Abstract
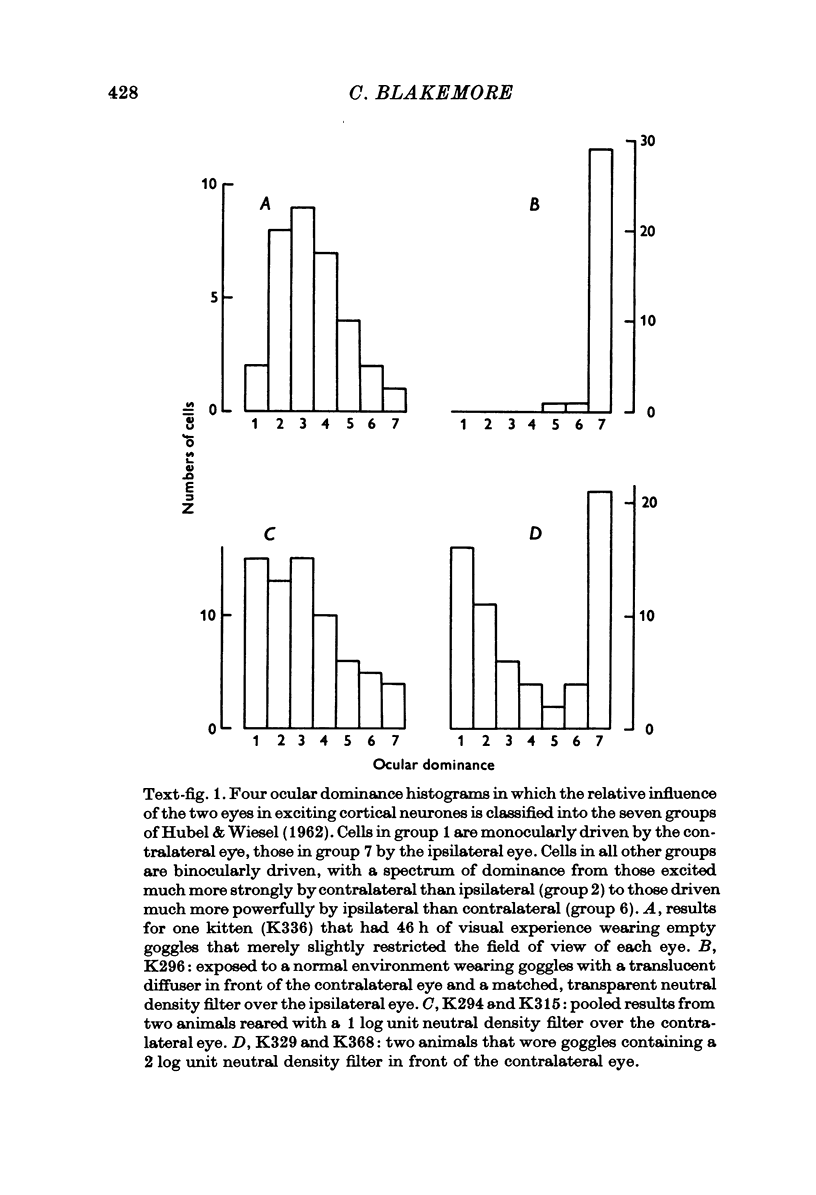
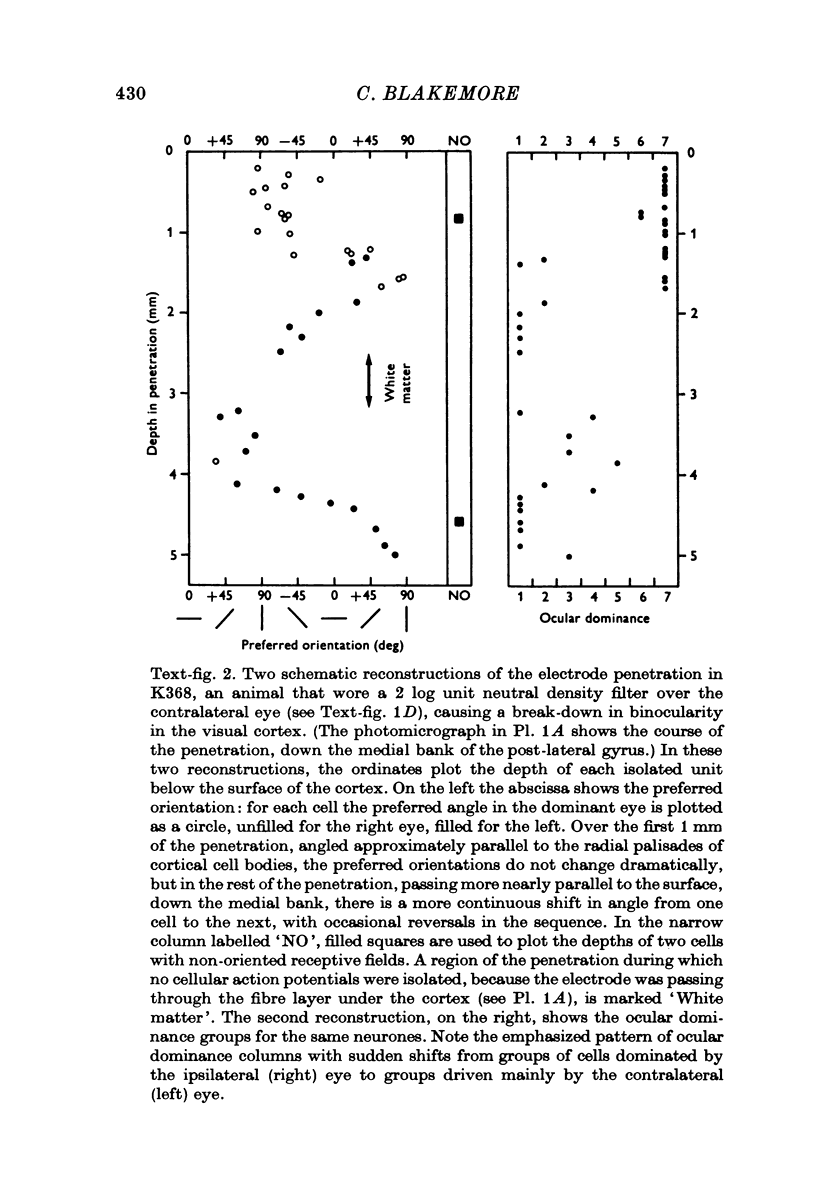
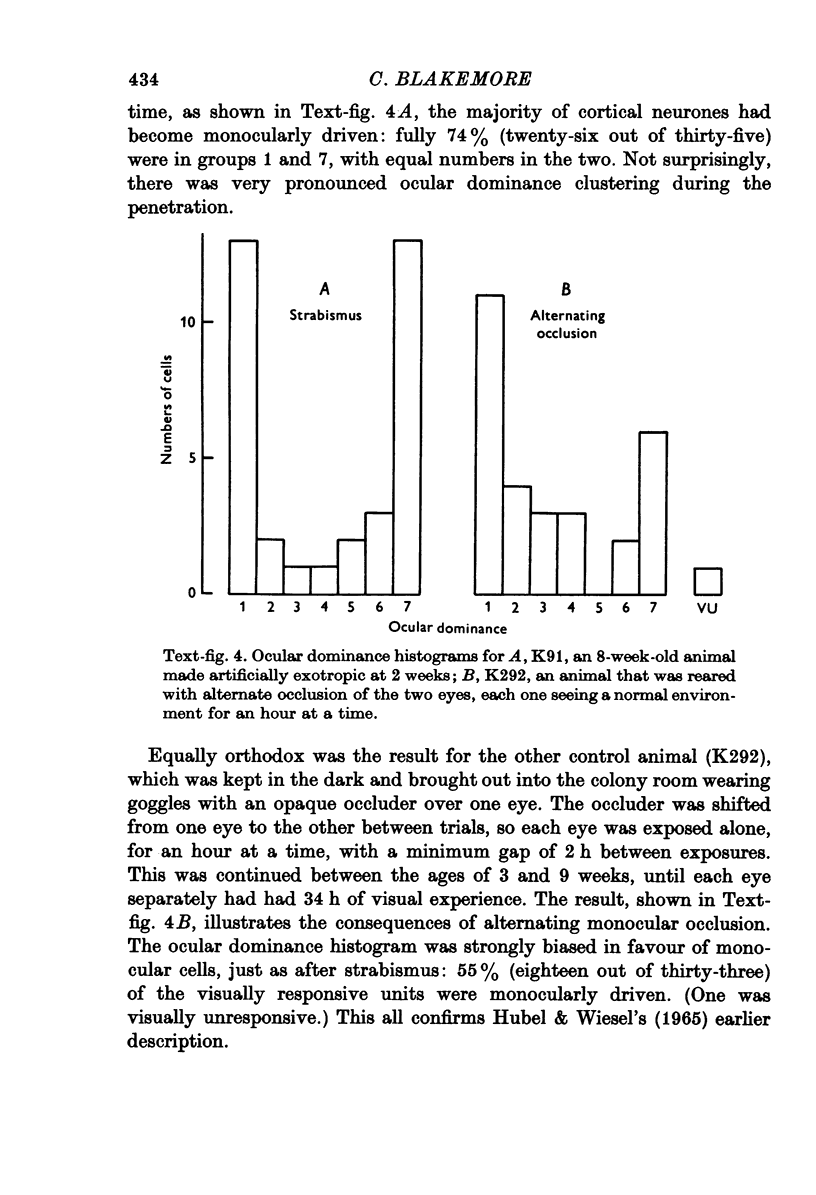
1. In young kittens, cortical neurones, which are usually binocularly driven, have their binocularity reduced if one eye is covered, or if the eyes are made strabismic or alternately occluded. Some of the factors causing these changes were analysed. 2. If the contrast of one retinal image is abolished with no difference in mean illumination, the input from that eye is virtually lost. 3. If one eye merely has its mean retinal illumination attenuated, that eye does not specifically lose its influence in the cortex, although there is a reduction in the proportion of binocular units. This change might partly be due to a difference in the timing of signals from the two eyes but is more likely to be caused by a difference in the strength of the discharges. 4. There is little change in binocularity if one image is dimmed but contrast is absent from both. 5. If contours of very different orientation fall simultaneously on corresponding retinal regions, binocularity breaks down, as in the case of strabismus or when different patterns are presented to the two eyes. But as long as the patterns on corresponding retinal points have similar orientation, even if the visual axes are misaligned, binocularity can be maintained. 6. If the eyes are not stimulated simultaneously, binocularity is reduced, even if the contours falling on the two retinae (at different times) are identical. 7. Roughly simultaneous stimulation, with roughly congruent patterns on the two receptive fields, are needed for the upkeep of binocular connexions on to cortical cells.
Full text
PDF



















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albus K. A quantitative study of the projection area of the central and the paracentral visual field in area 17 of the cat. II. The spatial organization of the orientation domain. Exp Brain Res. 1975 Dec 22;24(2):181–202. doi: 10.1007/BF00234062. [DOI] [PubMed] [Google Scholar]
