Abstract
The causative agent of Johne's disease is Mycobacterium avium subsp. paratuberculosis. This is a chronic, debilitating gastrointestinal disorder that affects ruminants and is responsible for significant economic loss. The specimen processing method that combines C18-carboxypropylbetaine (CB-18) treatment and lytic enzyme decontamination has been shown to improve the diagnosis of mycobacterioses. This processing method was applied to the isolation of M. avium subsp. paratuberculosis from ruminant tissue samples. The BACTEC 12B liquid culture system was used but was supplemented with 1% egg yolk emulsion, 4 μg of mycobactin J, and 0.5% pyruvate (12B/EMP) for use in conjunction with this method. The final concentration of antibiotics used was 10 μg of vancomycin, 30 μg of amphotericin B, and 20 μg of nalidixic acid (VAN) per ml. A 7H10-based solid medium was also used that included mycobactin J, pyruvate, and VAN but excluded the egg yolk emulsion (7H10/MPV). Several M. avium subsp. paratuberculosis isolates were examined during the evaluation of this processing method. It was observed that treatment with lytic enzymes stimulated the growth of M. avium subsp. paratuberculosis; however, the growth of one isolate was markedly inhibited due to the presence of vancomycin. Subsequently, the vancomycin concentration in the VAN formulation was reduced to 2 μg/ml. A blinded panel of 60 previously characterized tissue samples from bovine and bison were then processed and analyzed by smear and culture. Historically, 31 and 37 specimens were classified as positive by histology and culture, respectively. The overall sensitivity and specificity of smear relative to culture following CB-18 processing were 97.6 and 89.5%, respectively. The 12B/EMP/VAN liquid culture system recovered M. avium subsp. paratuberculosis from 39 specimens, whereas 7H10/MPV and Herrold's egg yolk media recovered M. avium subsp. paratuberculosis from 26 and 16 specimens, respectively. The average times to positive were 7.4 ± 8.3, 29.9 ± 2.6, and 24 ± 0 days, respectively. The contamination rates were 4.8, 22.6, and 20.0%, respectively.
The etiologic agent of Johne's disease (enterocolitis) is Mycobacterium avium subsp. paratuberculosis (2). Johne's disease is characterized by weight loss and chronic diarrhea, as well as losses in productivity and fecundity. As a result, this disease causes significant economic loss in agrarian economies. Estimates in the United States suggest that the dairy industry loses approximately $222 million annually due to the impact of this single disease (18). One of the most significant hurdles in managing Johne's disease is that there is no rapid, accurate, and reliable test for the diagnosis of subclinically ill animals.
Serologic tests have reasonable sensitivity diagnosing cattle with clinical disease but have poor sensitivity overall. For example, the sensitivity of one commercial test was greater than 87% for animals with positive fecal cultures, but when subclinically ill, nonshedding animals were included in testing, the overall sensitivity dropped to 47% (4). Alternatively, application of a gamma interferon stimulation assay has shown greater promise: over 93% of subclinically ill, shedding animals could be detected, but only 72% of subclinically ill, nonshedding animals could be detected (1). Inhibitors in ruminant fecal material have dramatically hampered application of nucleic acid amplification tests for diagnosis (17). Definitive diagnosis is still considered isolation of viable bacilli by culture. Unfortunately, cultural isolation of M. avium subsp. paratuberculosis remains difficult for two reasons. The first reason is the fastidious nature and slow growth of this species (11, 19); cultures must be supplemented with mycobactin J and egg yolk emulsion and held for 16 weeks before being reported as negative. Second, contamination rates can be excessive due to the high concentration of contaminants in fecal material (16). This latter effect requires extreme decontamination measures (11).
Recently, a method for processing human respiratory specimens using the Zwitterionic detergent C18-carboxypropylbetaine (CB-18) was reported to improve the recovery of mycobacteria (12). The companion article also reported the development of a cocktail of lytic enzymes that contained lysozyme, Zymolyase, Cytophaga, and Trichoderma extracts (LZCT) to further combat contamination (13). As a first step toward applying the CB-18 processing and lytic enzyme decontamination methods to the detection of M. avium subsp. paratuberculosis, the present report evaluates the recovery of M. avium subsp. paratuberculosis from a blinded panel of historically characterized tissue specimens.
MATERIALS AND METHODS
Mycobacterial isolates .
Several different M. avium subsp. paratuberculosis isolates were used in these experiments. For in vitro evaluations the M. avium subsp. paratuberculosis type strain ATCC 19698 was used, as were the M. avium subsp. paratuberculosis isolates ATCC 19851 and ATCC 43015 (American Type Culture Collection, Rockville, Md.). All isolates were maintained on Herrold's egg yolk media (HEYM) supplemented with mycobactin J and pyruvate (Difco Laboratories, Detroit, Mich.).
Solid media.
Solid media used either HEYM slants or 7H10 slants or plates supplemented with 4 μg of mycobactin J (Allied Monitor, Fayette, Mo.), 0.5% pyruvate (Sigma Chemical Co., St. Louis, Mo.), and 2 μg of vancomycin, 30 μg of amphotericin B, and 20 μg of nalidixic acid (antibiotic mixture is called VAN) (all antibiotics from Sigma Chemical Co.) per ml. The combination of VAN with the preceding substances is termed 7H10/MPV. 7H10/MPV plates were made in house using 7H10 powder and oleic acid-albumin-dextrose-catalase supplement according to the instructions of the manufacturer (Becton Dickinson, Cockeysville, Md.).
Liquid culture.
