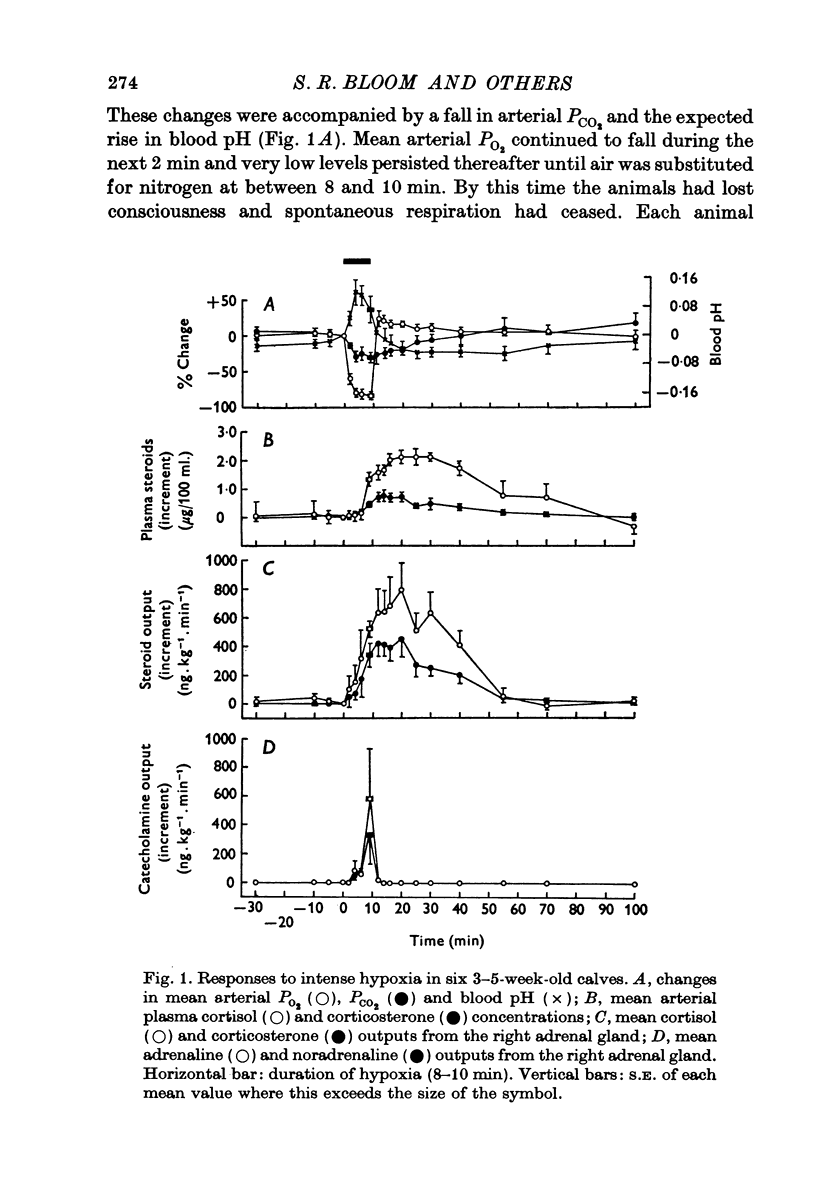
Abstract
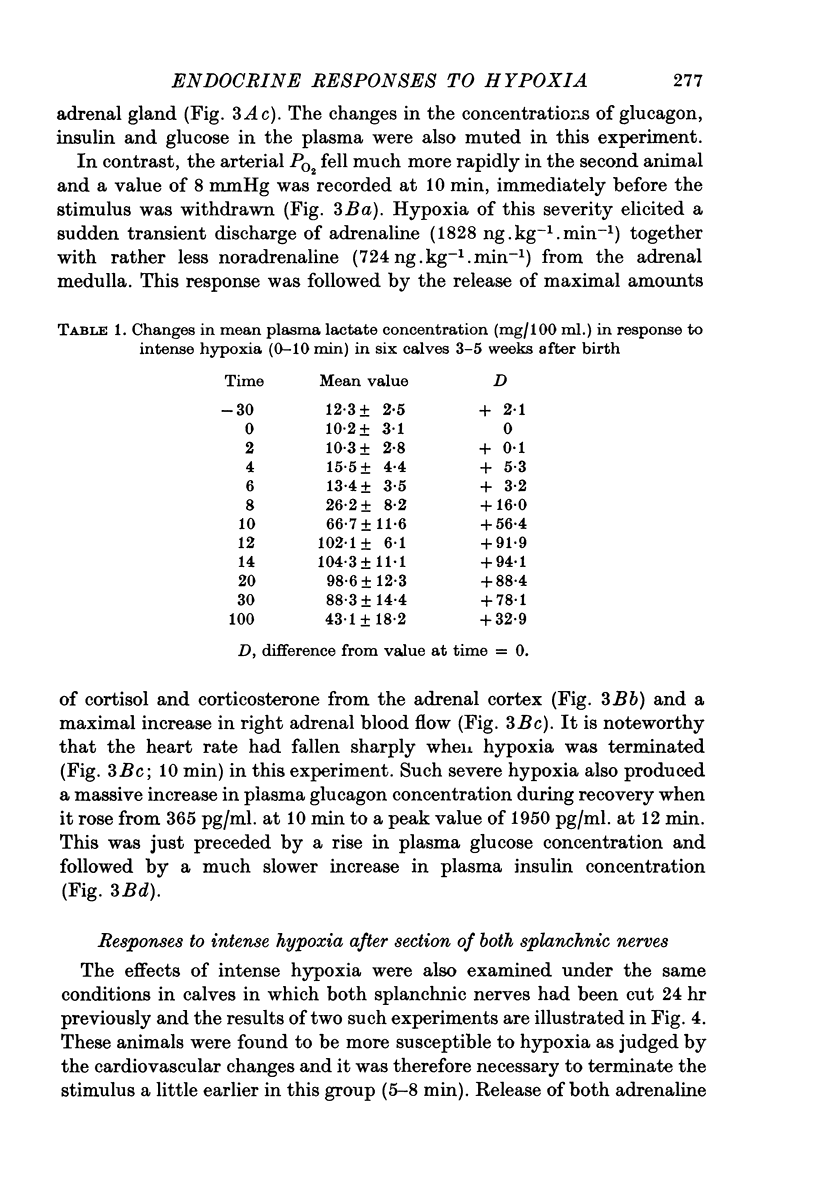
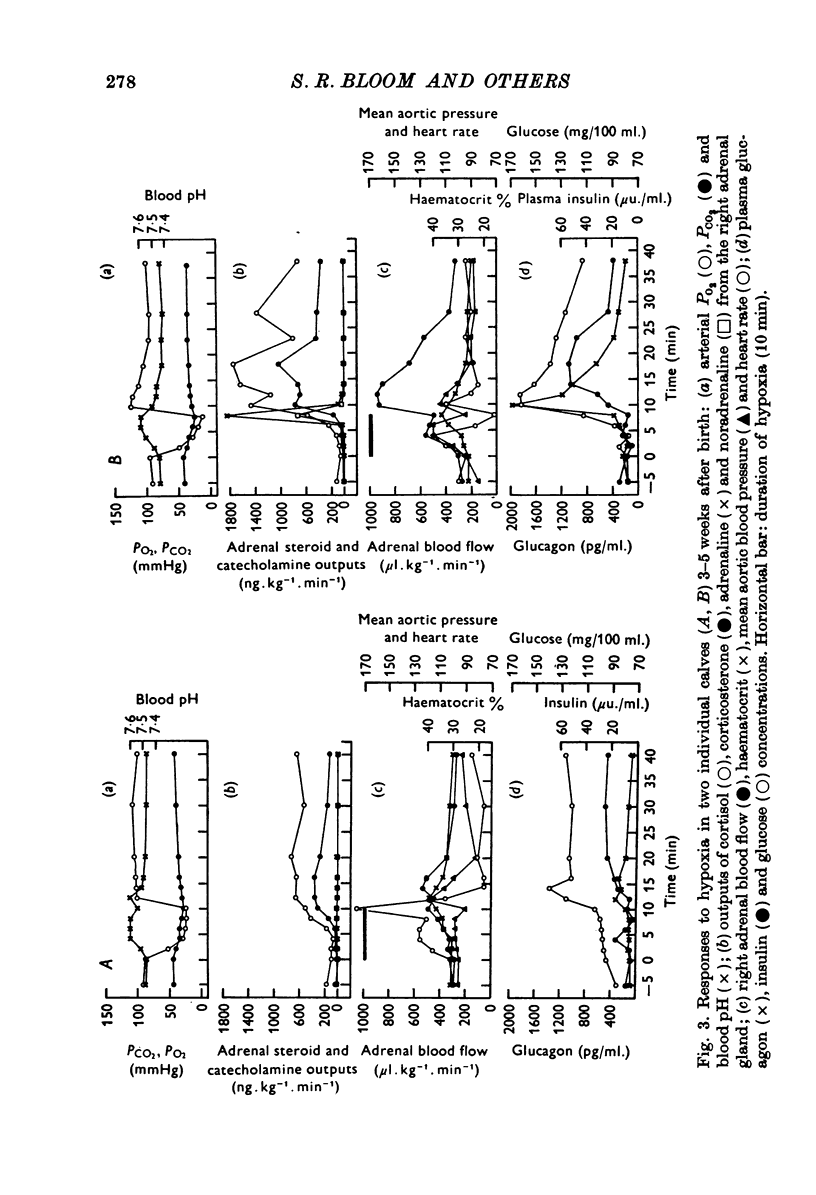
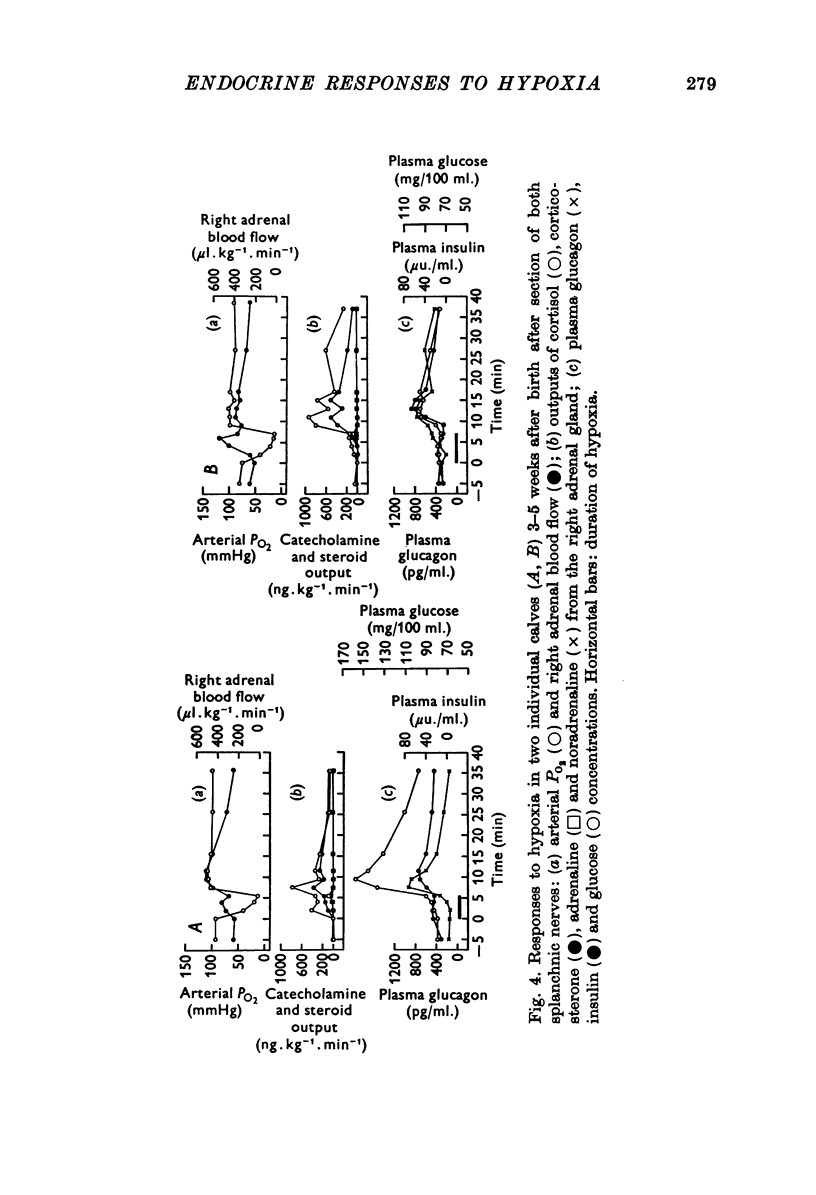
1. Pancreatic and adrenal responses to intense hypoxia have been examined in conscious unrestrained calves 3-5 weeks after birth. 2. The outputs of both cortisol and corticosterone from the right adrenal gland rose steadily in response to hypoxia and this cortical secretory response was accompanied by a pronounced increase in blood flow through the gland. The changes in both steroid output and adrenal blood flow corresponded with those which occur in response to supramaximal doses of corticotrophin in calves of the same age. 3. Neither adrenaline nor noradrenaline were released in significant amounts from the adrenal medulla until the arterial PO2 had fallen below 15 mmHg. Such severe hypoxia caused secretion of catecholamines at rates comparable with those recorded during maximal stimulation of the sympathetic innervation to the gland in anaesthetized calves. The response to intense hypoxia in these conscious calves differed from that which occurs under anaesthesia in that the amount of adrenaline released was invariably greater than that of noradrenaline. 