Abstract
Ninety-five household contacts (aged 2 months to 73 years) of patients with enteropathic hemolytic-uremic syndrome (HUS) were investigated for the presence of immunoglobulin (Ig) G antibodies to Shiga toxins Stx2 and Stx1 by Western blot assay. Thirty-one percent of the household contacts and 19% of 327 controls had anti-Stx2 IgG (heavy and light chain [H + L]), 5 and 8%, respectively, had anti-Stx1 IgG (H + L), and 3 and 2%, respectively, had both anti-Stx2 and anti-Stx1 IgG (H + L). The incidence of infections with Stx-producing Escherichia coli (STEC) was determined based on the following diagnostic criteria: STEC isolation, detection of stx gene sequences, free fecal Stx in stool filtrates, and serum IgM antibodies against E. coli O157 lipopolysaccharide. Evidence of STEC infection was observed in 25 household contacts, of whom 18 (72%) were asymptomatic and represented a potential source of infection. Six of 13 (46%) household contacts with Stx2-producing E. coli O157:H7 in stool culture developed anti-Stx2 IgG (H + L), compared to 71% of Stx2-associated HUS cases. In individuals showing anti-Stx2 IgG (H + L), the antibody response was directed against the B subunit in 69% of household contacts and 71% of controls, in contrast to 28% of HUS patients. In this investigation controls had a significant increase of the median of IgM antibodies to O157 lipopolysaccharide (LPS) with age, up to the fifth decade. The lack of disease in household contacts with B subunit-specific antibodies, as well as the significantly higher median of anti-O157 LPS IgM antibodies in controls beyond 4.9 years of age, suggests a protective role for anti-Stx and anti-O157 LPS antibodies.
The enteropathic form of hemolytic-uremic syndrome (HUS) is of growing public health importance. Worldwide, outbreaks and sporadic cases of infections with Shiga toxin (Stx)-producing Escherichia coli (STEC) O157 and non-O157 strains are increasing (9, 14, 17, 25, 33, 59). STEC infections can be asymptomatic or present as diarrhea, hemorrhagic colitis, or HUS (26, 27, 29, 32, 38, 45).
Human STEC strains produce Stx1, Stx2, or Stx2 variants alone or in combination (29, 55). All members of the Stx protein family are structurally and functionally closely related. They consist of the A subunit (≈32 kDa), which is cleaved by the mammalian, membrane-anchored protease furin (15) to yield an enzymatically active A1 fragment of ≈27.5 kDa and noncovalently linked, five identical receptor-binding B subunits (≈7.5 kDa) (29, 37). The A and B subunits of each Stx type can be differentiated by specific immune sera.
Few investigators have addressed the prevalence of anti-Stx antibodies in patients and in healthy (control) populations using sensitive assays, and none have examined persons with mild STEC infections. In HUS patients, the frequency of neutralizing antibodies to Stx1 ranged from 9% in Germany (6) to 20% in the United States (2). Control populations showed a frequency of Stx1 neutralizing antibodies of 2.5% in Germany (6), and 10.6% in the United States (2). The detection of Stx1-neutralizing antibodies correlated well with the detection of immunoglobulin (Ig) G (heavy and light chain [H + L]) antibodies to Stx1, measured by an enzyme-linked immunosorbent assay (ELISA) (30). More recently, Reymond et al. demonstrated that the Western blot assay (WBA) detected IgG (H + L) antibodies against Stx1 with greater specificity and sensitivity than the Stx-neutralizing antibody assay and ELISA (43).
The STEC-induced immune response to Stx2 is still poorly understood. Several investigators showed that serum samples of virtually all HUS patients and controls neutralized Stx2 in vitro (6, 8). Stx2 but not Stx1 appears to be neutralized by nonimmune factors, such as the high-density lipoprotein fraction in serum (8). In order to circumvent this nonspecific neutralization, we used Western blotting technology to detect IgG (H + L) antibodies to Stx2 and demonstrated that 71% of children with Stx2-associated E. coli infection in Germany exhibit anti-Stx2 IgG (H + L) antibodies, compared to 10% of the age-matched control group (35). Furthermore, 85% of the anti-Stx2-reactive patient sera recognized the A subunit and 15% recognized the B subunit of Stx2. In contrast, 45% of the reactive control samples recognized the Stx2 A and 55% recognized the Stx2 B subunit (35). The reason for this difference is not yet clear.