- Albus K. Predominance of monocularly driven cells in the projection area of the central visual field in cat's striate cortex. Brain Res. 1975 May 23;89(2):341–347. doi: 10.1016/0006-8993(75)90725-8. [DOI] [PubMed] [Google Scholar]
- Barlow H. B., Levick W. R. Changes in the maintained discharge with adaptation level in the cat retina. J Physiol. 1969 Jun;202(3):699–718. doi: 10.1113/jphysiol.1969.sp008836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow H. B., Levick W. R. Three factors limiting the reliable detection of light by retinal ganglion cells of the cat. J Physiol. 1969 Jan;200(1):1–24. doi: 10.1113/jphysiol.1969.sp008679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore C., Cooper G. F. Development of the brain depends on the visual environment. Nature. 1970 Oct 31;228(5270):477–478. doi: 10.1038/228477a0. [DOI] [PubMed] [Google Scholar]
- Blakemore C., Van Sluyters C. V., Movshon J. A. Synaptic competition in the kitten's visual cortex. Cold Spring Harb Symp Quant Biol. 1976;40:601–609. doi: 10.1101/sqb.1976.040.01.056. [DOI] [PubMed] [Google Scholar]
- Blakemore C., Van Sluyters R. C. Innate and environmental factors in the development of the kitten's visual cortex. J Physiol. 1975 Jul;248(3):663–716. doi: 10.1113/jphysiol.1975.sp010995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore C., Van Sluyters R. C., Peck C. K., Hein A. Development of cat visual cortex following rotation of one eye. Nature. 1975 Oct 16;257(5527):584–586. doi: 10.1038/257584a0. [DOI] [PubMed] [Google Scholar]