Liquid cultures used the BACTEC 12B/460TB system (Becton Dickinson). 12B cultures were supplemented with 300 μl of a solution containing egg yolk emulsion (Remel, Lenexa, Kans.), mycobactin J, and pyruvate (the mixture is termed EMP) and antibiotics. While the EMP/antibiotic supplement was made as a large stock for addition to multiple 12B vials, the ratio of the components in one 300-μl portion was 100 μl of 50% egg yolk, 100 μl of 12B-VAN antibiotics (see below), 90 μl of 25% pyruvate, and 10 μl of mycobactin J at 400 μg/ml in 75% ethanol. Therefore, the final concentration or amount of each added component in a 12B culture vial after inoculation was approximately 1% egg yolk, 4 μg of mycobactin J, 0.5% pyruvate, and VAN. The 12B-VAN antibiotic supplement was made as a concentrate and stored frozen at −20°C until use. Immediately prior to use a 100-μl aliquot was thawed and 1 ml of standard PANTA reconstitution fluid (RF) (Becton Dickinson) was directly added to the 12B-VAN stock. A 100-μl portion of the 12B-VAN cocktail was then mixed with a 200-μl portion of EMP and was added directly to 12B vials to achieve the desired final concentration. Where indicated, the individual antibiotic components were tested without the other antibiotics. In those instances, individual stocks were made and added to 12B vials in a similar manner. Where indicated, vancomycin was used at 10, 5, 2.5, and 1.25 μg/ml. In this instance the 10-μg/ml stock was serially diluted 1:1 in RF and was added to 12B vials in a manner similar to that described above.
Growth curves.
In all in vitro experiments where the growth of an isolate was characterized, a 0.5 MacFarland standard was initially prepared as previously described (13) and was diluted 20,000:1 in water for use. Four replicate cultures were inoculated for each test condition or supplement evaluated. Inoculations were typically 200 to 300 μl per 12B bottle. Unless indicated otherwise, all liquid cultures were supplemented with EMP. Where indicated, cultures were also supplemented with antibiotics and/or CB-18. Antibiotics were added as described above, whereas CB-18 was added in 100-μl portions in 1:50 isopropanol:water to a final concentration of 20 μg/ml as previously described (13).
Cultures were monitored and analyzed as previously described (13, 14). A growth index (GI) greater than or equal to 15 was considered positive. Growth curves were plotted as the average of the four replicates and were analyzed using a two-tailed, heteroscedastic Student's t test. All positive cultures were subjected to Kinyoun staining to confirm the presence of acid-fast bacilli (AFB) using recommended procedures (10). In all experiments the identity of random samples was confirmed using the M. avium AccuProbe test (Gen-Probe, San Diego, Calif.), IS900 PCR, or biochemical analysis (10).
CB-18 processing of tissue specimens.
Tissue specimens (approximately 1 or 2 g) were cut at either the University of Pennsylvania School of Veterinary Medicine in Kennett Square, Pa. (UPenn), or at the U.S. Department of Agriculture—Animal Research Service, National Animal Disease Center (NADC) in Ames, Iowa, from frozen blocks of previously characterized samples and shipped frozen to the Quest Diagnostics facility in Baltimore, Md., where they were processed with CB-18 (as described below). Specimens were blinded throughout the duration of the study, but results were reported in real time.
Immediately prior to processing, specimens were thawed and macerated in disposable tissue grinders (Sage Products, Crystal Lakes, Ill.) and were then clarified using Spin X-II columns (Corning Science Products, Boston, Mass.) as previously described (15). Macerated specimens were processed with 1 mM CB-18 in Tris-citrate buffer (50 mM Tris-HCl, 12.5 mM citrate, pH 7.6, and 1.5 mM NaCl) containing 0.25% N-acetyl-l-cysteine (NALC) (wt/vol) using a protocol modified from that previously described (15). The Tris-citrate was formulated as a 20-fold stock as previously described, and CB-18 (Ecochem Research, Inc., Chaska, Minn.) was made as a 100 mM concentrate in 1:1 isopropanol:water as previously described (13). Immediately prior to use, the buffer was diluted 20:1, the CB-18 was diluted 100:1, and the NALC concentration was brought to 0.25% by the addition of dry powder.
Following addition of the CB-18 solution, specimens were immediately subjected to centrifugation at 3,800 × g for 20 min at 30°C. After centrifugation specimens were decanted and resuspended in 1.5 ml of sterile water. Four drops of the resuspended sediment was placed on a microscope slide for acid-fast staining using auramine-rhodamine according to the instructions of the manufacturer (Becton Dickinson). Smear values were reported according to guidelines recommended by the Centers for Disease Control and Prevention (10). Subsequently, 500 μl of sediment was transferred to a tube containing 500 μl of the lytic enzyme resuspension buffer previously described (i.e., 2× LZCT as follows: lysozyme at 2 mg/ml; lyticase at 1,000 U/ml; Cytophaga extract at 0.2 mg/ml; and Trichoderma extract at 2 mg/ml [all enzymes and extracts from Sigma Chemical Co.] in 100 mM Tris-HCl, 25 mM citrate, pH 7.6, 3 mM NaCl, 10 mM NALC, and 0.15% lecithin [13]). The sediment-LZCT mixture was incubated at 37°C for 20 min and was then used for inoculations. A 300-μl portion was first used to inoculate one 12B/EMP/VAN culture, and then duplicate 7H10/MPV plates and duplicate HEYM slants were each inoculated with 200-μl portions of the sediment. Liquid cultures were monitored every other day for 2 weeks and then once weekly for a total of 16 weeks. Solid media were examined weekly for a total of 16 weeks. The presence of AFB was confirmed in all positive cultures by Kinyoun staining, and the identity of all isolates was characterized using the M. avium AccuProbe test or IS900 PCR or both.
Previous characterization of tissues.
Previous characterizations of these tissues were performed at the UPenn facility, the NADC facility, or both. IS900 PCR analysis of isolates following CB-18 processing was performed at the UPenn facility.
The following criteria were used to evaluate tissues histologically by acid-fast staining: a tissue was deemed negative if no AFB were observed and + if very few AFB were observed, most of which were in the lamina propria. A tissue was considered ++ if a moderate number of AFB were scattered throughout lymph node tissues or in the lamina propria and mucosa of ileal tissues. A sample was judged to be +++ if a marked number of AFB were observed in the lamina propria, mucosa, and submucosa of ileal tissues and throughout lymph nodes with areas of granulomatous lesions.
