Abstract
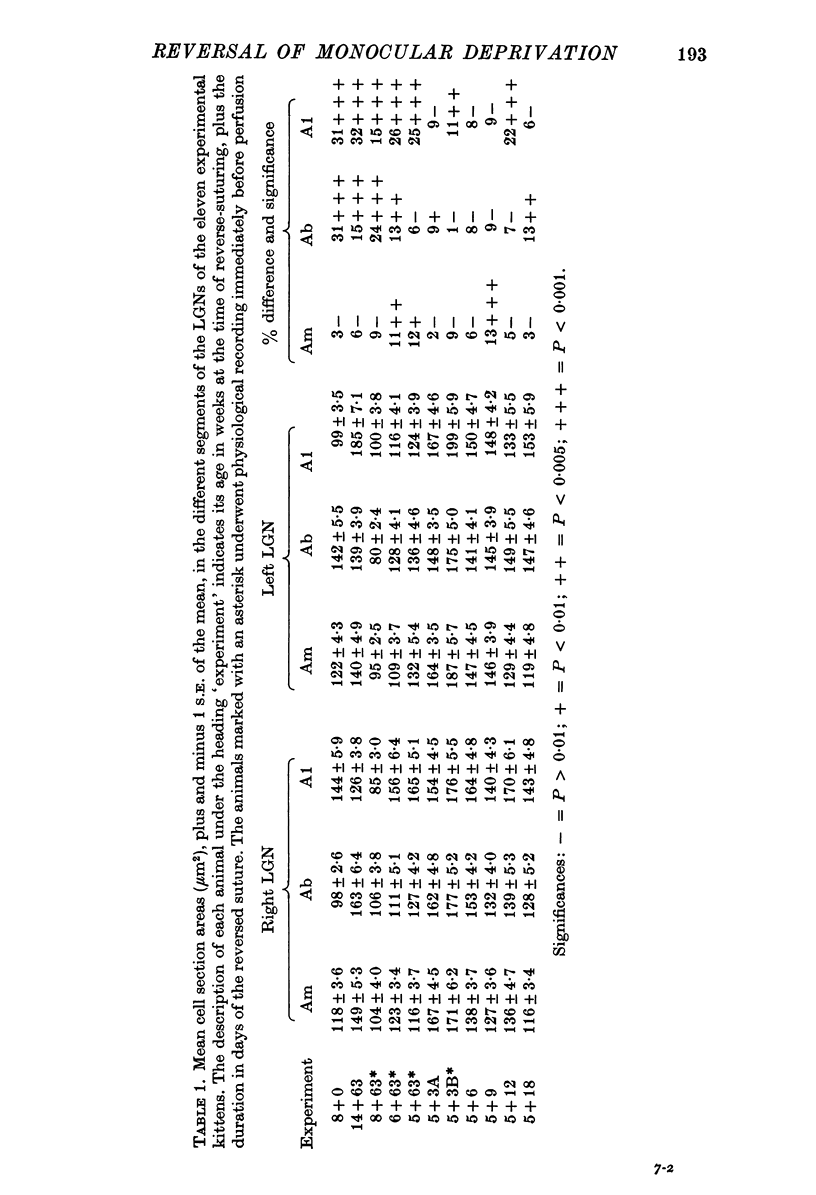
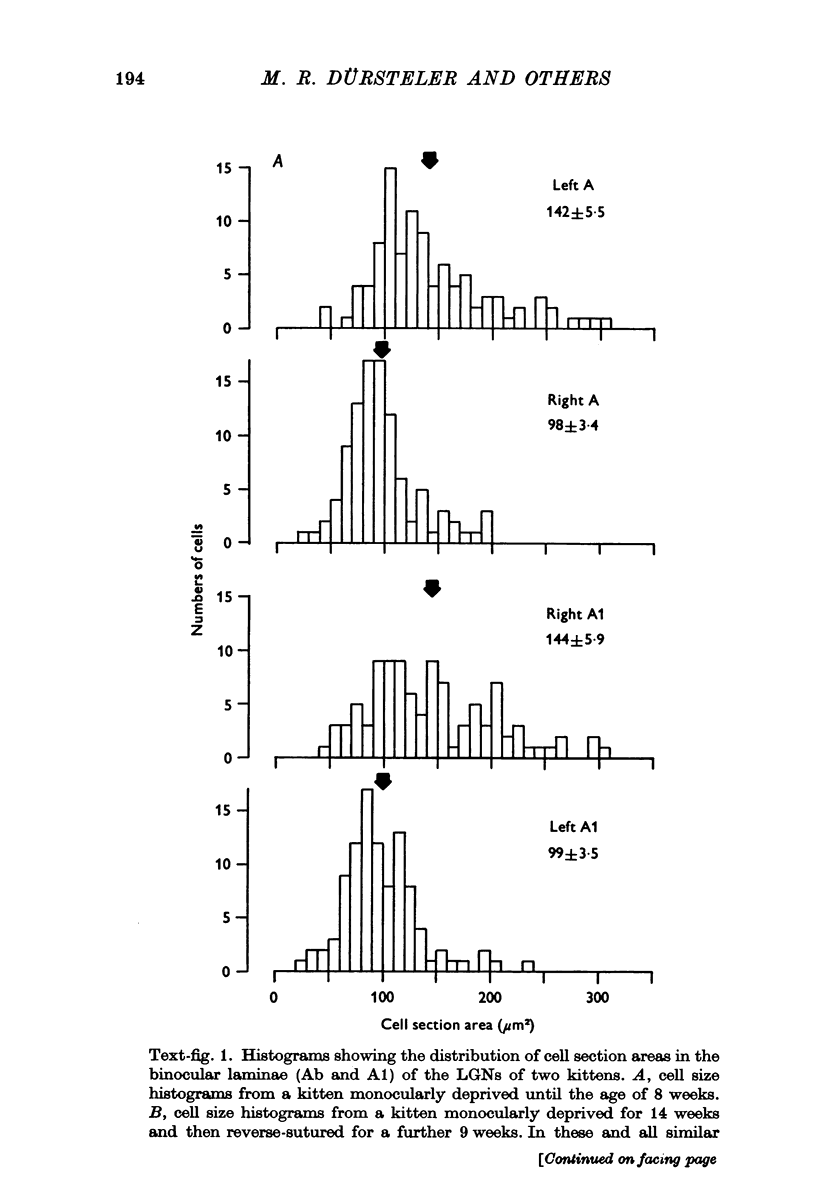
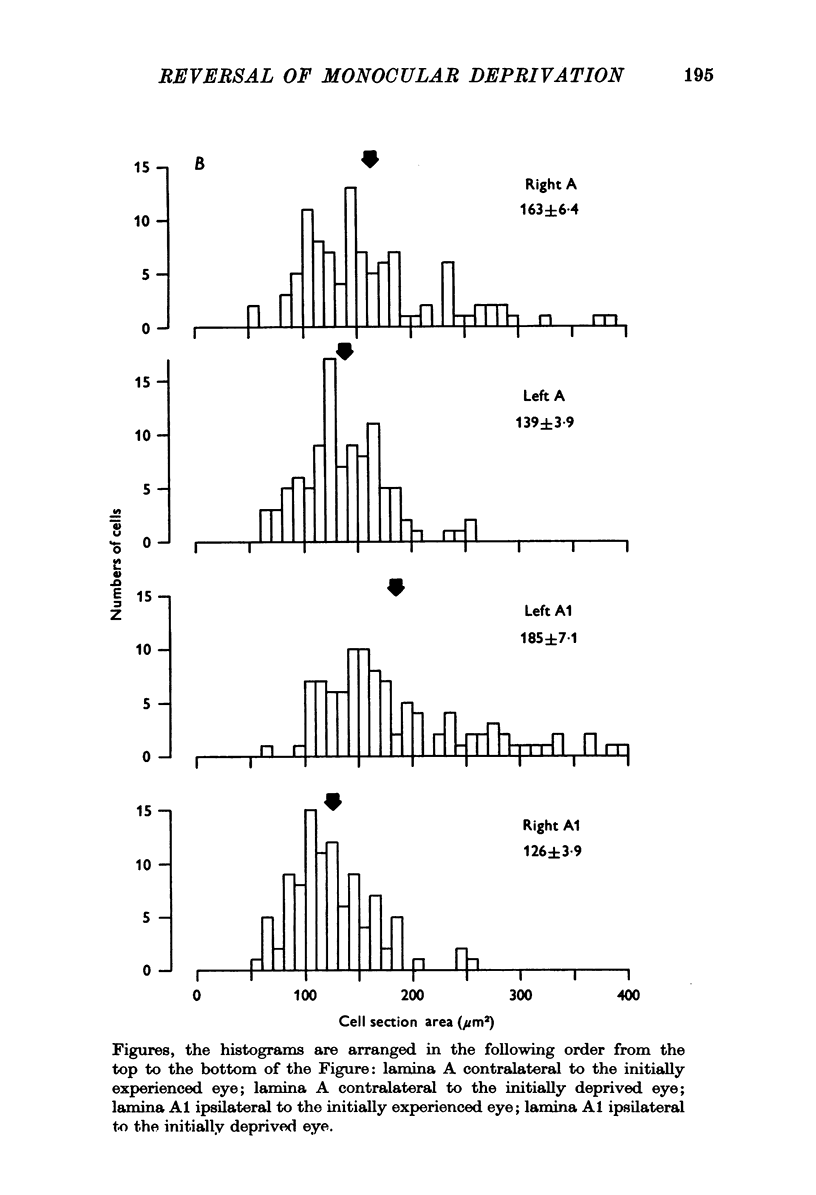
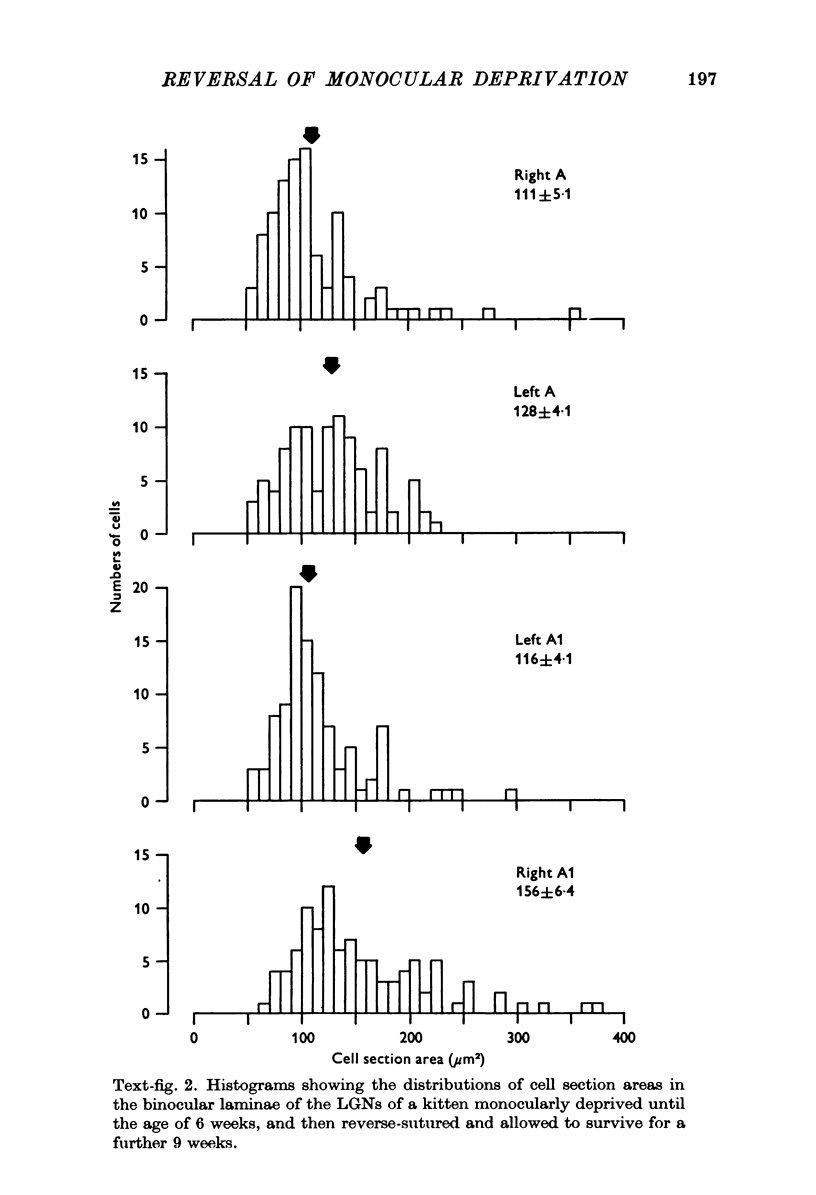
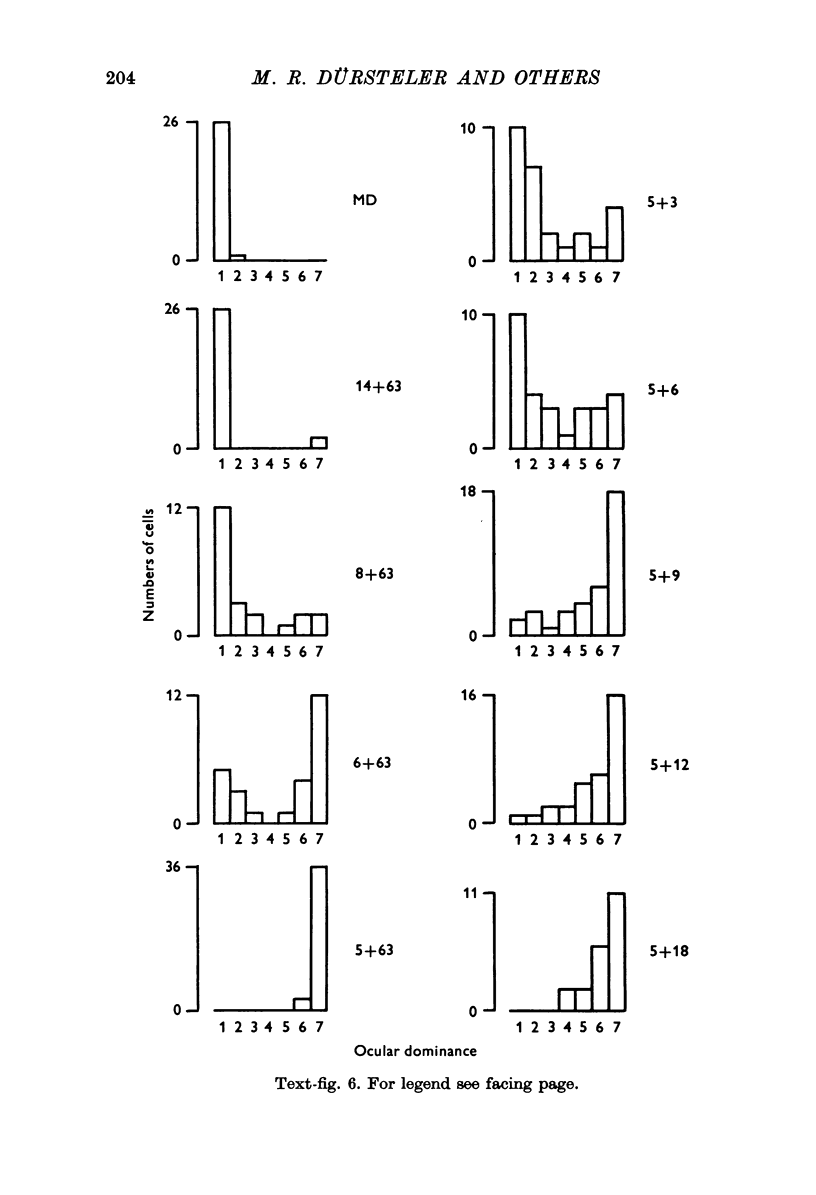
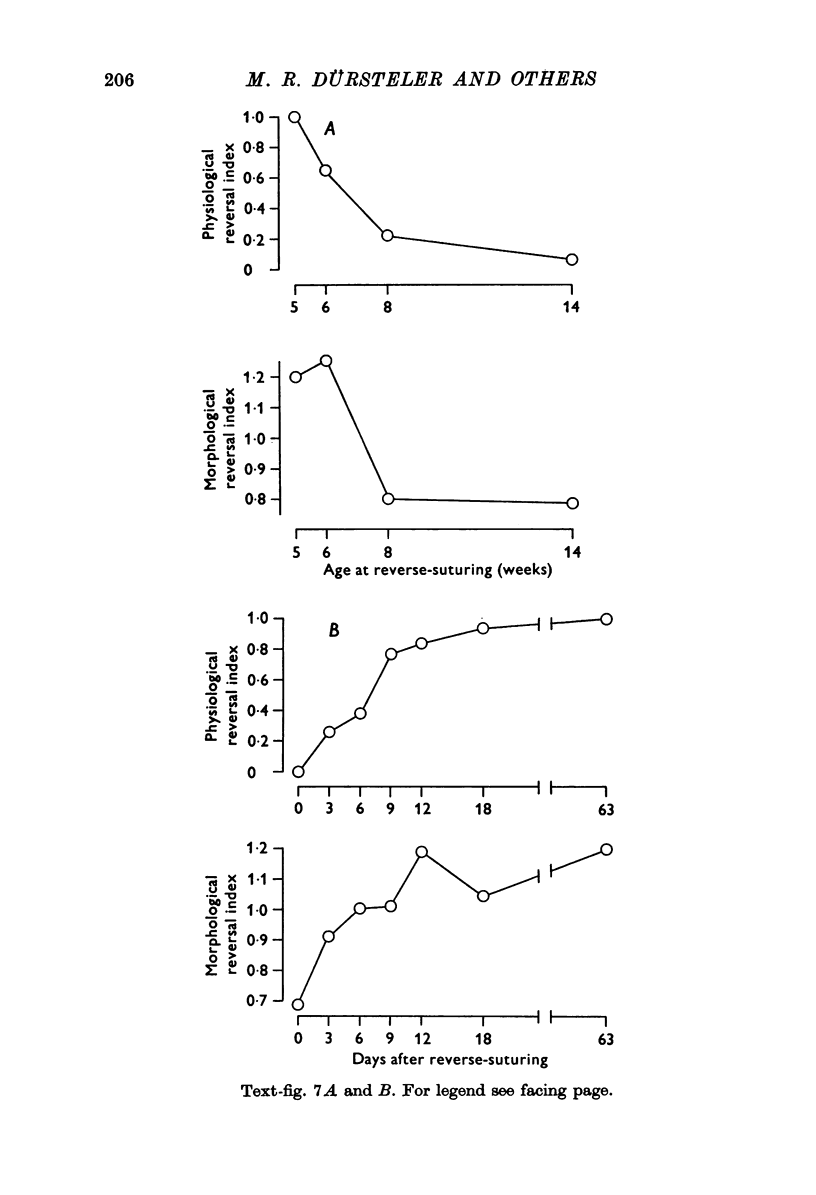
1. Eleven kittens were deprived of vision in one eye until the age of between 5 and 14 weeks. Their eyes were then reverse-sutured, they were allowed to survive for a further 3-63 days, and their brains were then examined histologically. 2. Measurement of the cross-sectional area of cells in the lateral geniculate nucleus (LGN) showed that when the reversal of lid suture was performed at the age of 8 or 14 weeks, the mean cell size was smaller in laminae connected to the initially closed right eye than it was in other laminae. 3. When the reversal of lid suture took place at 5 or 6 weeks of age there was a reversal of interlaminar size differences: the initially deprived eye was then connected to laminae containing larger cells. Even within 3 days after the reversal of lid suture, most of the morphological effects of the initial suture had been abolished, and they were fully reversed within 12 days. 4. These results are compared with physiological changes in the visual cortex of these and similarly reared animals.