- Blakemore C., Van Sluyters R. C. Reversal of the physiological effects of monocular deprivation in kittens: further evidence for a sensitive period. J Physiol. 1974 Feb;237(1):195–216. doi: 10.1113/jphysiol.1974.sp010478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. RECEPTIVE FIELDS OF CELLS IN STRIATE CORTEX OF VERY YOUNG, VISUALLY INEXPERIENCED KITTENS. J Neurophysiol. 1963 Nov;26:994–1002. doi: 10.1152/jn.1963.26.6.994. [DOI] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. Receptive fields, binocular interaction and functional architecture in the cat's visual cortex. J Physiol. 1962 Jan;160:106–154. doi: 10.1113/jphysiol.1962.sp006837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry G. H., Bishop P. O., Coombs J. S. Inhibitory and sub-liminal excitatory receptive fields of simple units in cat striate cortex. Vision Res. 1969 Oct;9(10):1289–1296. doi: 10.1016/0042-6989(69)90116-3. [DOI] [PubMed] [Google Scholar]
- Hirsch H. V., Spinelli D. N. Modification of the distribution of receptive field orientation in cats by selective visual exposure during development. Exp Brain Res. 1971 Jun 29;12(5):509–527. doi: 10.1007/BF00234246. [DOI] [PubMed] [Google Scholar]
- Hubel D. H., Wiesel T. N. Binocular interaction in striate cortex of kittens reared with artificial squint. J Neurophysiol. 1965 Nov;28(6):1041–1059. doi: 10.1152/jn.1965.28.6.1041. [DOI] [PubMed] [Google Scholar]
- Hubel D. H., Wiesel T. N. Sequence regularity and geometry of orientation columns in the monkey striate cortex. J Comp Neurol. 1974 Dec 1;158(3):267–293. doi: 10.1002/cne.901580304. [DOI] [PubMed] [Google Scholar]
- Hubel D. H., Wiesel T. N. The period of susceptibility to the physiological effects of unilateral eye closure in kittens. J Physiol. 1970 Feb;206(2):419–436. doi: 10.1113/jphysiol.1970.sp009022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Movshon J. A., Blakemore C. Functional reinnervation in kitten visual cortex. Nature. 1974 Oct 11;251(5475):504–505. doi: 10.1038/251504a0. [DOI] [PubMed] [Google Scholar]
