Abstract
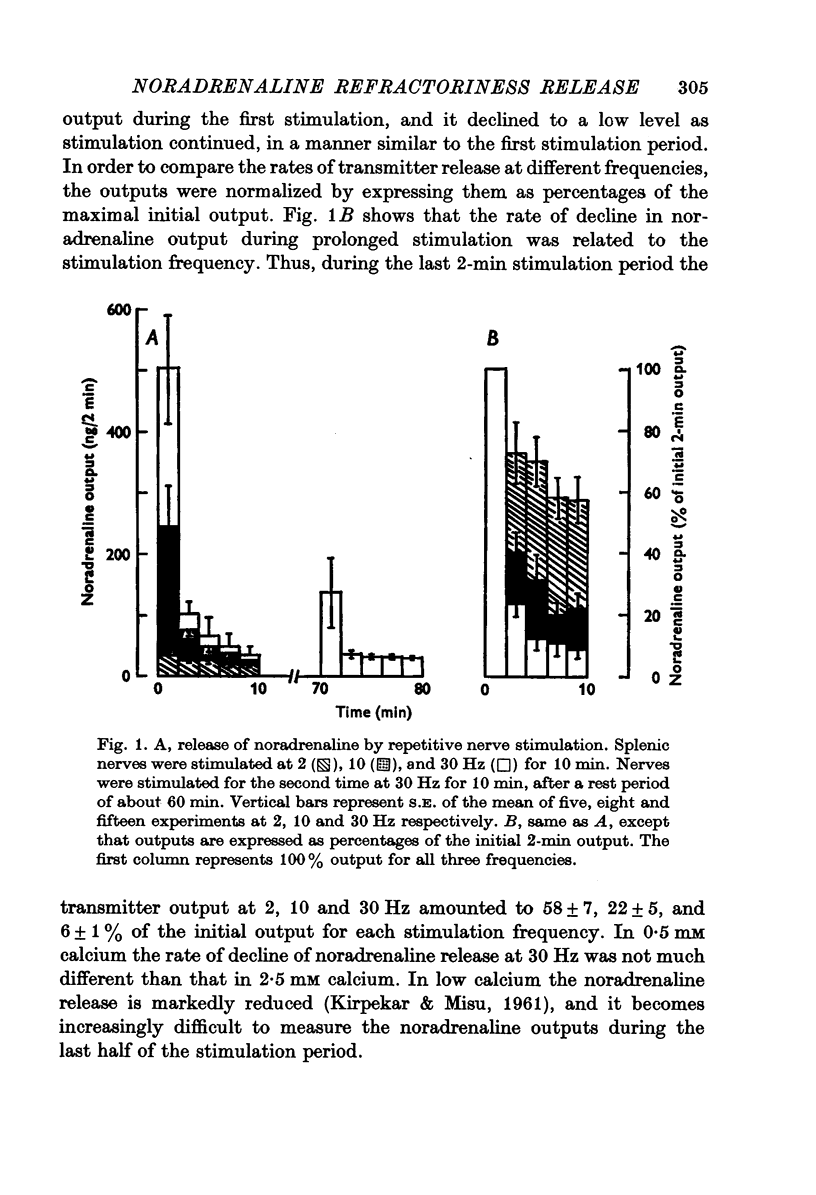
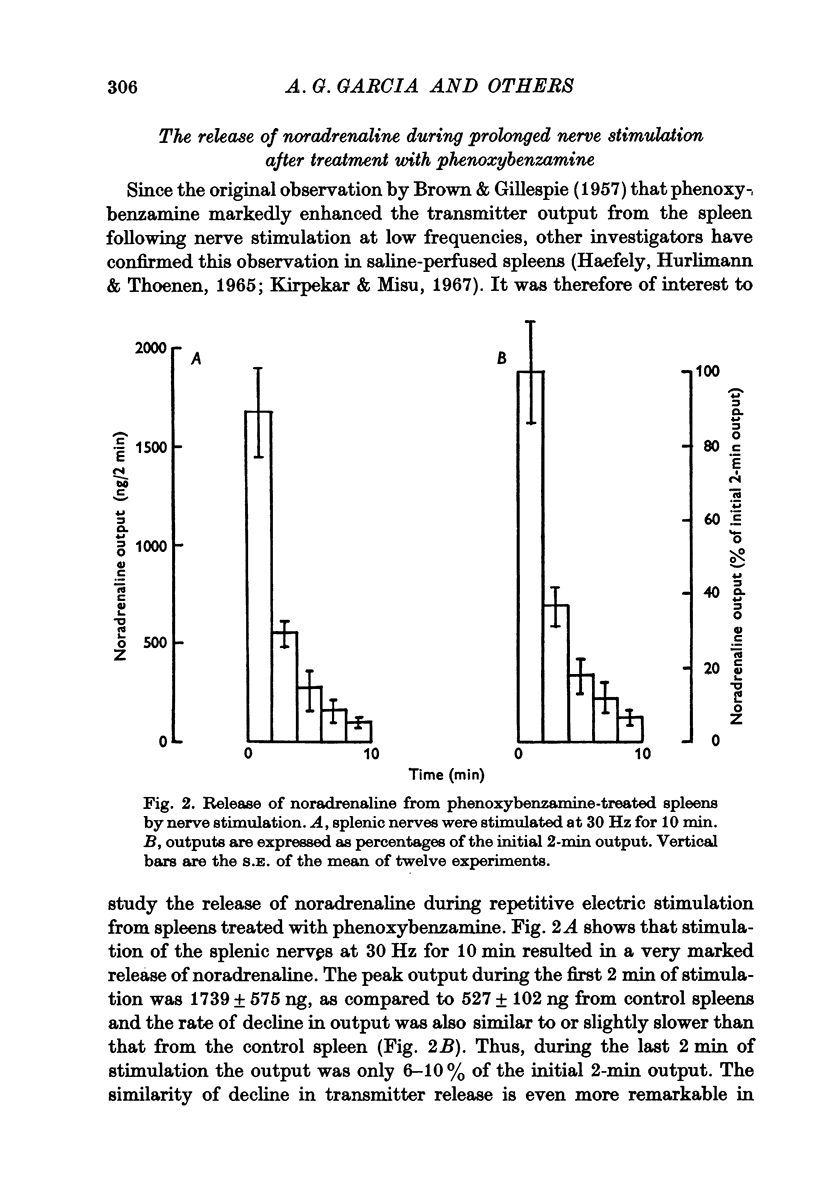
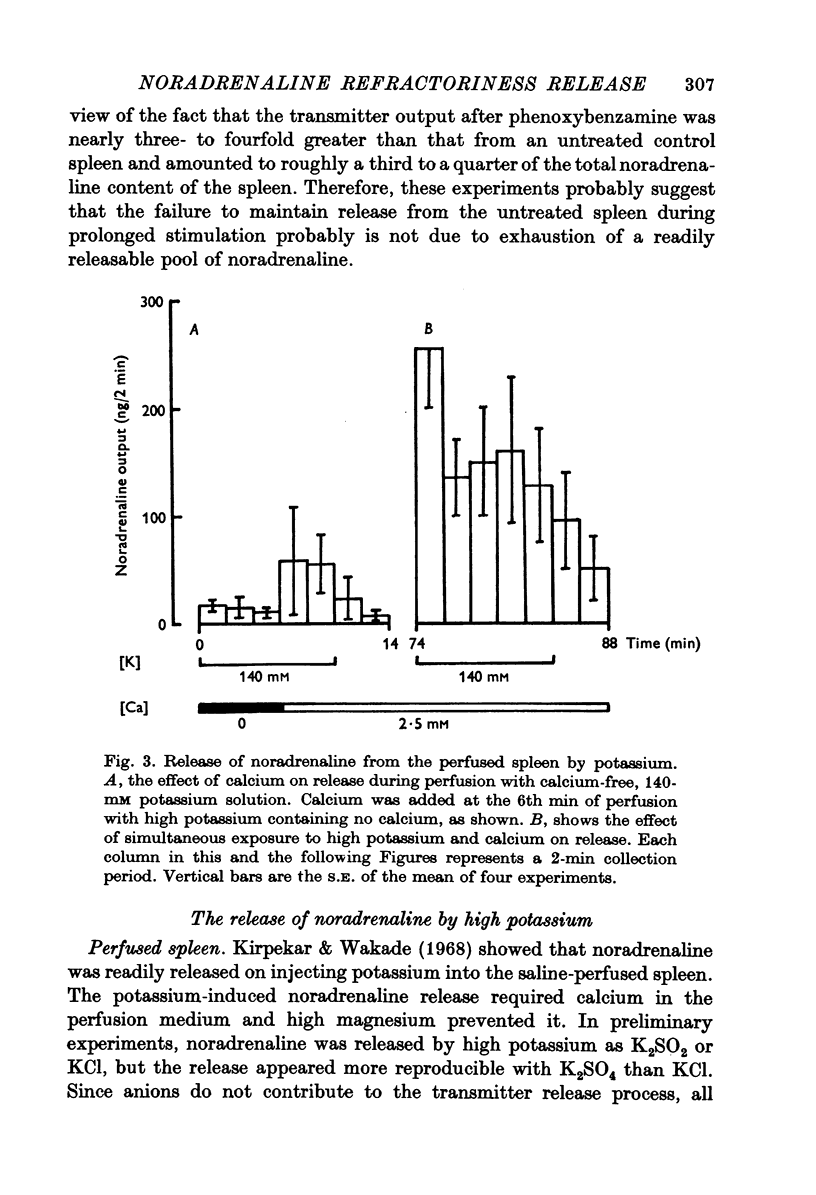
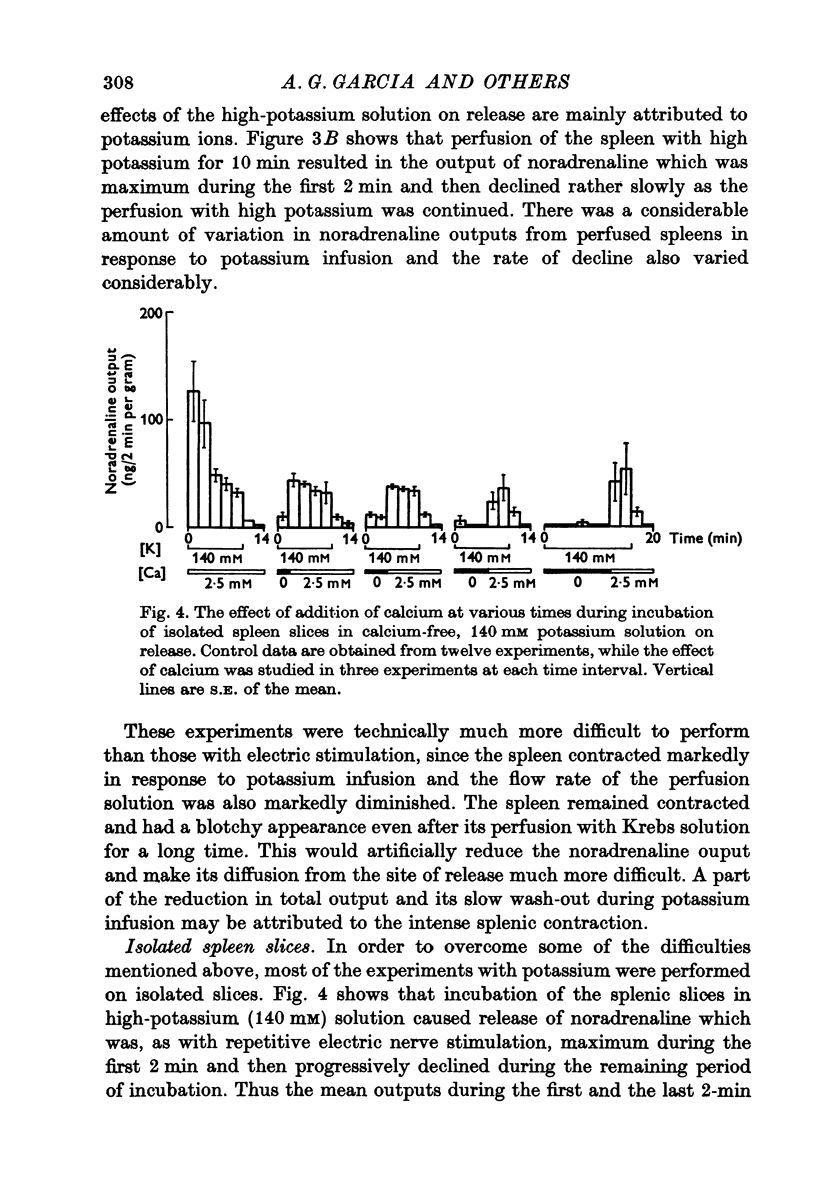
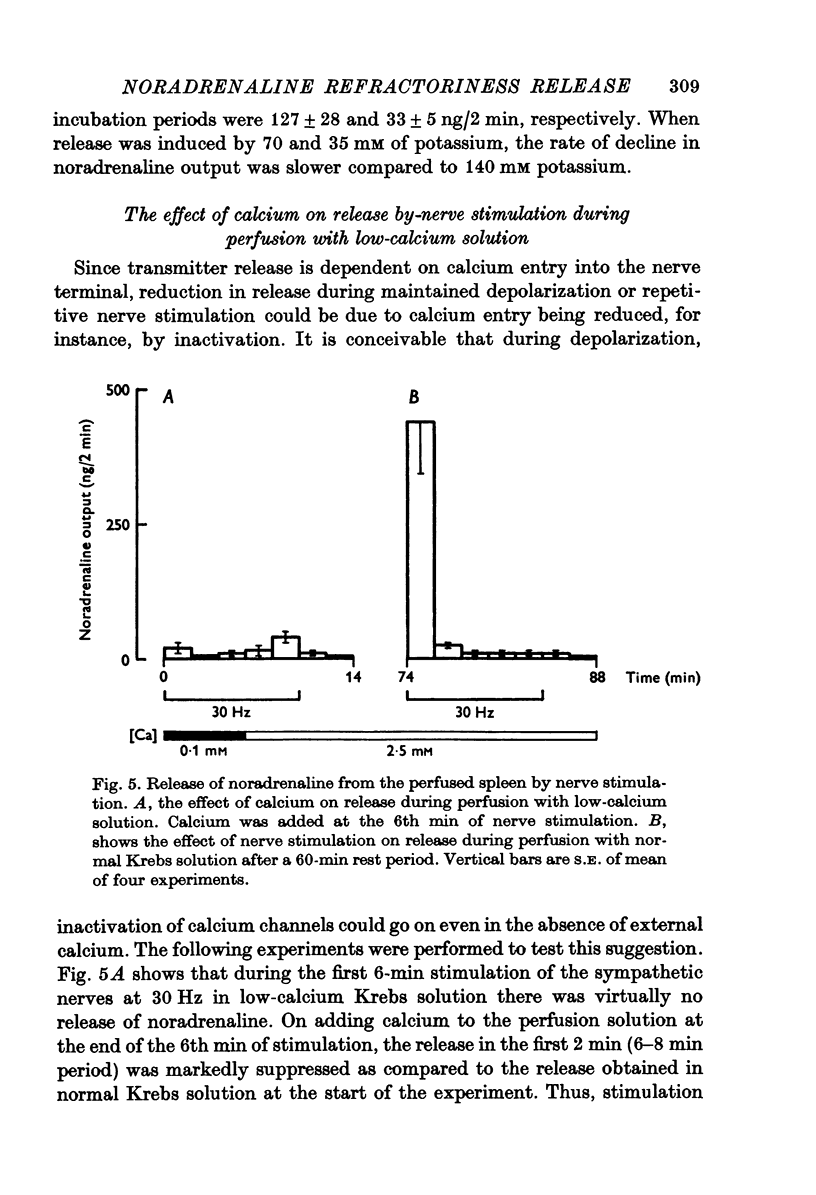
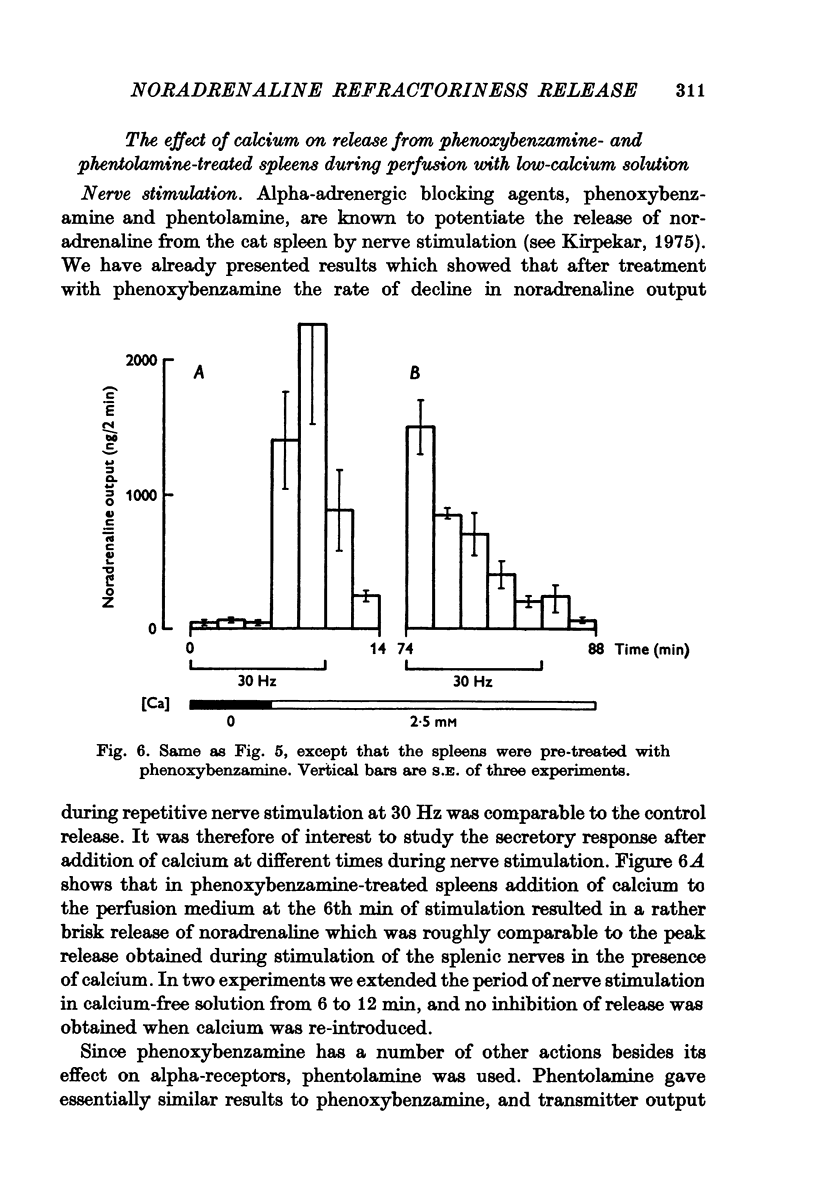
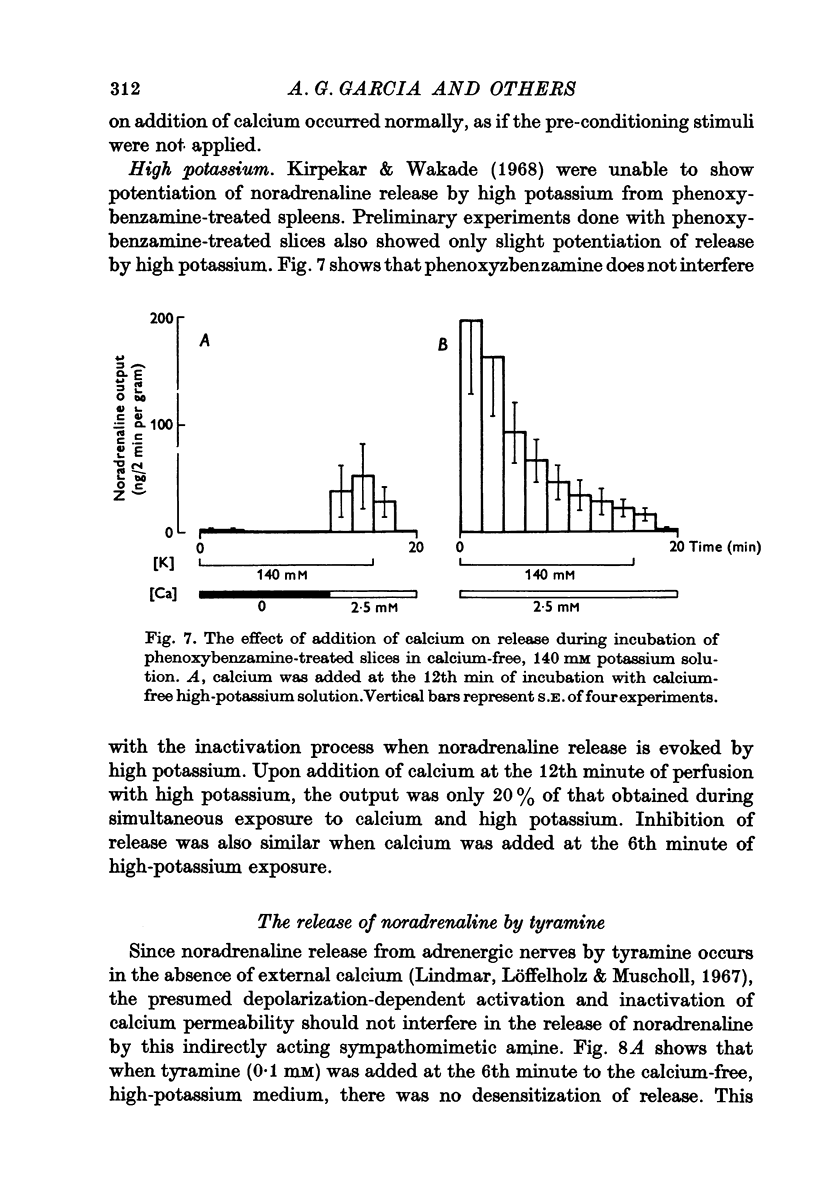
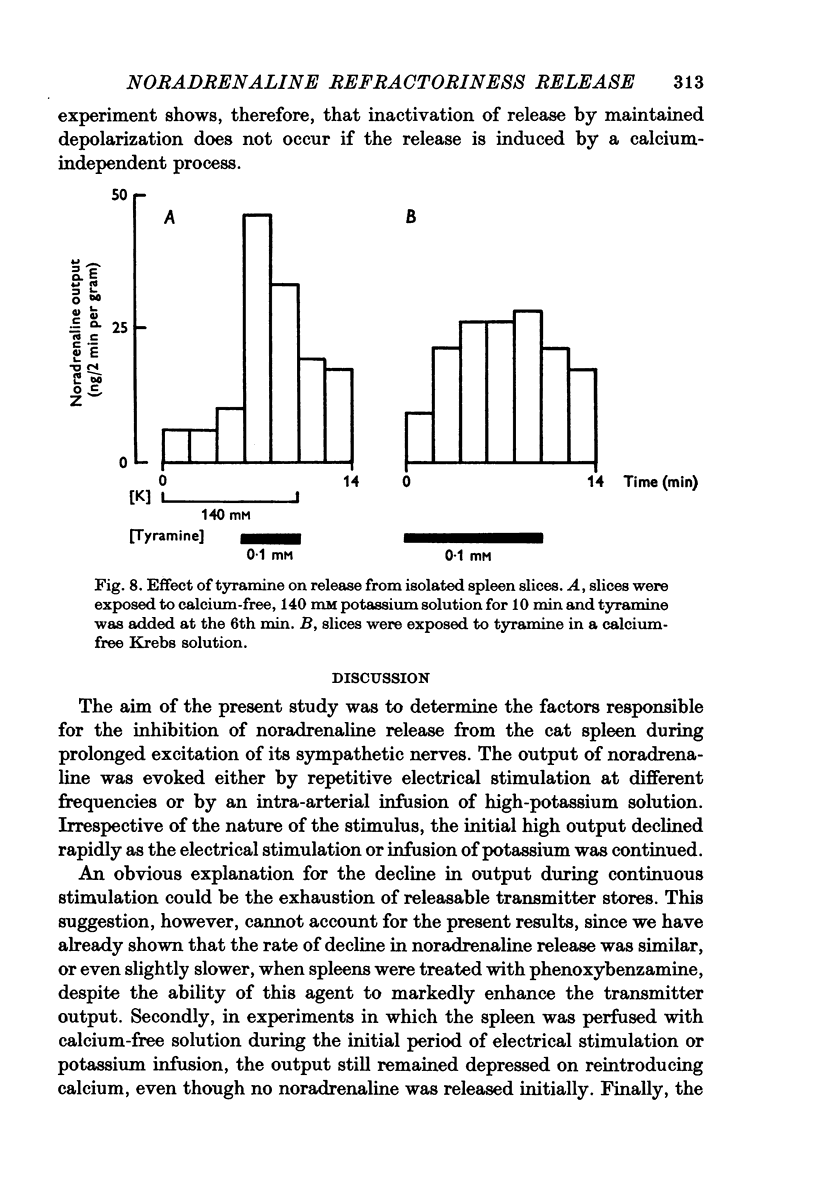
1. Release of noradrenaline from the perfused cat spleen, or from isolated spleen slices, in response to prolonged nerve stimulation or maintained depolarization by potassium was measured. 2. Prolonged stimulation of the splenic nerves at 2, 10 and 30 Hz for 10 min evoked release, which was maximum during the first 2 min, and then declined during the remaining period of stimulation. When noradrenaline release was induced by high potassium from the perfused spleen or from isolated slices, it followed a similar time course to nerve stimulation. Similar results were obtained from phenoxybenzamine-treated spleens, using both modes of stimulation. 