Full text
PDF




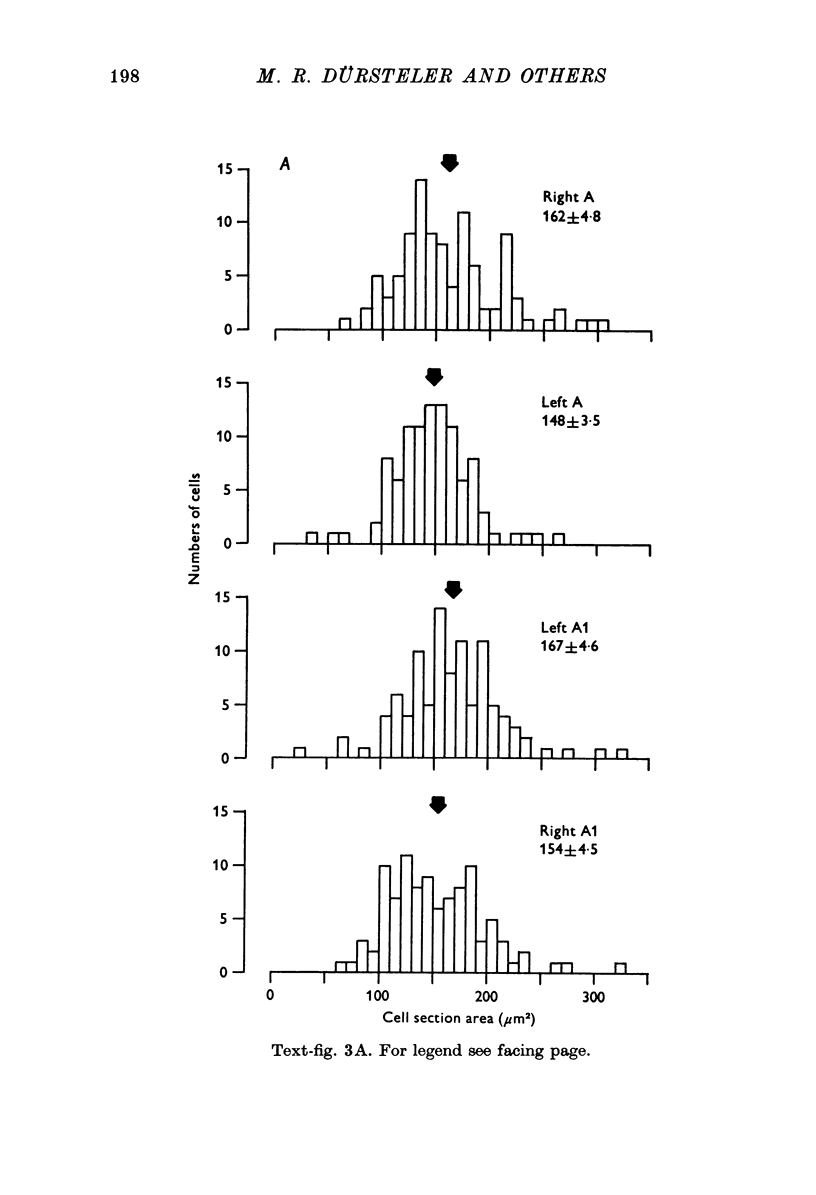
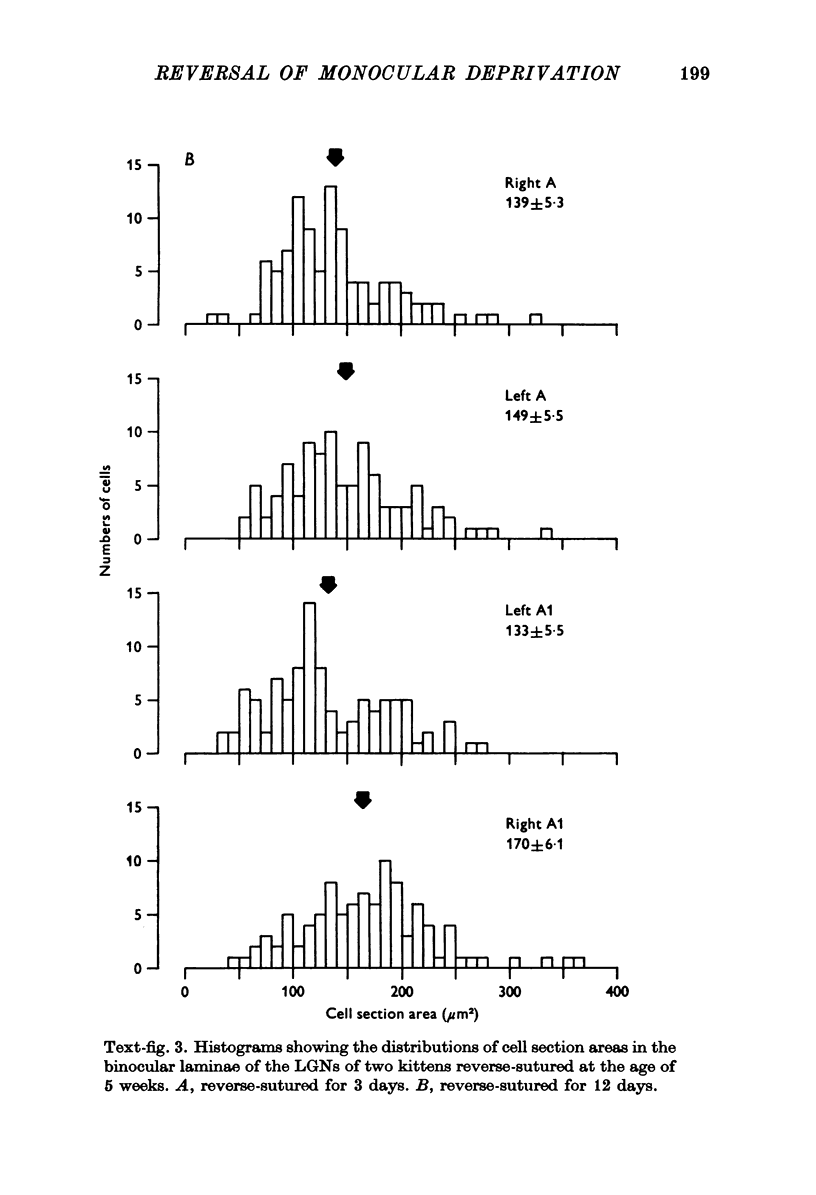
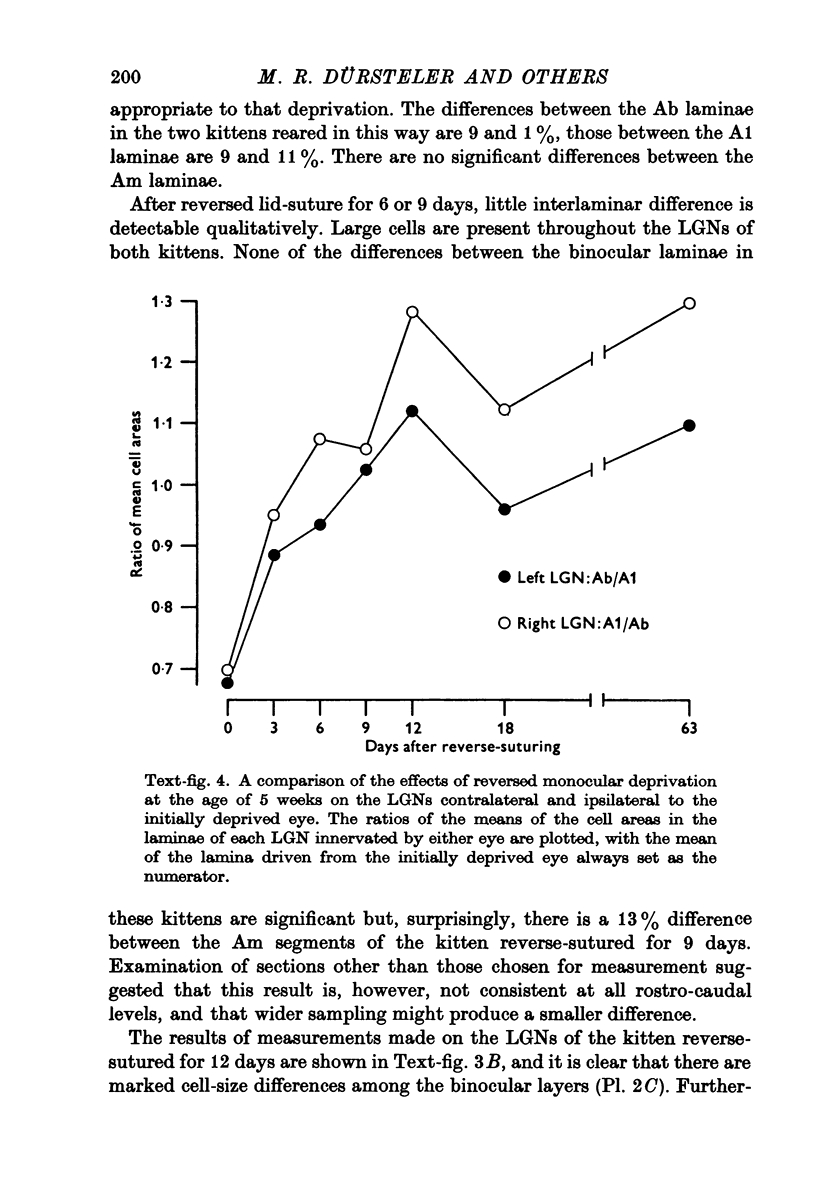
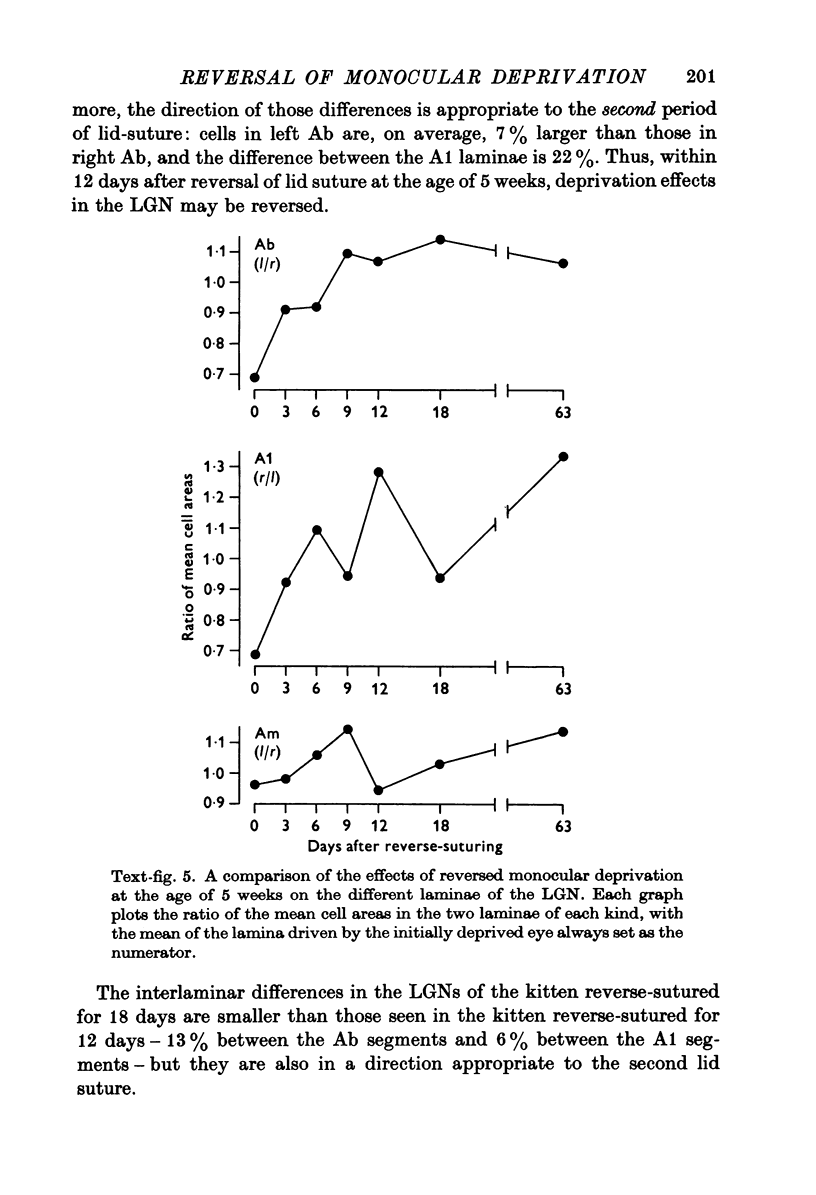







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BISHOP P. O., KOZAK W., LEVICK W. R., VAKKUR G. J. The determination of the projection of the visual field on to the lateral geniculate nucleus in the cat. J Physiol. 1962 Oct;163:503–539. doi: 10.1113/jphysiol.1962.sp006991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore C., Van Sluyters R. C. Reversal of the physiological effects of monocular deprivation in kittens: further evidence for a sensitive period. J Physiol. 1974 Feb;237(1):195–216. doi: 10.1113/jphysiol.1974.sp010478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COOK W. H., WALKER J. H., BARR M. L. A cytological study of transneuronal atrophy in the cat and rabbit. J Comp Neurol. 1951 Apr;94(2):267–291. doi: 10.1002/cne.900940207. [DOI] [PubMed] [Google Scholar]
- Chow K. L., Stewart D. L. Reversal of structural and functional effects of long-term visual deprivation in cats. Exp Neurol. 1972 Mar;34(3):409–433. doi: 10.1016/0014-4886(72)90038-6. [DOI] [PubMed] [Google Scholar]
- Cragg B. G. The development of synapses in the visual system of the cat. J Comp Neurol. 1975 Mar 15;160(2):147–166. doi: 10.1002/cne.901600202. [DOI] [PubMed] [Google Scholar]
- Freeman D. N., Marg E. Visual acuity development coincides with the sensitive period in kittens. Nature. 1975 Apr 17;254(5501):614–615. doi: 10.1038/254614a0. [DOI] [PubMed] [Google Scholar]
- Garey L. J., Fisken R. A., Powell T. P. Effects of experimental deafferentation on cells in the lateral geniculate nucleus of the cat. Brain Res. 1973 Mar 30;52:363–369. doi: 10.1016/0006-8993(73)90672-0. [DOI] [PubMed] [Google Scholar]
