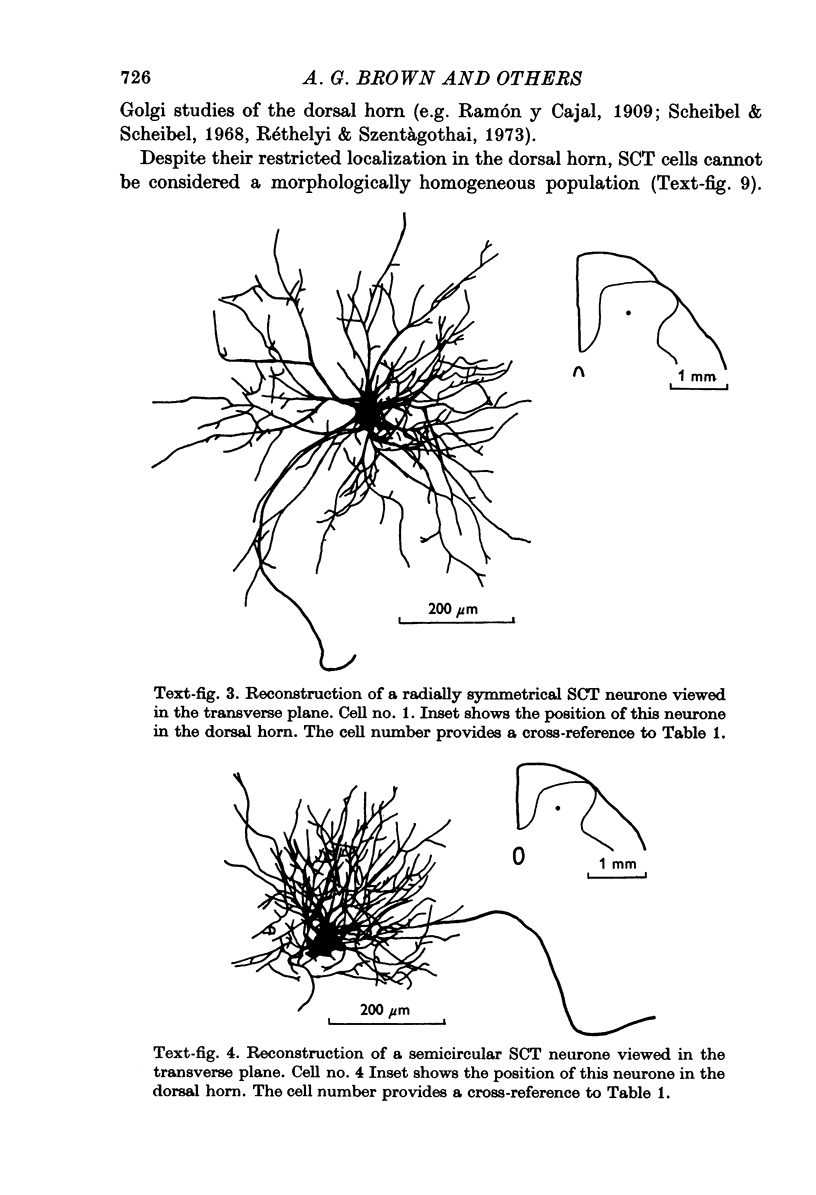
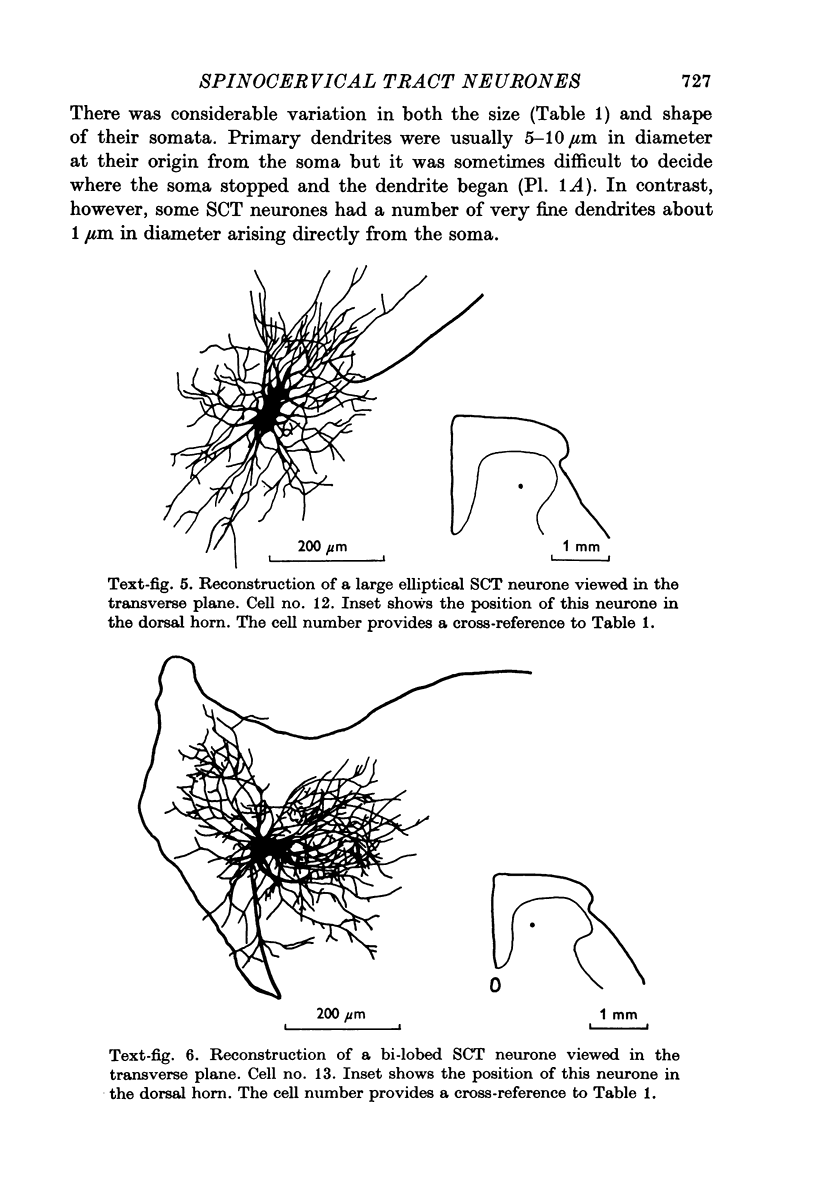
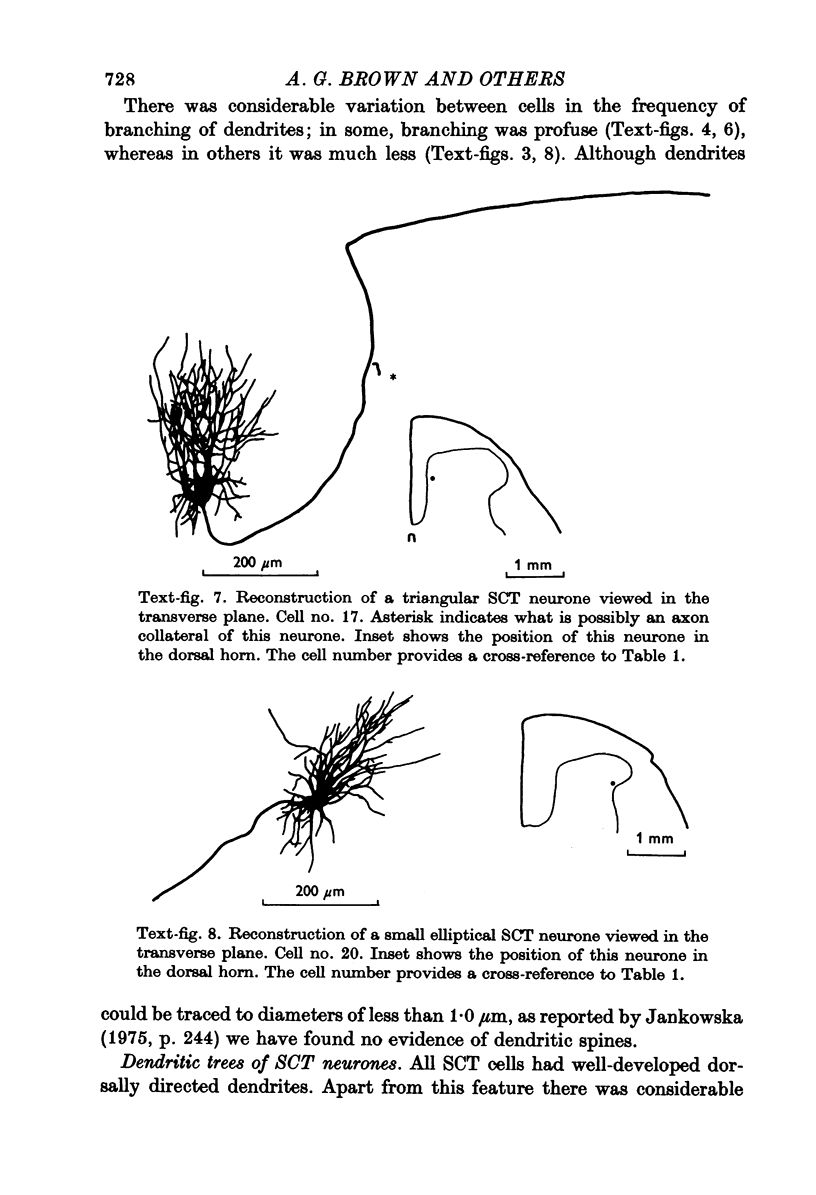
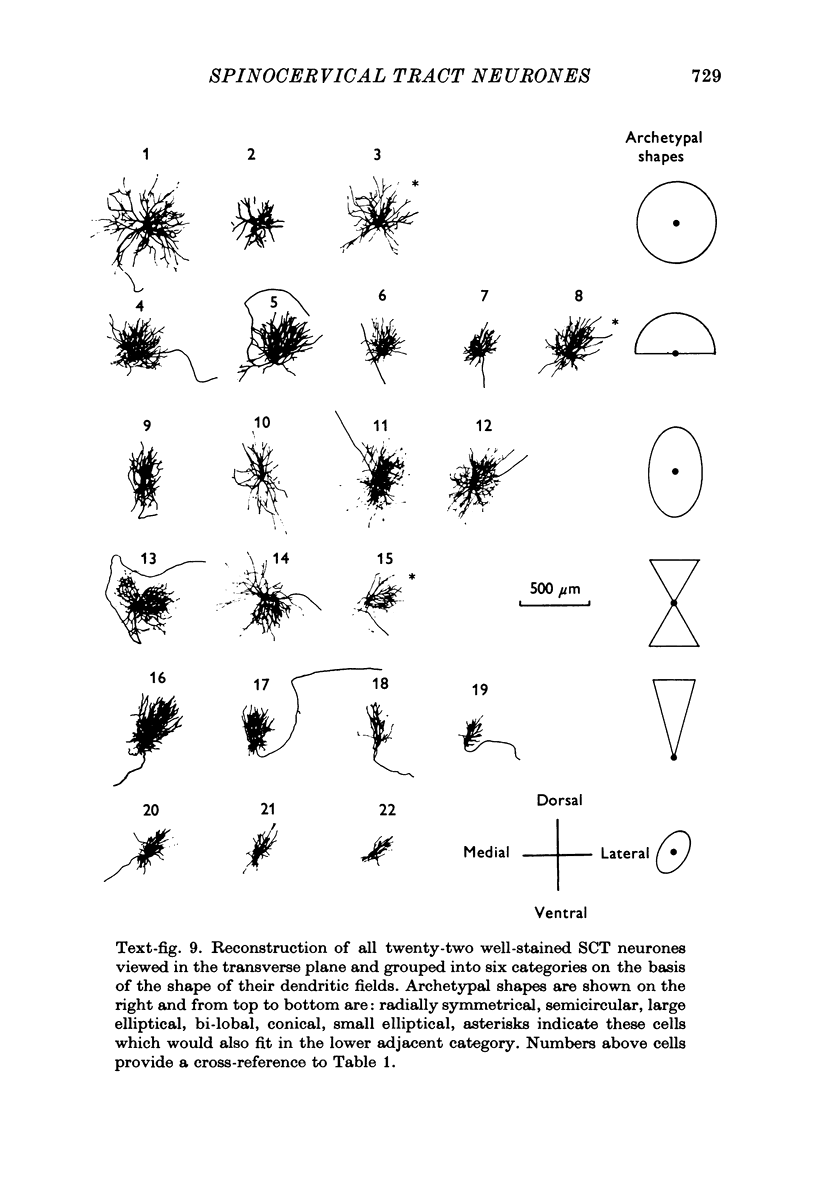
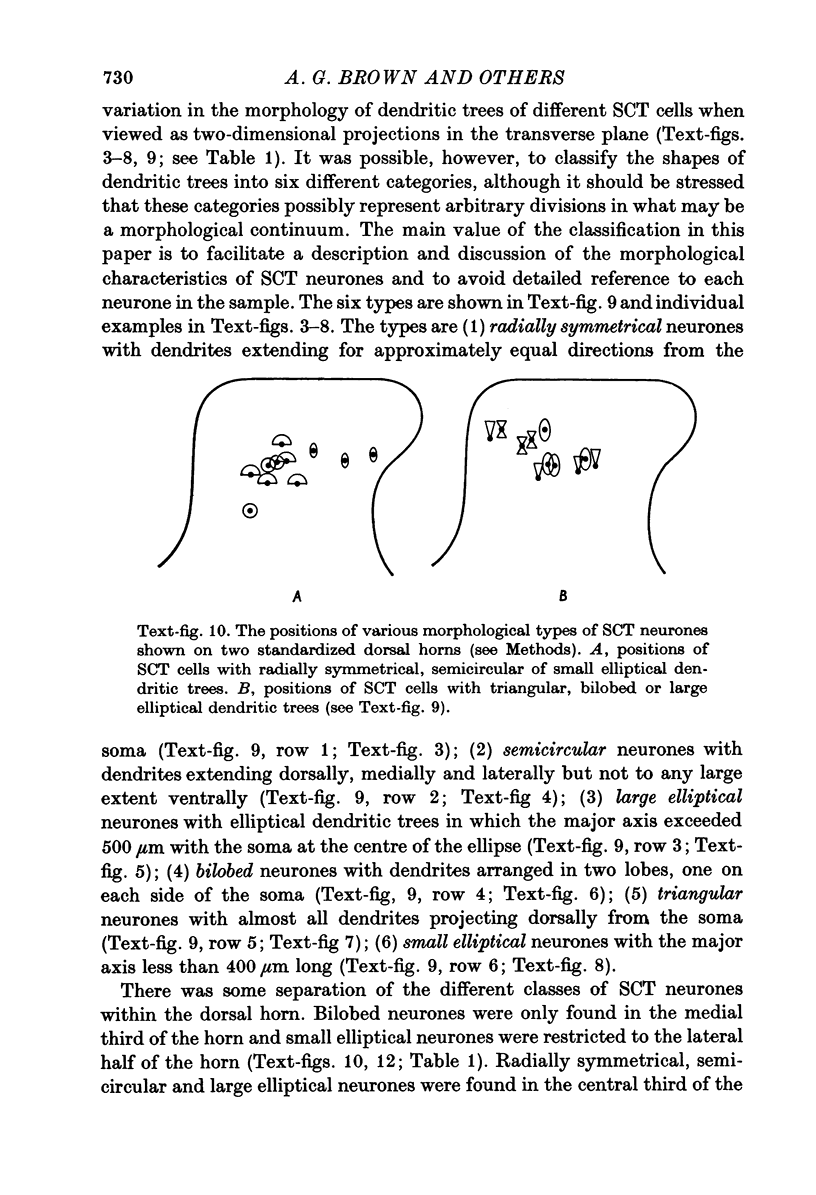
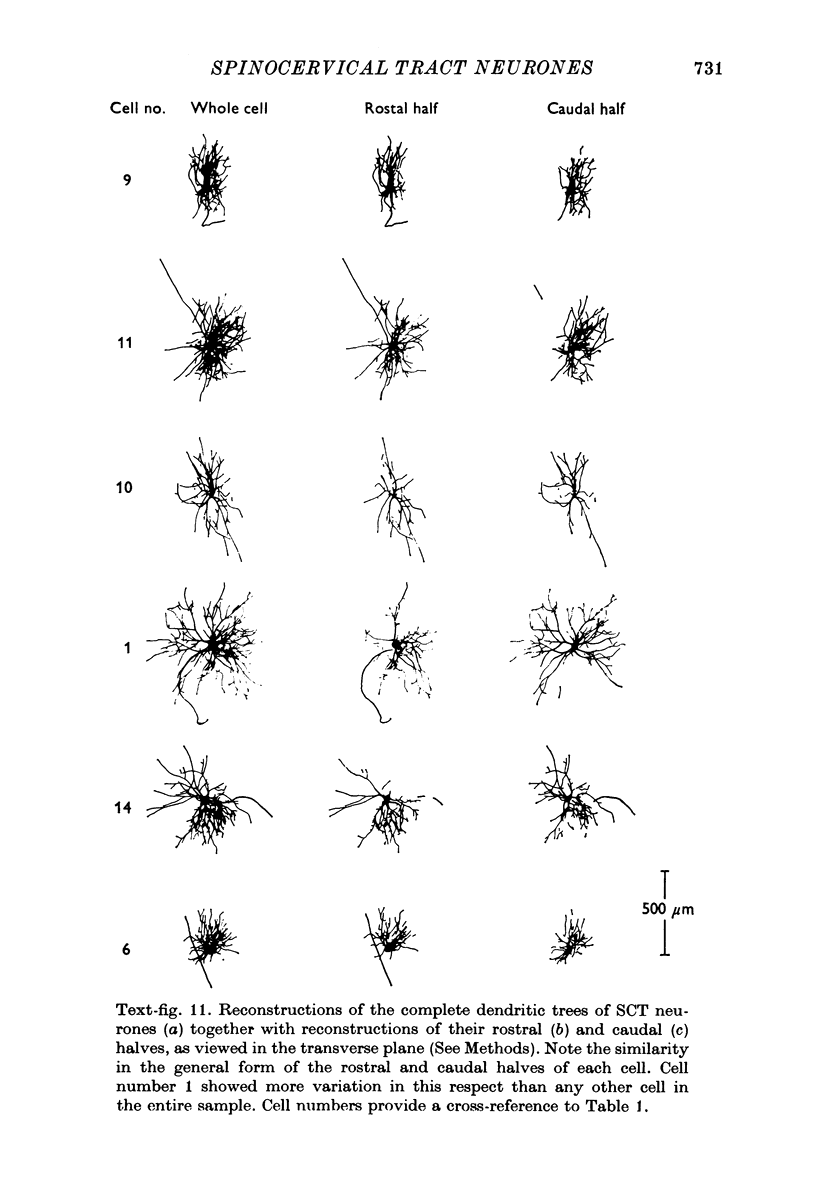
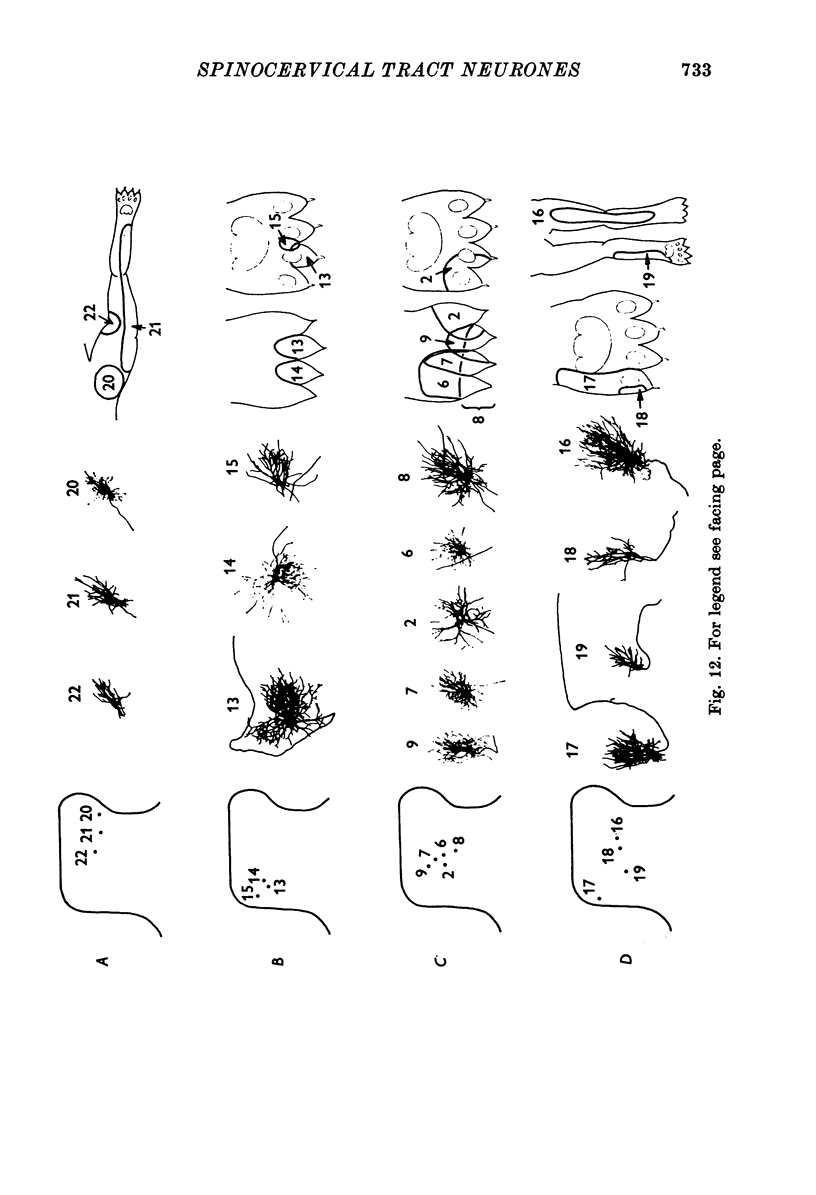
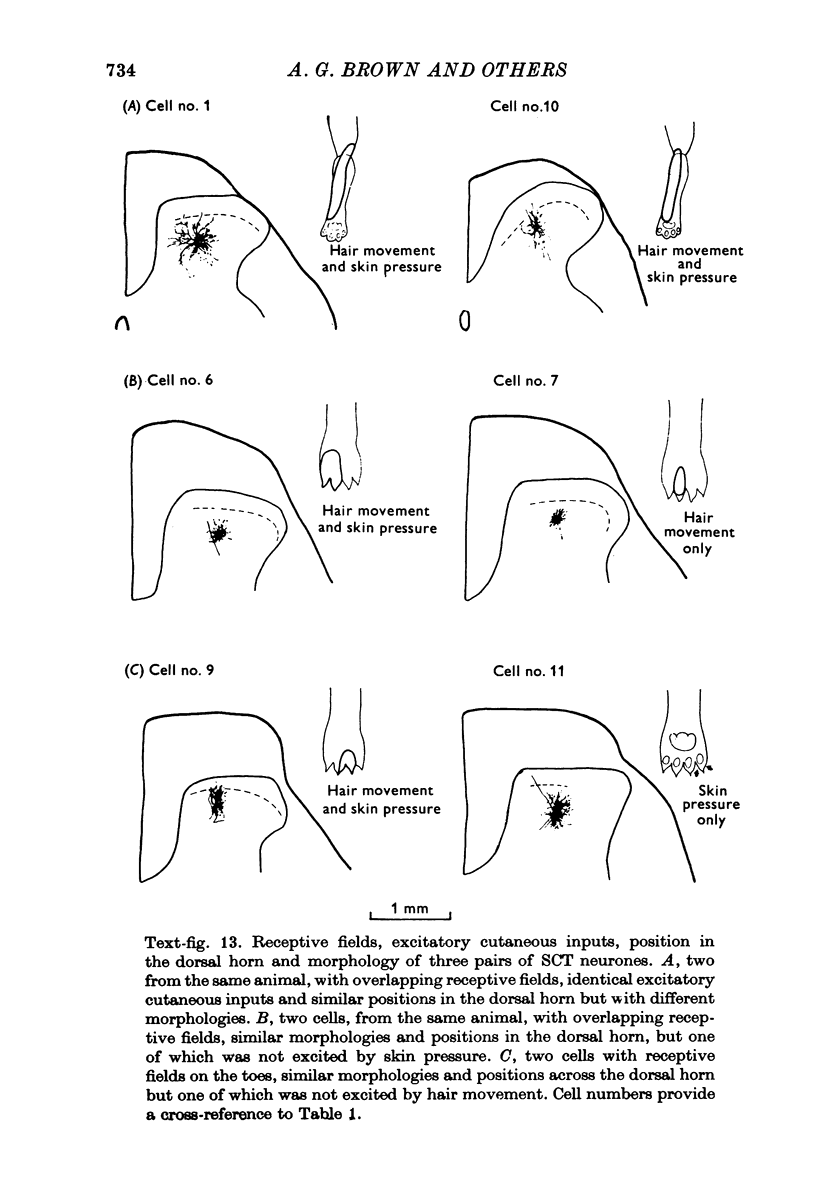
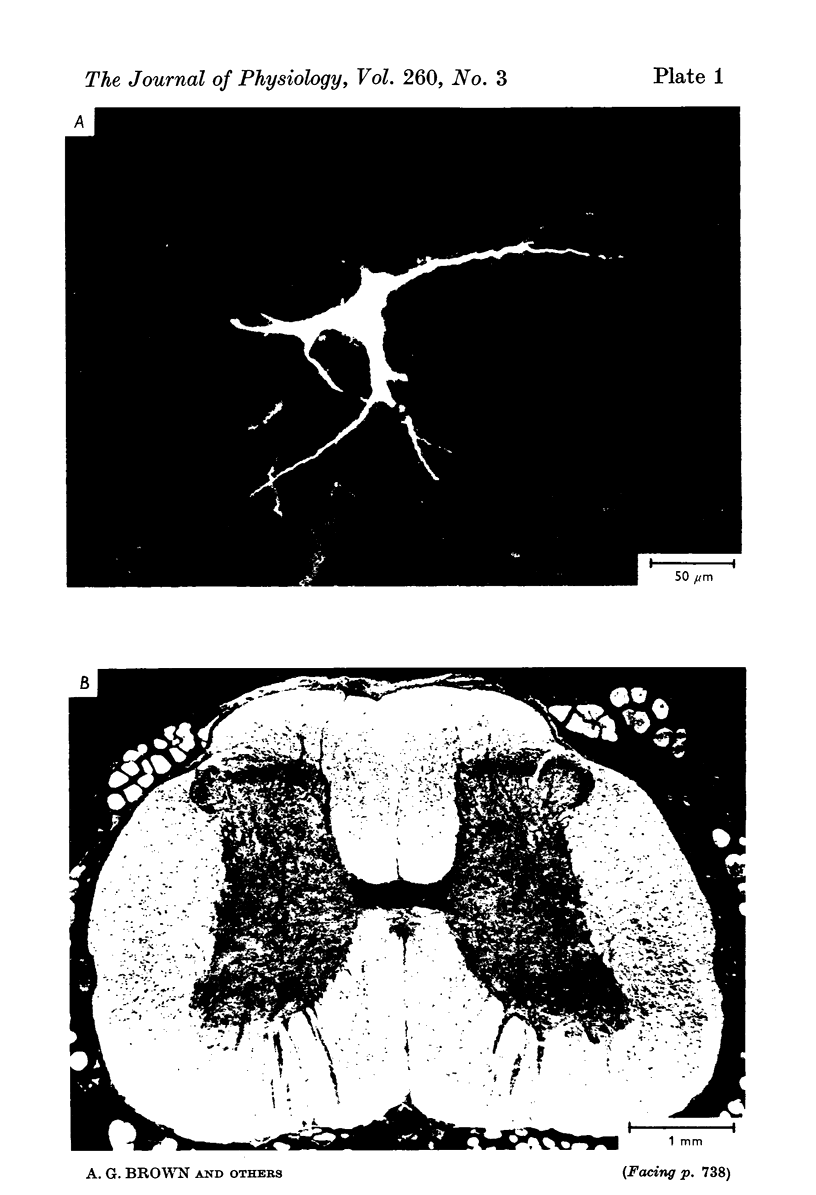
1. The morphology of physiologically identified spinocervical tract (SCT) neurones was studied using the intracellular injection of Procion dyes in anesthetized and decerebrate cats. 2. Extracellular recordings were made from SCT neurones at depths between 1000 and 2850 mum