4. Severe hypoxia produced a rapid but transient increase in plasma glucagon concentration, followed by a pronounced rise in plasma glucose concentration in animals with abundant liver glycogen. No change in plasma insulin concentration was observed during hypoxia although it rose subsequently in response to hyperglycaemia. 5. Bilateral section of the splanchnic nerves virtually abolished the release of catecholamines in response to hypoxia but the adrenal cortical and pancreatic responses did not appear to be affected.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albano J. D., Ekins R. P., Maritz G., Turner R. C. A sensitive, precise radioimmunoassay of serum insulin relying on charcoal separation of bound and free hormone moieties. Acta Endocrinol (Copenh) 1972 Jul;70(3):487–509. doi: 10.1530/acta.0.0700487. [DOI] [PubMed] [Google Scholar]
- Assan R., Slusher N. Structure-function and structure-immunoreactivity relationships of the glucagon molecule and related synthetic peptides. Diabetes. 1972 Aug;21(8):843–855. doi: 10.2337/diab.21.8.843. [DOI] [PubMed] [Google Scholar]
- Bloom S. R., Edwards A. V., Hardy R. N., Malinowska K. W., Silver M. Endocrine responses to insulin hypoglycaemia in the young calf. J Physiol. 1975 Jan;244(3):783–803. doi: 10.1113/jphysiol.1975.sp010826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom S. R., Edwards A. V., Hardy R. N., Malinowska K. W., Silver M. Proceedings: Endocrine responses to hypoxia in the conscious calf. J Physiol. 1976 Jan;254(1):29P–30P. [PubMed] [Google Scholar]