Tissues were prepared for cultural analysis using the hexadecyl pyridinium chloride (HPC) double-incubation method (20; R. H. Whitlock, J. B. Bruce, P. A. Spencer, and L. T. Hutchinson, Abstr. 69th Ann. Meet. Conf. Res. Workers, abstr. 134, p. 24, 1988) and were inoculated on HEYM slants with mycobactin J in quadruplicate. Slants were held at 35 to 37°C for 16 weeks before being discarded. All positive cultures were first subjected to acid-fast staining to confirm the presence of AFB and were then subcultured onto HEYM slants with or without mycobactin J. All AFB-positive isolates were further characterized by IS900 PCR.
DNA extraction and PCR analysis.
Paraffin-embedded sections for each tissue processed by the NADC were cut at 5 μm. Two sections were cut per sample and placed into 1.5-ml sterile, RNase- and DNase-free microcentrifuge tubes. Tubes were wiped off to remove excess paraffin. Gloves and blades were changed between each pair of tissue blocks to reduce cross-contamination. Tubes were centrifuged at 16,000 × g for 1 min to pellet sections. Pellets were then resuspended in 200 μl of sterile 50 mM Tris buffer, pH 8.3, with 1 mM EDTA and 0.5% Tween 20. Tubes were placed in a boiling water bath for 10 min, followed by an ethanol bath in dry ice for 1 or 2 min. This step was repeated two times, and tubes were centrifuged at 3,000 × g for 20 min to pellet the tissue. Proteinase K (0.2 mg/ml) was added to each tube, and samples were incubated overnight at 42°C. Samples were then snap-frozen in a cold ethanol bath, followed by boiling to inactivate the proteinase K. Tubes were then centrifuged at 3,000 × g for 10 min to pellet debris. An equal volume of ProCipitate (LigoChem, Fairfield, N.J.) was added to the supernatant of the sample being extracted, and samples were mixed gently for 5 min, left for 1 min, and centrifuged for 2 min at 16,000 × g. Twenty-five microliters of 4 M LiCl was added to the supernatant, followed by 750 μl of cold 100% ethanol. Samples were mixed by inversion and placed in a −70°C freezer for 30 min to 1 h. Tubes were then centrifuged at 16,000 × g for 30 min. After decanting the ethanol, pellets were washed in cold 70% ethanol, followed by centrifugation at 16,000 × g for 15 min. Ethanol was then decanted from pellets, and tubes were inverted to dry for 15 min. DNA pellets were resuspended in 200 μl of Ultrapure RNase- and DNase-free H2O (Life Technologies, Inc., Rockville, Md.) and were amplified using the PCR amplification conditions previously described (7).
RESULTS
Growth curves.
Three M. avium subsp. paratuberculosis isolates (ATCC 19851, ATCC 19698, and ATCC 43015) were initially used to examine growth conditions in culture. Based on the work of others (3, 5, 6, 9, 21; C. B. Bartley, M. T. Sweeney, and M. R. Plaunt, Abstr. 98th Gen. Meet. Am. Soc. Microbiol., abstr. U-69, p. 507, 1998), various additives to the BACTEC 12B culture vials were initially evaluated individually and in combination for their ability to influence growth of these M. avium subsp. paratuberculosis isolates. These included the presence or absence of mycobactin J at 4 μg per 12B vial (≈ 1 μg/ml); pyruvate at a 0, 0.1, or 0.5% final concentration; egg yolk emulsion at a 0, 0.1, 1, 2.5, 5, or 10% final concentration; and the VAN antibiotic formulation. In general, these results were unremarkable and consistent with the results of others (data not shown). Therefore, the first-generation 12B supplement used in the studies herein incorporated 1% egg yolk emulsion (final), 4 μg of mycobactin J, 0.5% pyruvate (final), and the VAN antibiotics where vancomycin was added to a final concentration of 10 μg/ml. In addition, VAN was formulated to include the recommended concentration of PANTA RF typically used in the BACTEC 12B liquid culture system.
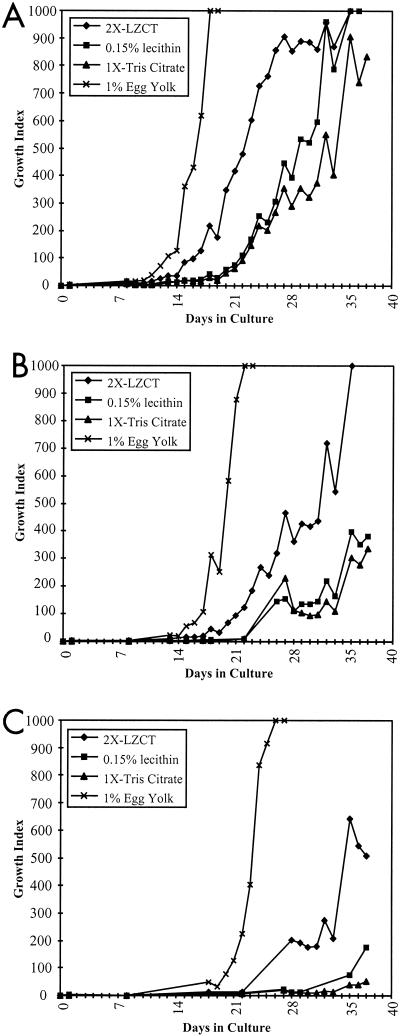
The lytic enzyme resuspension buffer developed for use in conjunction with the CB-18 processing method contains components not typically used in the isolation of M. avium subsp. paratuberculosis (12, 13). Therefore, the effects of these components on M. avium subsp. paratuberculosis growth were carefully evaluated. In these experiments a 0.5 MacFarland standard of each of the three ATCC isolates evaluated (19698, 19698, and 43015) was made and was then serially diluted in 10-fold increments to generate mock sediments. Final dilutions were made in Tris-citrate buffer (above), 2× LZCT resuspension buffer (13), or Tris-citrate buffer supplemented with 0.15% lecithin (i.e., the 2× LZCT resuspension buffer without the lytic enzymes). These mock sediments were then used to inoculate quadruplicate 12B vials; however, the buffer-only sediments were inoculated into 12B vials with or without 1% egg yolk. Each isolate was evaluated at dilutions of 100:1, 1,000:1, and 10,000:1. When using these experimental conditions the ATCC 43015 isolate grew very poorly (data not shown). Typical growth curves of the ATCC 19851 and ATCC 19698 isolates are shown in Fig. 1 (ATCC 19851 results shown). The inoculum used (i.e., the number of CFU inoculated into each 12B culture bottle) for this isolate was approximately 35,000 CFU (Fig. 1A), 3,500 CFU (Fig. 1B), and 350 CFU (Fig. 1C).