- Garey L. J., Fisken R. A., Powell T. P. Observations on the growth of cell sin the lateral geniculate nucleus of the cat. Brain Res. 1973 Mar 30;52:359–362. doi: 10.1016/0006-8993(73)90671-9. [DOI] [PubMed] [Google Scholar]
- Garey L. J., Jones E. G., Powell T. P. Interrelationships of striate and extrastriate cortex with the primary relay sites of the visual pathway. J Neurol Neurosurg Psychiatry. 1968 Apr;31(2):135–157. doi: 10.1136/jnnp.31.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garey L. J., Powell T. P. The projection of the retina in the cat. J Anat. 1968 Jan;102(Pt 2):189–222. [PMC free article] [PubMed] [Google Scholar]
- Guillery R. W. Binocular competition in the control of geniculate cell growth. J Comp Neurol. 1972 Jan;144(1):117–129. doi: 10.1002/cne.901440106. [DOI] [PubMed] [Google Scholar]
- Guillery R. W. Patterns of fiber degeneration in the dorsal lateral geniculate nucleus of the cat following lesions in the visual cortex. J Comp Neurol. 1967 Jul;130(3):197–221. doi: 10.1002/cne.901300303. [DOI] [PubMed] [Google Scholar]
- Guillery R. W., Stelzner D. J. The differential effects of unilateral lid closure upon the monocular and binocular segments of the dorsal lateral geniculate nucleus in the cat. J Comp Neurol. 1970 Aug;139(4):413–421. doi: 10.1002/cne.901390403. [DOI] [PubMed] [Google Scholar]
- Guillery R. W. Survival of large cells in the dorsal lateral geniculate laminae after interruption of retinogeniculate afferents. Brain Res. 1971 May 21;28(3):541–544. doi: 10.1016/0006-8993(71)90062-x. [DOI] [PubMed] [Google Scholar]