The major sources of food-borne STEC infections are undercooked ground beef and unpasteurized milk (17). However, frequently STEC infections and HUS cannot be linked to particular foods or recognized outbreaks. It is increasingly appreciated that person-to-person transmission plays an important role in institutional settings such as hospitals (29), nursing homes (10, 39), and day care centers (3, 41, 53) and among family members (28, 34). In fact, person-to-person transmission may be more important than contaminated food in sporadic enteropathic HUS (49). However, rates of STEC transmission and resultant illness are largely unknown.
One way to estimate the spreading of STEC infections is to investigate household contacts for STEC shedding. Because the detection of fecal STEC excretion is often limited to a short period of time (22, 56), household contacts can also be monitored for the appearance of antibodies to Stx or STEC-specific antigens. Previous studies attempting to isolate STEC and to detect an immune response among family members and other household contacts of HUS patients showed varied results. For example, in Argentina 42% of healthy household contacts of HUS patients showed Stx1-neutralizing antibodies (31), while in the Netherlands only 4% of the healthy household contacts had Stx1-neutralizing antibodies (58). Although the vast majority of prototypic E. coli O157:H7 and E. coli O157:H− isolates produce Stx2, alone or in combination with Stx2c (37), there are no data available about the frequency of anti-Stx2 antibodies in household contacts of HUS patients.
STEC O157 induces an O-group-specific immune response that has been successfully used diagnostically (6, 11). Due to the diversity of STEC serotypes, however, the usefulness is limited where other serotypes prevail (9, 33, 54). We hypothesized that use of the WBA, especially for the detection of antibodies to Stx2, will improve the identification of infected family members of children with enteropathic HUS. We further hypothesized that the anti-Stx antibody profile differs in patients with HUS, diarrhea, and asymptomatic STEC infections. The objectives of this study therefore were (i) to investigate the frequency of STEC infections in household contacts of HUS patients using STEC isolation and stx genotyping, free Stx (FStx) in stool filtrates, and E. coli O157 lipopolysaccharide (LPS) IgM antibodies in serum samples; (ii) to investigate the prevalence of antibodies to Stx2 and to Stx1 by WBA in index cases with HUS, household contacts, and age-stratified controls; and (iii) to compare the presence of serum antibodies to the A and B subunits of Stx2 and Stx1 in symptomatic (HUS and diarrhea) and asymptomatic individuals.
MATERIALS AND METHODS
Subjects studied. (i) HUS patients.
The diagnosis of HUS was based on the presence of microangiopathic hemolytic anemia (hemoglobin < 10 g/dl) with thrombocytopenia (platelets < 150/nl) and acute nephropathy (serum creatinine above the upper limit for age) according to the criteria of Fong et al. (13). HUS patients were 0.3 to 14.8 years old {median, 3.5 years; mean, 3.9 ± 2.9 years [1 standard deviation (SD)]}. Patients were treated at the Department of Pediatrics of the University Hospital of Hamburg (n = 19) between 1991 and 1997 and the University Children's Hospital of Erlangen-Nürnberg (n = 7) between 1993 and 1994. Some data concerning some of these patients and household contacts have been published previously (34, 35; K. Ludwig, H. Ruder, H. D. Rott, and H. Karch, letter, Lancet 347:196-197, 1996).
(ii) Household contacts.