FIG. 1.
Growth curves of ATCC 19581 at three different input levels wherein bottles were supplemented with 4 μg of mycobactin J and either 2× LZCT, 0.15% lecithin, 1× Tris-citrate, or 1% egg yolk emulsion, as indicated. The numbers of input CFU were approximately 34,757 ± 12,010 CFU/bottle (A), 3,476 ± 1,201 CFU/bottle (B), and 348 ± 120 CFU/bottle (C). LZCT is the lytic enzyme decontamination mixture previously described (13).
When the 1% egg yolk was included as a component of the culture media, both the time-to-positive (GI ≥ 15) and the time-to-maximum GIs (GI = 999) were significantly different from all other conditions, regardless of the input (Fig. 1) for both ATCC 19851 and ATCC 19698. In 1% egg yolk both the ATCC 19851 and ATCC 19698 isolates reached the maximum GI within the evaluation period (i.e., 6 weeks) at all input levels. Interestingly, in all evaluations with these two isolates the 2× LZCT series provided more robust growth than either the Tris-citrate buffer without egg yolk or 0.15% lecithin series. In no instance were the 0.15% lecithin series results significantly different from the Tris-citrate buffer series results without egg yolk.
Analysis of VAN antibiotic supplement.
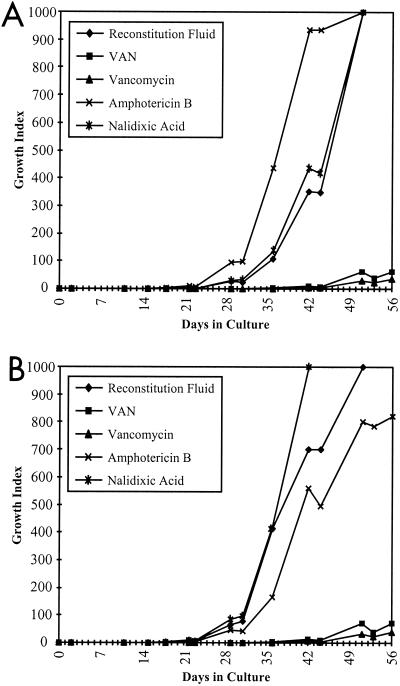
The inability to generate consistent growth curves with the ATCC 43015 isolate was examined. An evaluation of the individual components of the VAN supplement in the 12B/EMP culture system was undertaken (Fig. 2A). The differences in the time-to-positive and the time-to-maximum GIs between either VAN or vancomycin and the controls or other antibiotics (amphotericin B or nalidixic acid) were invariably significant. In contrast, the differences between the RF series, the amphotericin B series, and the nalidixic acid series, with respect to the time-to-positive and the time-to-maximum GIs, were not consistently significant between experiments, regardless as to whether CB-18 was present or not. The presence of CB-18 at 20 μg/ml did not hinder the growth of this isolate, nor did CB-18 act to enhance susceptibility to VAN, vancomycin, amphotericin B, or nalidixic acid (Fig. 2B). This was in contrast to observations reported with other mycobacteria (13). Since the VAN series results paralleled the results of the vancomycin series, it was concluded that the presence of vancomycin was solely responsible for the depressed growth patterns observed with the ATCC 43015 isolate.
FIG. 2.
Growth curves of ATCC 43015 wherein bottles were supplemented with 4 μg of mycobactin J, 1% egg yolk emulsion and RF, and either VAN, vancomycin, amphotericin B, or nalidixic acid as indicated. Bottles also contained either Tris-citrate buffer (A) or 20 μg of CB-18/ml (B).
Vancomycin titration.
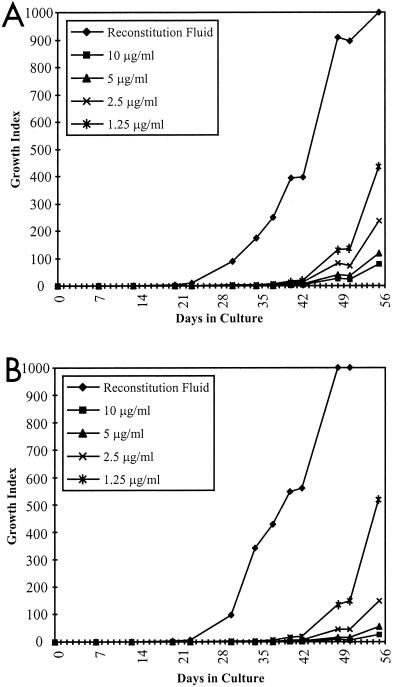
Because vancomycin is a potent antimicrobial and has potential value with respect to its ability to reduce cultural contamination rates, subsequent experiments serially reduced the vancomycin concentration by halves from 10 μg/ml to 1.25 μg/ml (Fig. 3). Even at 1.25 μg/ml, vancomycin significantly altered both the time-to-positive and the time-to-maximum GIs of the ATCC 43015 isolate relative to the control. For example, at 1.25 μg of vancomycin/ml, the time-to-positive was delayed by almost 3 weeks when compared to the time for the control. Because of the potential for vancomycin to impact contamination, subsequent studies involving the 12B/EMP/VAN culture system used vancomycin at 2 μg/ml.