3. Stimulation of the splenic nerves in calcium-free Krebs solution did not release noradrenaline. If calcium was introduced at a later stage during stimulation, the release was markedly diminished. In phenoxybenzamine- or phentolamine-treated spleens, stimulation of the nerves in the presence of calcium evoked a secretory response which was comparable to the one produced by introduction of calcium after a few minutes of nerve stimulation. 4. Simultaneous application of calcium plus high potassium always produced a much greater secretion of noradrenaline than application of calcium after a few minutes of potassium depolarization. Release of noradrenaline by potassium from phenoxybenzamine-treated spleens was also much greater if calcium and potassium were added simultaneously than addition of calcium after a few minutes of potassium depolarization. 5. In the presence of maintained depolarization by potasssium, tyramine was effective in causing release of noradrenaline.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BROWN G. L., GILLESPIE J. S. The output of sympathetic transmitter from the spleen of the cat. J Physiol. 1957 Aug 29;138(1):81–102. doi: 10.1113/jphysiol.1957.sp005839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Meves H., Ridgway E. B. Calcium entry in response to maintained depolarization of squid axons. J Physiol. 1973 Jun;231(3):527–548. doi: 10.1113/jphysiol.1973.sp010247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Meves H., Ridgway E. B. Effects of manganese and other agents on the calcium uptake that follows depolarization of squid axons. J Physiol. 