Household contacts were defined as persons who lived in the same household or visited the family frequently around the time of the diarrhea of the index child. The 95 household contacts (one to eight individuals per index case), aged 2 months to 73 years (median, 32 years; mean, 31 ± 19 years [1 SD]) agreed to participate in the study. The age distribution of household contacts is provided in Fig. 1. Household contacts were identified as parents (n = 46; 25 mothers, 21 fathers), siblings (n = 25; 14 male, 11 female), grandparents (n = 16; 10 female, 6 male), other relatives (n = 5; 2 uncles and 3 cousins), playmates (n = 2), and 1 child-minder. All household contacts were enrolled within the first week after admission of the index cases to the hospital, including 61 enrolled within the first 48 h.
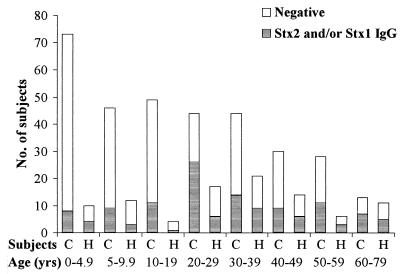
FIG. 1.
Age distribution of controls (C; n = 327) and household contacts (H; n = 95) showing no antibody response or IgG (H + L) against Stx2 and/or Stx1.
(iii) Controls.
Controls representing the regions where the index patients originated were evaluated: 289 individuals were from Hamburg in northern Germany, and 38 were from Erlangen in southern Germany (Bavaria). Control samples were solicited from kindergartens (routine blood samples obtained prior to hepatitis B immunization) and from the outpatient endocrinology clinic (evaluation for growth disorders). Additional samples were from adult volunteers. Controls (n = 327) were 1 month to 77 years old (median, 16 years; mean, 23 ± 20 years [1 SD]). The age distribution of the controls is presented in Fig. 1. None of the controls had known kidney disease or recent diarrhea or was related to an HUS case.
Sera used in the study.
All blood samples were drawn with the informed consent of parents and participants in accordance with the ethical guidelines of the Children's Hospital of the University Hospital of Hamburg-Eppendorf and Erlangen. Samples were separated directly, and sera were stored at −20°C until use. Sera were investigated for IgG antibodies, heavy- and light-chain specific (H + L), against Stx2 and Stx1 and for IgM antibodies against E. coli O157 LPS antibodies.
(i) HUS patients.
Overall 137 serum samples from 26 patients with enteropathic HUS were evaluated. The first serum sample of each patient (n = 26) was taken at the time of admission, which corresponded to 1 to 13 days (median, 7 days) and 0 to 8 days (median, 1 day) after the onset of diarrhea and after the clinical diagnosis of HUS, respectively. Follow-up serum samples (n = 111) were taken up to 12 months after the onset of diarrhea and HUS.
(ii) Household contacts.
The first serum sample was drawn 0 to 6 days (median, 1 day; mean, 1.8 ± 1.7 [1 SD]) after the index case was admitted to the hospital. Sixty-one follow-up samples collected 2 to 5 weeks later were available from 32 household contacts.
(iii) Controls.
Single serum samples from each control (n = 327) were drawn during the same time period as the patients' and household contacts' samples.
Stool studies.
All stool samples of HUS patients from Hamburg were investigated for bacterial enteric pathogens, including Campylobacter, Shigella, Salmonella, and Yersinia spp., enteropathogenic and enterohemorrhagic E. coli, and rotavirus and adenovirus in the University Hospital of Hamburg (Institut für Medizinische Mikrobiologie und Immunologie, Universitätsklinikum Hamburg-Eppendorf, Universität Hamburg, Hamburg, Germany). Additionally, all stool samples from all 26 HUS patients and 95 household contacts were investigated for STEC in a reference laboratory (Institut für Hygiene und Mikrobiologie der Universität Würzburg, Würzburg, Germany), as outlined below.
Stool specimens from HUS patients were collected 1 to 13 days (median, 7 days) after the onset of diarrhea, which corresponded to 0 to 8 days (median, 1 day) after the clinical diagnosis of HUS. Stool specimens from household contacts were collected within the first week (range, 0 to 7 days; median, 1 day) after admission of the HUS patient to the hospital, which corresponded to 3 to 20 days (median, 9 days) after the onset of diarrhea of the index case. The majority of stool samples (61 of 95) were collected within the first 48 h, after the index case had been admitted.