FIG. 3.
Growth curves of ATCC 43015 wherein bottles were supplemented with 4 μg of mycobactin J, 1% egg yolk emulsion and RF, and vancomycin at either 10, 5, 2.5, or 1.25 μg/ml as indicated. All bottles were inoculated with approximately 285 ± 17 CFU/bottle with either Tris-citrate buffer (A) or 20 μg of CB-18/ml (B).
Processing tissue samples.
In order to evaluate the CB-18 processing method, a panel of 60 tissue samples that had been previously characterized by histology, culture, and IS900 PCR were processed with the CB-18 method. Specimens were processed in three sets. The first set consisted of 12 tissue specimens from both bison and bovine. These first 12 were analyzed by smear and culture using 12B/EMP/VAN and 7H10/MPV; however, the vancomycin concentration was 10 μg/ml, and these specimens were not inoculated onto HEYM slants. The second set consisted of 28 bison tissue specimens, and the third set consisted of 20 bovine tissue specimens. The second and third groups (Σ = 48) were also analyzed using 12B/EMP/VAN and 7H10/MPV; however, the vancomycin concentration was reduced to 2 μg/ml, and these specimens were also inoculated onto HEYM slants.
Of the 60 tissue samples submitted for processing with CB-18, a total of 28 were from bovine and 32 were from bison. Of the bovine tissues, 16 were derived from the ileum, 2 were derived from the jejunum, and 10 were lymph nodes (5 intercostal [IC] and 5 medial). Of the bison tissues submitted, a total of 27 were ileum while the remaining 5 were IC lymph nodes. The NADC analyzed all tissues by histology and IS900 PCR, and UPenn performed IS900 PCR analyses on the 32 bison tissues only. Processing and cultural analysis of bovine tissues were performed by the NADC, whereas processing and cultural analysis of bison tissues were performed by UPenn. Of the 60 specimens submitted for analysis, 31 (51.7%) were originally classified as AFB positive by histology, and 37 (61.7%) were reported as culture positive on HEYM slants following processing with HPC. Of the 60 specimens analyzed by IS900 PCR by the NADC, 40 (66.7%) were identified as positive. Of the 32 bison tissues analyzed by IS900 PCR by UPenn, 12 (37.5%) were reported as positive.
When processed by the CB-18 method, 42 processed sediments (70.0%) were positive by acid-fast staining using fluorochromes. Of these 42, 30 (71.4%) were also positive by histology. An additional 12 tissues that were negative upon histologic examination were positive by acid-fast staining following CB-18 processing. Eight of these 12 tissues were confirmed as positive by both culture and IS900 PCR (only one was previously culture positive), two were confirmed culture positive following processing with CB-18 (both were previously culture positive), and one was confirmed IS900 PCR positive by the NADC. Therefore, there was only one specimen that had no confirming result. This remaining positive smear was considered false positive, as this specimen was from a known negative control animal (see below). Only one specimen was smear negative following CB-18 processing but was positive for AFB by histology (this sample [IRT identification no. 10] is discussed below). Therefore, using any culture result as the “gold standard” (i.e., either HPC or CB-18), the sensitivity and specificity of smear following CB-18 specimen processing would be 97.6 and 89.5%, respectively.
When processed by the CB-18 method a total of 40 (66.7%) specimens were culture positive: 39 (62.9%) were culture positive using 12B/EMP/VAN, 26 (41.9%) were positive using 7H10/MPV, and 16 (33.3%) were positive using HEYM. The average times to positive (± standard deviation) for the three different media were 7.4 ± 8.3, 29.9 ± 2.6, and 24 ± 0 days, respectively. The median times to positive were 3.0, 31, and 24 days, respectively, and the ranges in times to positive were 1 to 40, 24 to 31, and 0 days, respectively. The contamination rates on the three different media were 5.0, 23.3, and 20.8%, respectively.
Six specimens were reported as 12B/EMP/VAN culture negative following CB-18 processing but were culture positive historically (Table 1). One of these specimens (IRT- identification no. 28) was positive on HEYM only following CB-18 processing (Table 2). In four of the five other discrepancies, there was at least one confirming IS900 PCR result, and in two of these five discrepancies, there were confirming histologic results. Further examination of these five discrepant specimens revealed the following: (i) the bison no. 9 ileum tissue (IRT identification no. 10) processed by UPenn presented with two colonies after 6 months of incubation; (ii) the bison no. 52 ileum tissue (IRT identification no. 35) processed by UPenn presented with one colony after 16 weeks of incubation; and (iii) the source of IRT identification no. 24 and IRT identification no. 38 was bison no. 40. While the false-negative results on the first two were most probably due to sampling error resulting from low tissue load in the samples tested, the bison no. 40 false-negative results appear to be due to the presence of pyruvate: recovery of M. avium subsp. paratuberculosis from other tissue sources from bison no. 40 (e.g., upper, lower, and midjejunal lymph node specimens) could be accomplished only if pyruvate was omitted from medium formulations (data not shown). The false-negative culture result from the IC lymph node of bison no. 50 (IRT identification no. 40) remains unexplained, as the smear of this specimen was 4+ and as all subsequent attempts to isolate M. avium subsp. paratuberculosis from this tissue or other tissues from this animal (n = 5) were successful. When additional samples of bison no. 9, no. 44, and no. 52 were processed with CB-18 and lytic enzymes, only the ileum of bison no. 52 generated culture-positive results.
TABLE 1.