1973 Jun;231(3):511–526. doi: 10.1113/jphysiol.1973.sp010246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Rink T. J. Catecholamine release from bovine adrenal medulla in response to maintained depolarization. J Physiol. 1975 Dec;253(2):593–620. doi: 10.1113/jphysiol.1975.sp011209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., Middleton J. An electrophysiological analysis of the effects of amine-uptake blockers and alpha-adrenoceptor blockers on adrenergic neuromuscular transmission. Br J Pharmacol. 1975 Sep;55(1):87–95. doi: 10.1111/j.1476-5381.1975.tb07615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dearnaley D. P., Geffen L. B. Effect of nerve stimulation on the noradrenaline content of the spleen. Proc R Soc Lond B Biol Sci. 1966 Dec 13;166(1004):303–315. doi: 10.1098/rspb.1966.0101. [DOI] [PubMed] [Google Scholar]
- Haefely W., Hürlimann A., Thoenen H. Relation between the rate of stimulation and the quantity of noradrenaline liberated from sympathetic nerve endings in the isolated perfused spleen of the cat. J Physiol. 1965 Nov;181(1):48–58. doi: 10.1113/jphysiol.1965.sp007744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedqvist P., Stjärne L. The relative role of recapture and of de novo synthesis for the maintenance of neurotransmitter homeostasis in noradrenergic nerves. Acta Physiol Scand. 1969 Jul;76(3):270–283. doi: 10.1111/j.1748-1716.1969.tb04470.x. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. Tetrodotoxin-resistant electric activity in presynaptic terminals. J Physiol. 1969 Aug;203(2):459–487. doi: 10.1113/jphysiol.1969.sp008875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. The effect of prolonged depolarization on synaptic transfer in the stellate ganglion of the squid. J Physiol. 