- Guillery R. W. The effect of lid suture upon the growth of cells in the dorsal lateral geniculate nucleus of kittens. J Comp Neurol. 1973 Apr 15;148(4):417–422. doi: 10.1002/cne.901480402. [DOI] [PubMed] [Google Scholar]
- HAYHOW W. R. The cytoarchitecture of the lateral geniculate body in the cat in relation to the distribution of crossed and uncrossed optic fibers. J Comp Neurol. 1958 Aug;110(1):1–63. doi: 10.1002/cne.901100102. [DOI] [PubMed] [Google Scholar]
- Hubel D. H., Wiesel T. N. Binocular interaction in striate cortex of kittens reared with artificial squint. J Neurophysiol. 1965 Nov;28(6):1041–1059. doi: 10.1152/jn.1965.28.6.1041. [DOI] [PubMed] [Google Scholar]
- Hubel D. H., Wiesel T. N. The period of susceptibility to the physiological effects of unilateral eye closure in kittens. J Physiol. 1970 Feb;206(2):419–436. doi: 10.1113/jphysiol.1970.sp009022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUPFER C., PALMER P. LATERAL GENICULATE NUCLEUS: HISTOLOGICAL AND CYTOCHEMICAL CHANGES FOLLOWING AFFERENT DENERVATION AND VISUAL DEPRIVATION. Exp Neurol. 1964 May;9:400–409. doi: 10.1016/0014-4886(64)90074-3. [DOI] [PubMed] [Google Scholar]
- LaVail J. H., LaVail M. M. Retrograde axonal transport in the central nervous system. Science. 1972 Jun 30;176(4042):1416–1417. doi: 10.1126/science.176.4042.1416. [DOI] [PubMed] [Google Scholar]
- Mitchell D. E., Giffin F., Wilkinson F., Anderson P., Smith M. L. Visual resolution in young kittens. Vision Res. 1976;16(4):363–366. doi: 10.1016/0042-6989(76)90197-8. [DOI] [PubMed] [Google Scholar]
- Movshon J. A., Blakemore C. Functional reinnervation in kitten visual cortex. Nature. 1974 Oct 11;251(5475):504–505. doi: 10.1038/251504a0. [DOI] [PubMed] [Google Scholar]