Summary of culture-positive results
| CB-18-based culture medium | Comparison of culture results (no. of samples) for:
|
||||
|---|---|---|---|---|---|
| Total processed | Previous culture only | Previous culture and CB-18 culture | CB-18 culture only | CB-18 (% of total)c | |
| 12B/EMP/VANa | 60 | 6 | 31 | 8 | 86.7 |
| 7H10/MPVb | 60 | 12 | 25 | 1 | 68.4 |
| HEYM | 48 | 16 | 15 | 1 | 50 |
12B/EMP/VAN, BACTEC 12B liquid culture supplemented with 1% egg yolk emulsion, 4 μg of mycobactin J, 0.5% pyruvate, and VAN. Concentration of vancomycin was 10 or 2 μg/ml.\
7H10/MPV, 7H10-based solid media supplemented with oleic acid-albumin-dextrose-catalase, 4 μg of mycobactin J, 0.5% pyruvate, and VAN. Concentration of vancomycin was 10 or 2 μg/ml.\
These percentages represent the number of culture-positive results using the indicated media following CB-18 processing, divided by the total number of culture-positive results (i.e., including those specimens that were historically culture positive).
TABLE 2.
Discrepant specimens
| Discrepant specimen type | IRT identifi- cation no. | NADC identifi- cation no. | Source | Typeb | Previous characterizationa by:
|
CB-18 processing result by:
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histology | Culture | IS900 PCR
|
Smearc | 12B/EMPVANd | 7H10/MPVe | HEYMe | ||||||
| NADC | UPenn | |||||||||||
| Specimens missed by CB-18 | 10 | 9 | Bison | Ileum | + | ± | ± | + | Neg | Neg | Neg | ND |
| 24 | 40 | Bison | Ileum | Neg | + | Neg | Neg | Neg | Neg | Neg | Neg | |
| 28 | 44 | Bison | Ileum | Neg | + | + | ++ | Neg | Neg | Cont | +/Cont | |
| 35 | 52 | Bison | Ileum | Neg | ± | + | ++ | Neg | Neg | Cont | Neg | |
| 38 | 40 | Bison | LN (IC) | Neg | + | Neg | ± | Neg | Neg | Neg | Neg | |
| 40 | 50 | Bison | LN (IC) | + | + | + | ++ | 4+ | Neg | Neg | Neg | |
| Specimens missed previously | 16 | 31 | Bison | Ileum | Neg | Neg | + | Neg | ± | 12/Cont | Cont | + |
| 18 | 34 | Bison | Ileum | Neg | Neg | + | Neg | 1+ | 9 | Neg | Neg | |
| 19 | 35 | Bison | Ileum | Neg | Neg | + | Neg | 2+ | 7 | Neg | Neg | |
| 20 | 36 | Bison | Ileum | Neg | Neg | + | Neg | 2+ | 7 | Neg | Cont | |
| 27 | 43 | Bison | Ileum | Neg | Neg | + | Neg | Neg | 28 | Cont | Cont | |
| 29 | 46 | Bison | Ileum | Neg | Neg | + | Neg | ± | 12 | Neg | Cont | |
| 30 | 47 | Bison | Ileum | Neg | Neg | + | Neg | 1+ | 7 | Cont | Neg | |
| 31 | 48 | Bison | Ileum | Neg | Neg | ± | Neg | ± | 7 | + | Cont | |
LN (IC) = IC lymph node.\
See Materials and Methods for detailed definitions of + values for histologic results. For previous culture results: ± indicates fewer than 10 colonies recovered from this specimen; + indicates 10 to 75 colonies per slant recovered from this specimen; and ++ indicates that all slants had >75 colonies. For previous amplification results: ± indicates marginally positive or questionable result; + indicates moderately or obviously positive; ++ indicates a strongly positive result; and ND = not done.\
Smear values were reported as per the recommendations of Kent and Kubica (10).\
Numbers indicate time to positive (in days), and Cont indicates that the culture was lost to contamination. Neg, negative.\
“+” indicates that the culture was AFB positive; Cont indicates that the culture was lost to contamination; and ND = not done.
In contrast, there were eight specimens reported as culture positive following CB-18 processing that were historically culture negative (Table 2). Seven of these discrepancies were also smear positive following CB-18 processing, two of these discrepancies were also culture positive on one of the solid formulations following CB-18 processing, and in all eight instances there was a confirming IS900 PCR result from the NADC on these specimens.
The BACTEC 12B/EMP/VAN culture system was by far the most sensitive culture system. Of all culture-positive specimens following CB-18 processing, the 12B/EMP/VAN formulation missed only one specimen. In this one instance (IRT identification no. 28), only the HEYM slant was positive and the 7H10/MPV plate was contaminated. Subsequent analysis of this isolate revealed that this result was due primarily to susceptibility to vancomycin and also to nalidixic acid, albeit to a lesser degree. Growth curves using this isolate were similar to that observed for ATCC 43015 in Fig. 3A (data not shown).
When we compared the time-to-positive results of the tissue panel with the growth curves of the ATCC 19851 isolate, while bearing in mind that a positive smear requires approximately 10,000 AFB per ml (8, 22), the times to positive of the tissue specimens in the 12B/EMP/VAN culture system were in excellent agreement with the respective smear results (data not shown). For example, of the 37 specimens that were smear positive and culture positive in 12B/EMP/VAN, 21 were reported to have a smear value of 4+. The average time to detection in this group of 21 specimens was approximately 3 days, with a range of 1 to 10 days, suggesting an inoculum of several hundred thousand bacilli (only 2 of these 21 had a time to positive greater than 4 days). Of the 16 specimens that had smear values of either ±, 1+, 2+, or 3+, the average time to detection was approximately 10 days, with a range of 3 to 24 days, suggesting an inoculum of tens of thousands of bacilli. The two specimens that were smear negative and 12B/EMP/VAN positive were positive in 28 and 40 days, suggesting an inoculum below 100 bacilli.