- Movshon J. A. Reversal of the physiological effects of monocular deprivation in the kitten's visual cortex. J Physiol. 1976 Sep;261(1):125–174. doi: 10.1113/jphysiol.1976.sp011551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson C. R., Freeman R. D. Progressive changes in kitten striate cortex during monocular vision. J Neurophysiol. 1975 Jan;38(1):26–32. doi: 10.1152/jn.1975.38.1.26. [DOI] [PubMed] [Google Scholar]
- Peck C. K., Blakemore C. Modification of single neurons in the kitten's visual cortex after brief periods of monocular visual experience. Exp Brain Res. 1975;22(1):57–68. doi: 10.1007/BF00235411. [DOI] [PubMed] [Google Scholar]
- Sherman S. M. Development of interocular alignment in cats. Brain Res. 1972 Feb 25;37(2):187–203. doi: 10.1016/0006-8993(72)90666-x. [DOI] [PubMed] [Google Scholar]
- Sherman S. M., Guillery R. W., Kaas J. H., Sanderson K. J. Behavioral, electrophysiological and morphological studies of binocular competition in the development of the geniculo-cortical pathways of cats. J Comp Neurol. 1974 Nov 1;158(1):1–18. doi: 10.1002/cne.901580102. [DOI] [PubMed] [Google Scholar]
- Stryker M. P., Sherk H. Modification of cortical orientation selectivity in the cat by restricted visual experience: a reexamination. Science. 1975 Nov 28;190(4217):904–906. doi: 10.1126/science.1188372. [DOI] [PubMed] [Google Scholar]
- Tretter F., Cynader M., Singer W. Modification of direction selectivity of neurons in the visual cortex of kittens. Brain Res. 1975 Jan 24;84(1):143–149. doi: 10.1016/0006-8993(75)90808-2. [DOI] [PubMed] [Google Scholar]
- WIESEL T. N., HUBEL D. H. SINGLE-CELL RESPONSES IN STRIATE CORTEX OF KITTENS DEPRIVED OF VISION IN ONE EYE. J Neurophysiol. 1963 Nov;26:1003–1017. doi: 10.1152/jn.1963.26.6.1003. [DOI] [PubMed] [Google Scholar]
- Wiesel T. N., Hubel D. H. Comparison of the effects of unilateral and bilateral eye closure on cortical unit responses in kittens. J Neurophysiol. 1965 Nov;28(6):1029–1040. doi: 10.1152/jn.1965.28.6.1029. [DOI] [PubMed] [Google Scholar]