STEC isolation and stx genotyping.
All stool samples were examined for STEC, including E. coli O157, and traditional enteropathogenic E. coli. STEC of serogroup O157 were isolated using sorbitol-MacConkey agar and immunomagnetic separation as described previously (23). Sorbitol-negative colonies from sorbitol-MacConkey agar plates were confirmed biochemically as E. coli and tested for the presence of O157 antigen using a latex slide agglutination test (Oxoid, Wesel, Germany). To detect non-O157 STEC strains, bacterial growths from sorbitol-MacConkey agar were harvested into 1 ml of saline and screened for stx1 and stx2 gene sequences as described previously (24). To identify STEC strains from PCR-positive stool samples, 100 to 200 separate colonies were subjected to colony blot hybridization using digoxigenin-labeled DNA probes derived from strains C600 (H19J) and C600 (933W) using primer pairs KS7/KS8 for stx1 B (51) and GK3/GK4 for stx2 B and stx2c B (18, 24). Isolated STEC strains were serotyped as described previously (1). stx genotypes were determined using the above primer pairs (18, 24, 51). Differentiation of stx2 and stx2c genes was performed by restriction endonuclease analysis using HaeIII and FokI as described by Rüssmann et al. (50). Pulsed-field gel electrophoresis of genomic DNA was carried out according to the protocol of Böhm and Karch (7).
FStx.
Stool filtrates from all HUS patients and household contacts were investigated for the presence of free fecal cytotoxicity using Vero cell monolayers as described by Karmali et al. (26).
E. coli O157 LPS ELISA and O157 LPS immunoblotting.
All serum samples were tested for IgM antibodies against E. coli O157 LPS using ELISA methodology as previously described (6). First, breakpoints for the E. coli O157 LPS IgM ELISA for different age groups were established using serum samples from a total of 438 controls (healthy adult volunteers and children from the outpatient department at the University Children's Hospital Hamburg, who were evaluated for tall or small stature, as well as children from kindergartens with routine blood samples obtained prior to hepatitis B immunization). They were between 0.1 and 77 years of age and represented all age groups. These controls were divided into the following age groups: 0.1 to 4.9 years (n = 116) and 5 to 9.9 years (n = 63) for the first decade of life, and the second (n = 85), third (n = 51), fourth (n = 48), fifth (n = 30), sixth (n = 32), and seventh to eighth (n = 13) decades of life. Mean values and standard deviations calculated for each age group of healthy controls showed the homogeneity of variances (Levene test for variance homogeneity without a significant result).
As the distribution of ELISA readings was skewed with respect to a normal distribution , nonparametric tests were applied. Examination of the medians of the respective age groups yielded a significant effect, meaning that at least one of those groups had a significantly different median (Kruskal-Wallis test, P < 10−4). Inspection of the A405 values of the respective median A405 values of all age groups revealed that three strata could be defined: stratum I (0.1 to less than 5 years), stratum II (5 to less than 50 years), and stratum III (50 years and older). A three-way comparison yielded a statistical significance of P < 10−7. In posttesting, there were significant differences between stratum I and stratum II (P < 10−6) and between stratum II and stratum III (P < 10−4). Therefore, different cutoff levels were defined for the age groups 0.1 to 4.9, 5 to 49, and 50 to 77 years. In the age group of 0.1 to 4.9 years, the A405 value for IgM ranged from 0.000 to 0.359, and the cutoff (mean + 3 SD) was calculated to be 0.350.
For the controls in the age groups 5 to 49 years and 50 years and older, the A405 values for IgM ranged from 0.000 to 0.577 and 0.000 to 0.404, respectively, and the cutoff levels were calculated as 0.500 and 0.400, respectively. All serum samples were tested at least twice. Known positive and negative serum samples were run with each assay as controls.