- Bloom S. R., Edwards A. V., Vaughan N. J. The role of the autonomic innervation in the control of glucagon release during hypoglycaemia in the calf. J Physiol. 1974 Feb;236(3):611–623. doi: 10.1113/jphysiol.1974.sp010455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom S. R., Edwards A. V., Vaughan N. J. The role of the sympathetic innervation in the control of plasma glucagon concentration in the calf. J Physiol. 1973 Sep;233(2):457–466. doi: 10.1113/jphysiol.1973.sp010317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boddy K., Jones C. T., Mantell C., Ratcliffe J. G., Robinson J. S. Changes in plasma ACTH and corticosteroid of the maternal and fetal sheep during hypoxia. Endocrinology. 1974 Feb;94(2):588–591. doi: 10.1210/endo-94-2-588. [DOI] [PubMed] [Google Scholar]
- Comline R. S., Edwards A. V. The effects of insulin of the new-born calf. J Physiol. 1968 Sep;198(2):383–404. doi: 10.1113/jphysiol.1968.sp008613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comline R. S., Silver M. The development of the adrenal medulla of the foetal and new-born calf. J Physiol. 1966 Mar;183(2):305–340. doi: 10.1113/jphysiol.1966.sp007868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards A. V., Hardy R. N., Malinowska K. W. The effects of infusions of synthetic adrenocorticotrophin in the conscious calf. J Physiol. 1974 Jun;239(3):477–498. doi: 10.1113/jphysiol.1974.sp010579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards A. V., Hardy R. N., Malinowska K. W. The sensitivity of adrenal responses to synthetic adrenocorticotrophin in the conscious unrestrained calf. J Physiol. 1975 Mar;245(3):639–653. doi: 10.1113/jphysiol.1975.sp010866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards A. V. The glycogenolytic response to stimulation of the splanchnic nerves in adrenalectomized calves, sheep, dogs, cats and pigs. J Physiol. 1971 Mar;213(3):741–759. doi: 10.1113/jphysiol.1971.sp009412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIRAI K., ATKINS G., MAROTTA S. F. 17-HYDROXYCORTICOSTEROID SECRETION DURING HYPOXIA IN ANESTHETIZED DOGS. Aerosp Med. 1963 Sep;34:814–816. [PubMed] [Google Scholar]
- Kaneto A., Kosaka K. Stimulation of glucagon and insulin secretion by acetylcholine infused intrapancreatically. Endocrinology. 1974 Sep;95(3):676–681. doi: 10.1210/endo-95-3-676. [DOI] [PubMed] [Google Scholar]