- Movshon J. A. Reversal of the behavioural effects of monocular deprivation in the kitten. J Physiol. 1976 Sep;261(1):175–187. doi: 10.1113/jphysiol.1976.sp011552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Movshon J. A. Reversal of the physiological effects of monocular deprivation in the kitten's visual cortex. J Physiol. 1976 Sep;261(1):125–174. doi: 10.1113/jphysiol.1976.sp011551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson K. J. The projection of the visual field to the lateral geniculate and medial interlaminar nuclei in the cat. J Comp Neurol. 1971 Sep;143(1):101–108. doi: 10.1002/cne.901430107. [DOI] [PubMed] [Google Scholar]
- Sherman S. M., Guillery R. W., Kaas J. H., Sanderson K. J. Behavioral, electrophysiological and morphological studies of binocular competition in the development of the geniculo-cortical pathways of cats. J Comp Neurol. 1974 Nov 1;158(1):1–18. doi: 10.1002/cne.901580102. [DOI] [PubMed] [Google Scholar]
- Stent G. S. A physiological mechanism for Hebb's postulate of learning. Proc Natl Acad Sci U S A. 1973 Apr;70(4):997–1001. doi: 10.1073/pnas.70.4.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeckel K., Thoenen H. Retrograde axonal transport of nerve growth factor: specificity and biological importance. Brain Res. 1975 Feb 28;85(2):337–341. doi: 10.1016/0006-8993(75)90092-x. [DOI] [PubMed] [Google Scholar]
- WIESEL T. N., HUBEL D. H. EFFECTS OF VISUAL DEPRIVATION ON MORPHOLOGY AND PHYSIOLOGY OF CELLS IN THE CATS LATERAL GENICULATE BODY. J Neurophysiol. 1963 Nov;26:978–993. doi: 10.1152/jn.1963.26.6.978. [DOI] [PubMed] [Google Scholar]
- Wiesel T. N., Hubel D. H. Extent of recovery from the effects of visual deprivation in kittens. J Neurophysiol. 1965 Nov;28(6):1060–1072. doi: 10.1152/jn.1965.28.6.1060. [DOI] [PubMed] [Google Scholar]