To establish the sensitivity and specificity of the ELISA, homologous anti-O157 LPS and heterologous non-O157 LPS rabbit immune sera were run on each plate. Selected serum samples, especially those with a weak antibody response by ELISA, were also subjected to immunoblotting using O157 LPS as antigen as previously described (5). A serum sample was considered positive when the A405 value exceeded the mean plus 3 SD of the appropriate controls. Based on the evaluation of the 15 HUS patients harboring E. coli O157 in stool culture and the 327 controls used in this study, the O157 LPS IgM ELISA demonstrated a sensitivity of 100% (15 of 15) and a specificity of 98.5% by using a cutoff of 3 SD above the mean of the controls. Previous studies have shown that breakpoints derived in a manner similar to ours are highly sensitive and specific for diagnosing recent E. coli infection (5, 16, 42).
Shiga toxin preparation and purification.
Stx2 was purified from the E. coli strain R82(pJES) 120DH5α (kindly provided by J. E. Samuel, Department of Medical Microbiology and Immunology, College of Medicine, Texas A & M University, College Station, Tex.), and Stx1 was purified from JB28, an E. coli TB1 strain transformed by recombinant plasmid pUC19B containing the stx1 genes cloned from bacteriophage H19B (21, 44) (kindly provided by J. L. Brunton, University of Toronto, Toronto, Ontario, Canada) using sequential column chromatography (hydroxylapatite, chromatofocusing, and cibachron blue) as previously described (19, 35, 40). The Shiga toxin was resolved into its A and B subunits by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The 27-kDa band next to the A subunit is referred to as the A1 fragment (37). No protein contaminants were detected on the silver-stained SDS-PAGE (36), as shown previously (35).
WBA to detect IgG (H + L) against Stx2 and Stx1.
The WBA was adapted from the method of Towbin et al. (57). A standard concentration of 25 μg of Stx2 and Stx1 was separated on an SDS-PAGE gel using 9% stacking and 15% separating gels and electroblotted onto polyvinylidene difluoride (PVDF) membranes (Bio-Rad Laboratories, Hercules, Calif.) as recently described for Stx1 (35, 43). Membranes were cut into strips and blocked with 50 mM Tris-buffered saline (TBS; pH 7.4) containing 6% skim milk and 12% goat serum for Stx2 and 5% skim milk and 10% goat serum for Stx1 (35). Blots were incubated with diluted serum samples (human serum, 1:100, and immune rabbit serum 1:10,000) in TBS (containing 1% skim milk and 4% goat serum for Stx2 and 1% skim milk and 2% goat serum for Stx1) and developed for bound IgG (H + L) exactly as described using a chemiluminescent detection system (ECL; Amersham, Little Chalfont, United Kingdom). Each serum was tested at least twice. Blots were interpreted in a blinded fashion. A serum was considered positive by showing an anti-Stx2 and/or anti-Stx1 IgG (H + L) antibody response to at least one of the following: the A subunit, the A1 fragment, or the B subunit. To establish the specificity of the WBA, sera from rabbits immunized with Stx2 or Stx1 toxoids prepared by glutaraldehyde treatment were used (4, 35, 44).
Statistical methods.
The chi-square test, if necessary with Bonferroni correction, Fisher's exact test, Levene test, Kruskal-Wallis test, the Mann-Whitney U test, the t test, and the LeRoy test were used as indicated. Statistical testing was performed using SPSS version 10.0 (SPSS, Inc., Chicago, Ill.) on a standard personal computer.
RESULTS
Clinical data. (i) HUS patients.
Twenty-five of 26 HUS patients presented with diarrhea that was hemorrhagic in 22 cases. One patient presented with abdominal pain, nausea, and vomiting but no diarrhea. This patient showed STEC O157:H7 in stool culture and anti-O57 IgM antibodies in serum samples.
(ii) Household contacts.
Ten of 95 (10.5%) household contacts (3 parents, 6 siblings, and 1 playmate) related to five index cases (19%; one to five family members per index case) had watery diarrhea. None had bloody diarrhea. The onset of diarrhea in household contacts preceded the onset of diarrhea in the index case in three instances by up to 7 days and followed the index case in seven instances as late as 28 days (median, 1 day after the onset of diarrhea of the index case). Three symptomatic siblings of two index patients developed a hemolytic anemia, associated with a thrombocytopenia in one case.