- Kaneto A., Miki E., Kosaka K. Effects of vagal stimulation on glucagon and insulin secretion. Endocrinology. 1974 Oct;95(4):1005–1010. doi: 10.1210/endo-95-4-1005. [DOI] [PubMed] [Google Scholar]
- Lau C. Effects of O 2 -CO 2 changes on hypothalamoypophyseal-adrenocortical activation. Am J Physiol. 1971 Aug;221(2):607–612. doi: 10.1152/ajplegacy.1971.221.2.607. [DOI] [PubMed] [Google Scholar]
- Malinowska K. W., Hardy R. N., Nathanielsz P. W. Neonatal adrenocortical function and its possible relation to the uptake of macromolecules by the small intestine of the guinea-pig and rabbit. J Endocrinol. 1972 Nov;55(2):397–404. doi: 10.1677/joe.0.0550397. [DOI] [PubMed] [Google Scholar]
- Moncloa F., Donayre J., Sobrevilla L. A., Guerra-García R. Endocrine studies at high altitude. II. Adrenal cortical function in sea level natives exposed to high altitudes (4300 metersfor two weeks. J Clin Endocrinol Metab. 1965 Dec;25(12):1640–1642. doi: 10.1210/jcem-25-12-1640. [DOI] [PubMed] [Google Scholar]
- SILVER M. The output of adrenaline and noradrenaline from the adrenal medulla of the calf. J Physiol. 1960 Jun;152:14–29. doi: 10.1113/jphysiol.1960.sp006466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VON EULER U. S., FLODING I. A fluorimetric micromethod for differential estimation of adrenaline and noradrenaline. Acta Physiol Scand Suppl. 1955;33(118):45–56. [PubMed] [Google Scholar]