For quality control purposes and to evaluate the internal consistency of the methods, 10 specimens were derived from three control animals: four specimens were derived from a control negative bovine; four specimens were derived from a control positive bovine; and two specimens were from a different control positive bovine. Only two inconsistencies were observed among these 10 specimens: first, a jejunum specimen from the control negative bovine produced a 1+ smear value (described above). None of the cultures were positive from any of the four specimens from this animal. Second, a medial lymph node specimen from the first control positive bovine did not produce a positive result on HEYM (cultures were positive on both 12B/EMP/VAN and 7H10/MPV for this specimen). The former inconsistency was interpreted as a false-positive result, while the latter was interpreted as a false-negative result.
DISCUSSION
The goal of this research was to lay the basis for application of the CB-18 specimen processing method to the diagnosis of Johne's disease. As a first step toward this end, an evaluation of the interface between the CB-18 specimen processing method and contemporary culture techniques was required. Therefore, the advances made by other groups were used in a simple format. Specifically, mycobactin J was made in 75% ethanol (6) and was mixed with the egg yolk emulsion (5) and pyruvate (9; Bartley et al., Abstr. 98th Gen. Meet. Am. Soc. Microbiol. 1998). This was combined with the VAN antibiotic supplement (3) made in the standard BACTEC RF and then was added to BACTEC 12B vials to achieve the desired concentrations. During the evaluation of the 12B/EMP/VAN culture system, it was observed that the LZCT resuspension buffer provided a significant advantage with respect to supporting growth of this species of mycobacteria. Whereas the ability to support growth was not as substantive as that provided by 1% egg yolk, it was always significantly greater than either the buffer-only or the 0.15% lecithin supplements. This might result from either some heretofore-undefined growth factor or simply be a consequence of the natural activity of these enzymes. For example, the activity of these lytic enzymes might be such that they degrade culture and/or specimen components and/or contaminating microorganisms into constituents that are more readily absorbed and/or metabolized by M. avium subsp. paratuberculosis. Regardless, the lytic enzymes do not appear to be harmful to M. avium subsp. paratuberculosis but instead appear to provide some growth advantage.
After the interface of the 12B/EMP/VAN culture system with the CB-18 specimen processing method had been evaluated, a blinded panel of 60 previously characterized tissue specimens was processed. Acceptable sensitivities were obtained when the CB-18 processing method was combined with 12B/EMP/VAN as the primary culture system and with the 7H10/MPV solid media as the backup culture system. Perhaps the most remarkable result in these studies was the times to detection. In the 12B/EMP/VAN system three specimens were positive within 1 day, over 74% of the positive specimens in 12B/EMP/VAN had a GI above the cutoff in 1 week or less, and less than 13% of the positive specimens required more than 2 weeks of incubation. These results were probably due to a combination of the enhanced viability and recovery provided by CB-18-based specimen processing relative to the qualities of HPC-based specimen processing and the aforementioned advantages provided by the LZCT resuspension buffer.
Clearly, the 12B/EMP/VAN formulation provided the most consistent and robust results; however, inclusion of vancomycin (even at 2 μg/ml) still seemed problematic. For example, when the ATCC 43015 isolate was used, the time to positive was delayed by almost 3 weeks at 1.25 μg of vancomycin/ml under in vitro conditions (this was especially interesting since this was a human isolate). In addition, the false-negative result of IRT identification no. 28 in 12B/EMP/VAN was due to the susceptibility of this isolate to vancomycin. Therefore, susceptibility to vancomycin, while apparently not widespread, does appear to be a legitimate concern to the laboratory worker in regard to false-negative results. While removing or replacing vancomycin may reduce the time to positive for those isolates susceptible to vancomycin, the tradeoff between reducing the time to positive and increasing the contamination rate may be more critical with fecal specimens.
Whereas there was no contamination reported by the HPC method, the contamination rate for 12B/EMP/VAN was acceptable (4.8%); however, the contamination rates for 7H10/MPV and HEYM were high (23.3 and 20.8%, respectively). In five (38.5%) of the 13 instances where 7H10/MPV missed a 12B/EMP/VAN-positive specimen, the plate was contaminated, and in nine (47.4%) of the 19 instances where HEYM missed a 12B/EMP/VAN-positive specimen, the plate was contaminated. The contamination rate on 7H10/MPV was interesting since it contained the same antibiotic formulation as 12B/EMP/VAN but had a contamination rate comparable to that for the nonselective HEYM. The majority of these contaminants were mycologic (i.e., yeast and fungi). Regardless, while the lytic enzyme resuspension buffer functioned successfully when used in conjunction with VAN in 12B/EMP media, it was not as effective when used in conjunction with 7H10/MPV. This was unfortunate because the 7H10-based media had better overall sensitivity than the HEYM-based media. It is clear that the CB-18 processing method must be used in conjunction with the combination of the LZCT resuspension buffer and antibiotic-containing media. In summary, the solid media recently described (21) might be a better choice for backup (i.e., secondary) media for future evaluations.
The purpose of these experiments was to evaluate the utility of the CB-18 specimen processing method in regard to isolating M. avium subsp. paratuberculosis from tissues. The combination of CB-18 processing and LZCT treatment appeared to function effectively, and the experiments herein suggest that CB-18/lytic enzyme processing may provide an advantage with respect to time to positive. While processing of the tissue specimens was not performed on a side-by-side basis, the results were encouraging. A side-by-side comparison was recently completed and will be described in the near future.