STEC isolates.
STEC was identified in 18 of 26 (69%) stool samples from HUS patients. Isolates were serotyped as O157:H7 (n = 14), and O157:H−, O165:H−, O26, and O111 (H types not available), one isolate each (Table 1). The cytotoxic activity of the stool filtrates (FStx) correlated well with the hybridization results obtained with the stx2- and stx1-specific oligonucleotide probes (Table 1).
TABLE 1.
Correlations between stx genotypes, anti-Stx2 and anti-Stx1 immune responses, anti-0157 LPS IgM antibodies, and diarrheaa
| Group | STEC isolate | No. | FStx (no. of isolates) | Genotype | No. of subjects
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Diarrhea | Asymptomatic | IgG against:
|
IgM against O157 LPS | |||||||
| Stx2 | Stx1 | Stx2 and Stx1 | ||||||||
| HUS patients | O157:H7 | 14 | 14 | stx2b | 14c | 9 | 1 | 14 | ||
| O157:H− | 1 | 1 | stx2 | 1 | 1 | 1 | ||||
| O26:H n.t. | 1 | 1 | stx1 | 1 | ||||||
| O111:H n.t. | 1 | 1 | stx2 and stx1 | 1 | ||||||
| O165:H− | 1 | 1 | stx2 and stx2c | 1 | 1 | |||||
| None | 8 | 1 | N/A | 8 | 6 | 2 | 6 | |||
| Total | 26 | 19 | 26 | 16 | 2 | 2 | 21 | |||
| Household contacts | O157:H7 | 6 | 6 | stx2 | 6 | 4 | 4 | |||
| O157:H7 | 7 | 7 | stx2b | 7 | 2 | 3 | ||||
| O103:H2 | 1 | 1 | stx1 | 1 | 1 | |||||
| O156:H27 | 1 | 1 | stx1 | 1 | ||||||
| None | 3 | N/A | 3 | 1 | ||||||
| None | 77 | 1 | N/A | 77 | 22 | 4 | 3 | 9 | ||
| Total | 95 | 16 | 10 | 85 | 29 | 5 | 3 | 16 | ||
| Controls | 327 | n.d. | n.d. | 327 | 61 | 25 | 8 | 5 | ||
Abbreviations: n.t., not typed; N/A, not applicable; n.d., not done.\
Three patients' and four household contacts' isolates from stool samples also harbored the stx2c gene.\
One patient presented only with abdominal pain, nausea, and vomiting without diarrhea.
STEC isolates from 15 of 95 (16%) household contacts related to seven index cases belonged to three serovars, O157:H7 (n = 13), O103:H2 (1 case), and O156:H27 (1 case) (Table 1). The interval between admission of the index case to hospital and the sampling of STEC-positive stool specimens (n = 15) (mean, 2 ± 2.8 days [1 SD]; median, 0 days) and STEC-negative samples (n = 80) (mean, 2.5 ± 2.6 days [1 SD]; median, 1 day) was not significantly different in household contacts (P = 0.183; Mann-Whitney U test).
STEC O157:H7 isolates were detected significantly more frequently in stool samples from siblings (8 of 25) than in stool samples from parents (3 of 46) (P < 0.05, significant; Fisher's exact test) (Table 2). With the exception of family 7 (Table 3), stx genotypes were identical in each pair of index case and household contacts where STEC isolates were available (P < 0.05; chi-square test). Furthermore, the serotype, fermentation pattern, stx profile (presence of the stx2 gene only), and DNA fingerprinting restriction pattern (observed with XbaI-digested genomic DNA) in STEC isolates of two families (Table 3), including the isolates from one of these index (HUS) cases, suggested infection by the same strain in each family. The isolated O157:H7 strains in each family (from HUS patients and household contacts) had the same virulence factors (they produced Stx2 or Stx2 and Stx2c, enterohemorrhagic E. coli hemolysin, and possessed the eaeA genes). There was no epidemiological association between the families.