from the cord surface but neurones were only stained at depths between 1100 and 2400 mum. 3. The dendritic trees of stained SCT neurones were reconstructed in the transverse plane of the spinal cord. All SCT neurones had well developed dorsal dendrites but despite this it is not possible to consider the twenty-two SCT cells in out sample as consituting a morphologically homogenous population. 4. There was no correlation between the form of the dendritic trees and the depth of SCT neurones in the dorsal horn as determined both from measurements from the dorsal grey-white border and the position of cells with respect to the border between Rexed's laminae II and III. 5. Six types of SCT neurones were identified on the basis of the form of their dendritic trees as viewed in the transverse plane: (1) radially symmetrical, (2) semicircular, (3) large elliptical, (4) bilobed, (5) triangular, (6) small elliptical. Each of these types was found only in a certain region across the dorsal horn although any one region could contain more than one type. 6. Spinocervical tract neurones with small elliptical dendritic trees always had receptive fields encompassing part of the hip or thigh and were unique in being located in the lateral portions of the horn. 7. There was no correlation between the morphology of SCT neurones and their excitatory cutaneous inputs, receptive field size, axonal conduction velocity or depth in the dorsal horn.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown A. G., House C. R., Hume R. B. Physiology and morphology of identified spinal cord neurones. J Physiol. 1975 Jan;244(1):10P–12P. [PubMed] [Google Scholar]
- Brown A. G., House C. R., Rose P. K., Snow P. J. Proceedings: The cells of origin of the spinocervical tract. J Physiol. 1975 Jul;249(1):65P–66P. [PubMed] [Google Scholar]