REFERENCES
- 1.Billman-Jacobe, H., M. Carrigan, F. Cockram, L. A. Corner, I. J. Gill, J. F. Hill, T. Jessep, A. R. Milner, and P. R. Wood. 1992. A comparison of the interferon gamma assay with the absorbed ELISA for the diagnosis of Johne's disease in cattle. Aust. Vet. J. 69:25-28. [DOI] [PubMed] [Google Scholar]
- 2.Cocito, C., P. Gilot, M. Coene, M. de Kesel, P. Poupart, and P. Vannuffel. 1994. Paratuberculosis. Clin. Microbiol. Rev. 7:328-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Collins, M. T., K. B. Kenefick, D. C. Sockett, R. S. Lambrecht, J. McDonald, and J. B. Jorgensen. 1990. Enhanced radiometric detection of Mycobacterium paratuberculosis by using filter-concentrated bovine fecal specimens. J. Clin. Microbiol. 28:2514-2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Collins, M. T., D. C. Sockett, S. Ridge, and J. Cox. 1991. Evaluation of a commercial enzyme-linked immunosorbent assay for Johne's disease. J. Clin. Microbiol. 29:272-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cousins, D. V., R. J. Evans, and B. R. Francis. 1995. Use of BACTEC radiometric culture method and polymerase chain reaction for the rapid screening of faeces and tissues for Mycobacterium paratuberculosis. Aust. Vet. J. 72:458-462. [DOI] [PubMed] [Google Scholar]
- 6.Damato, J. J., and M. T. Collins. 1990. Growth of Mycobacterium paratuberculosis in radiometric, Middlebrook, and egg-based media. Vet. Microbiol. 22:31-42. [DOI] [PubMed] [Google Scholar]
- 7.Ellingson, J. L., J. R. Stabel, W. R. Bishai, R. Frothingham, and J. M. Miller. 2000. Evaluation of the accuracy and reproducibility of a practical PCR panel assay for rapid detection and differentiation of Mycobacterium avium subspecies. Mol. Cell. Probes 14:153-161. [DOI] [PubMed] [Google Scholar]
- 8.Hobby, G. L., A. P. Holman, M. D. Iseman, and J. M. Jones. 1973. Enumeration of tubercle bacilli in sputum of patients with pulmonary tuberculosis. Antimicrob. Agents Chemother. 4:94-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jorgensen, J. B. 1982. An improved medium for culture of Mycobacterium paratuberculosis from bovine faeces. Acta Vet. Scand. 23:325-335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kent, P. T., and G. P. Kubica. 1985. Public health mycobacteriology. A guide for the level III laboratory. Centers for Disease Control and Prevention, Atlanta, Ga.
- 11.Stabel, J. R. 1997. An improved method for cultivation of Mycobacterium paratuberculosis from bovine fecal samples and comparison to three other methods. J. Vet. Diagn. Investig. 9:375-380. [DOI] [PubMed] [Google Scholar]
- 12.Thornton, C. G., K. M. MacLellan, T. L. Brink, Jr., D. E. Lockwood, M. Romagnoli, J. Turner, W. G. Merz, R. S. Schwalbe, M. Moody, Y. Lue, and S. Passen. 1998. Novel method for processing respiratory specimens for detection of mycobacteria by using C18-carboxypropylbetaine: blinded study. J. Clin. Microbiol. 36:1996-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thornton, C. G., K. M. MacLellan, T. L. Brink, Jr., D. M. Wolfe, O. J. Llorin, and S. Passen. 1998. Processing respiratory specimens with C18-carboxypropylbetaine: development of a sediment resuspension buffer that contains lytic enzymes to reduce the contamination rate and lecithin to alleviate toxicity. J. Clin. Microbiol. 36:2004-2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thornton, C. G., K. M. MacLellan, T. L. Brink, Jr., and S. Passen. 1998. In vitro comparison of NALC-NaOH, Tween 80, and C18-carboxypropylbetaine for processing of specimens for recovery of mycobacteria. J. Clin. Microbiol. 36:3558-3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thornton, C. G., M. R. Cranfield, K. M. MacLellan, T. L. Brink, Jr., J. D. Strandberg, E. A. Carlin, J. B. Torrelles, J. N. Maslow, J. L. B. Hasson, D. M. Heyl, S. J. Sarro, D. Chatterjee, and S. Passen. 1999. Processing postmortem specimens with C18-carboxypropylbetaine and analysis by PCR to develop an antemortem test for Mycobacterium avium infections in ducks. J. Zoo Wildl. Med. 30:11-24. [PubMed] [Google Scholar]
- 16.Turcotte, C., B. W. Brooks, W. M. Dion, and R. Marenger. 1986. Bacterial and fungal contaminants interfering with the isolation of Mycobacterium paratuberculosis, p. 49-58. In American Association of Veterinary Laboratory Diagnosticians 29th Annual Proceedings. American Association of Veterinary Laboratory Diagnosticians, Davis, Calif.
- 17.van der Giessen, J. W., R. M. Haring, E. Vauclare, A. Eger, J. Haagsma, and B. A. van der Zeijst. 1992. Evaluation of the abilities of three diagnostic tests based on the polymerase chain reaction to detect Mycobacterium paratuberculosis in cattle: application in a control program. J. Clin. Microbiol. 30:1216-1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wells, S. J., S. L. Ott, and A. H. Seitzinger. 1998. Key health issues for dairy cattle—new and old. J. Dairy Sci. 81:3029-3035. [DOI] [PubMed] [Google Scholar]
- 19.Whipple, D. L., D. R. Callihan, and J. L. Jarnagin. 1991. Cultivation of Mycobacterium paratuberculosis from bovine fecal specimens and a suggested standardized procedure. J. Vet. Diagn. Investig. 3:368-373. [DOI] [PubMed] [Google Scholar]
- 20.Whitlock, R. H., A. E. Rosenberger, and P. A. Spencer. 1988. Laboratory culture techniques for Johne's disease: a critical evaluation of contamination and incubation times, p. 382-386. In Proceedings of the United States Animal Health Association. United States Animal Health Association, Richmond, Va.
- 21.Whittington, R. J., I. Marsh, S. McAllister, M. J. Turner, D. J. Marshall, and C. A. Fraser. 1999. Evaluation of modified BACTEC 12B radiometric medium and solid media for culture of Mycobacterium avium subsp. paratuberculosis from sheep. J. Clin. Microbiol. 37:1077-1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yeager, H., Jr., J. Lacy, L. R. Smith, and C. A. LeMaistre. 1967. Quantitative studies of mycobacterial populations in sputum and saliva. Am. Rev. Respir. Dis. 95:998-1004. [DOI] [PubMed] [Google Scholar]