1971 Jul;216(2):503–512. doi: 10.1113/jphysiol.1971.sp009537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirpekar S. M., Dixon W., Prat J. C. Inhibitory effect of manganese on norepinephrine release from the splenic nerves of cats. J Pharmacol Exp Ther. 1970 Jul;174(1):72–76. [PubMed] [Google Scholar]
- Kirpekar S. M., Misu Y. Release of noradrenaline by splenic nerve stimulation and its dependence on calcium. J Physiol. 1967 Jan;188(2):219–234. doi: 10.1113/jphysiol.1967.sp008135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirpekar S. M., Prat J. C., Puig M., Wakade A. R. Modification of the evoked release of noradrenaline from the perfused cat spleen by various ions and agents. J Physiol. 1972 Mar;221(3):601–615. doi: 10.1113/jphysiol.1972.sp009770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirpekar S. M., Wakade A. R. Release of noradrenaline from the cat spleen by potassium. J Physiol. 1968 Feb;194(3):595–608. doi: 10.1113/jphysiol.1968.sp008427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirpekar S. M., Yamamoto H. Release of noradrenaline and dopamine by nerve stimulation in the cat spleen perfused with 3 H-dopamine. Br J Pharmacol. 1971 Sep;43(1):86–96. doi: 10.1111/j.1476-5381.1971.tb07159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopin I. J., Breese G. R., Krauss K. R., Weise V. K. Selective release of newly synthesized norepinephrine from the cat spleen during sympathetic nerve stimulation. J Pharmacol Exp Ther. 1968 Jun;161(2):271–278. [PubMed] [Google Scholar]
- Lindmar R., Löffelholz K., Muscholl E. Unterschiede zwischen Tyramin und Dimethylphenylpiperzin in der Ca-Abhangigkeit und im zeitlichen Verlauf der Noradrenalin-Freisetzung am isolierten Kaninchenherzen. Experientia. 1967 Nov 15;23(11):933–934. doi: 10.1007/BF02136230. [DOI] [PubMed] [Google Scholar]
- Lorenz R. R., Vanhoutte P. M. Inhibition of adrenergic neurotransmission in isolated veins of the dog by potassium ions. J Physiol. 1975 Mar;246(2):479–500. doi: 10.1113/jphysiol.1975.sp010900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A. R., Ringham G. L. Synaptic transfer at a vertebrate central nervous system synapse. J Physiol. 1975 Oct;251(2):409–426. doi: 10.1113/jphysiol.1975.sp011101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R. Lanthanum ions abolish the "calcium response" of nerve terminals. Nature. 1971 Feb 5;229(5284):410–411. doi: 10.1038/229410a0. [DOI] [PubMed] [Google Scholar]
- Shellenberger M. K., Gordon J. H. A rapid, simplified procedure for simultaneous assay of norepinephrine, dopamine, and 5-hydroxytryptamine from discrete brain areas. Anal Biochem. 1971 Feb;39(2):356–372. doi: 10.1016/0003-2697(71)90426-x. [DOI] [PubMed] [Google Scholar]