- Brown P. B., Fuchs J. L. Somatotopic representation of hindlimb skin in cat dorsal horn. J Neurophysiol. 1975 Jan;38(1):1–9. doi: 10.1152/jn.1975.38.1.1. [DOI] [PubMed] [Google Scholar]
- Bryan R. N., Trevino D. L., Coulter J. D., Willis W. D. Location and somatotopic organization of the cells of origin of the spino-cervical tract. Exp Brain Res. 1973 Apr 30;17(2):177–189. doi: 10.1007/BF00235027. [DOI] [PubMed] [Google Scholar]
- Clark R., Ramsey R. L. A stereotaxic animal frame with stepping motor-driven micro-manipulator. J Physiol. 1975 Jan;244(1):5P–7P. [PubMed] [Google Scholar]
- Hongo T., Jankowska E., Lundberg A. Post-synaptic excitation and inhibition from primary afferents in neurones of the spinocervical tract. J Physiol. 1968 Dec;199(3):569–592. doi: 10.1113/jphysiol.1968.sp008669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E., Lindström S. Morphology of interneurones mediating Ia reciprocal inhibition of motoneurones in the spinal cord of the cat. J Physiol. 1972 Nov;226(3):805–823. doi: 10.1113/jphysiol.1972.sp010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REXED B. A cytoarchitectonic atlas of the spinal cord in the cat. J Comp Neurol. 1954 Apr;100(2):297–379. doi: 10.1002/cne.901000205. [DOI] [PubMed] [Google Scholar]
- REXED B. SOME ASPECTS OF THE CYTOARCHITECTONICS AND SYNAPTOLOGY OF THE SPINAL CORD. Prog Brain Res. 1964;11:58–92. doi: 10.1016/s0079-6123(08)64044-3. [DOI] [PubMed] [Google Scholar]
- Ralston H. J., 3rd The fine structure of neurons in the dorsal horn of the cat spinal cord. J Comp Neurol. 1968 Feb;132(2):275–302. doi: 10.1002/cne.901320205. [DOI] [PubMed] [Google Scholar]
- Ralston H. J., 3rd The organization of the substantia gelatinosa rolandi in the cat lumbosacral spinal cord. Z Zellforsch Mikrosk Anat. 1965 Jul 5;67(1):1–23. doi: 10.1007/BF00339273. [DOI] [PubMed] [Google Scholar]
- SZENTAGOTHAI J. NEURONAL AND SYNAPTIC ARRANGEMENT IN THE SUBSTANTIA GELATINOSA ROLANDI. J Comp Neurol. 1964 Apr;122:219–239. doi: 10.1002/cne.901220207. [DOI] [PubMed] [Google Scholar]
- Scheibel M. E., Scheibel A. B. Terminal axonal patterns in cat spinal cord. II. The dorsal horn. Brain Res. 1968 Jun;9(1):32–58. doi: 10.1016/0006-8993(68)90256-4. [DOI] [PubMed] [Google Scholar]
- Stretton A. O., Kravitz E. A. Neuronal geometry: determination with a technique of intracellular dye injection. Science. 1968 Oct 4;162(3849):132–134. doi: 10.1126/science.162.3849.132. [DOI] [PubMed] [Google Scholar]
- Van Essen D., Kelly J. Correlation of cell shape and function in the visual cortex of the cat. Nature. 1973 Feb 9;241(5389):403–405. doi: 10.1038/241403a0. [DOI] [PubMed] [Google Scholar]