TABLE 2.
Evidence of STEC O157 infection in various categories of household contacts by stool culture and anti-O157 LPS IgM antibodies
| Household contact category | No. | No. of contacts with:
|
||
|---|---|---|---|---|
| O157 isolate | No O157 isolate | Anti-O157 LPS IgM | ||
| Mothers | 25 | 2 | ||
| 23 | 3 | |||
| Fathers | 21 | 1 | 20 | |
| Grandparents | 16 | 16 | 5 | |
| Siblings | 25 | 8 | 6 | |
| 17 | 1 | |||
| Other relatives | 5 | 5 | ||
| Playmates | 2 | 1 | 1 | |
| Child-minder | 1 | 1 | 1 | |
| Total | 95 | 13 | 7 | |
| 82 | 9 | |||
TABLE 3.
STEC isolates and genotypes in stool specimens from 15 household contacts in relation to the seven HUS (index) patients
| Family no. | Household contacts
|
HUS patients
|
|||
|---|---|---|---|---|---|
| No. of contacts with isolate | Serotype | Genotype | Serotype | Genotype | |
| 1 | 1 | O157:H7 | stx2 | O157:H7 | stx2 |
| 2 | 3 | O157:H7 | stx2 and stx2c | O157:H7 | stx2 and stx2c |
| 3 | 1 | O157:H7 | stx2 and stx2c | O157:H7 | stx2 and stx2c |
| 1 | O156:H27 | stx1 | |||
| 4 | 2 | O157:H7 | stx2 | O157:H7 | stx2 |
| 5 | 1 | O157:H7 | stx2 | O157:H7 | stx2 |
| 6 | 5 | O157:H7 | stx2 | Negative | N/Aa |
| 7 | 1 | O103:H2 | stx1 | O157:H7 | stx2 |
N/A, not applicable.
Six of the 13 household contacts harboring STEC O157:H7 in stool culture had nonbloody diarrhea, while seven patients with STEC O157:H7 in stool samples were asymptomatic (Table 1). The mother with the STEC O103:H2 isolate had nonbloody diarrhea. The carrier of the STEC O156:H27 isolate had no clinical symptoms (Table 1). Taken together, STEC O157 and non-O157 serogroups were isolated in 7 of 10 symptomatic (70%) and in 8 of 85 asymptomatic (9%) household contacts (P < 0.0001, highly significant; Fisher's exact test) (Table 1).
Detection rates of FStx in stool filtrates from HUS patients and household contacts are shown in Table 1. Two individuals, a child with HUS and one sibling, had detectable FStx but no STEC isolate (Table 1). Overall, the detection of FStx strongly correlated with the isolation of STEC in HUS patients and in household contacts (P < 0.0001, Fisher's exact test), regardless of whether individuals carried STEC O157 or non-O157 serotypes and whether they had diarrhea or not (Table 1).
Antibodies to E. coli O157 LPS. (i) HUS patients.
Anti-O157 LPS IgM antibodies were detected in serum samples of all 15 HUS (index) patients with E. coli O157 isolates in stool culture, as well as in six of eight HUS patients without STEC O157 isolate (Table 1). Patients and household contacts whose stool samples contained STEC belonging to serogroups other than O157 (n = 5) did not have antibodies to the O157 LPS (Table 1).
(ii) Household contacts.
Sixteen of 95 (17%) household contacts exhibited anti-E. coli O157 IgM antibodies (Table 1). Of 13 STEC O157:H7-infected household contacts, seven (54%) had detectable E. coli O157 LPS IgM antibodies (4 of 6 with diarrhea, as well as 3 of 7 without diarrhea; not significant, Fisher's exact test) (Table 1). This contrasts with 100% (15 of 15) anti-O157 LPS IgM positivity in HUS patients. STEC infection of contact persons, symptomatic and asymptomatic, closely correlated with documented STEC O157 infection of the index case. Specifically, 22 of 95 household contacts had an E. coli O157:H7 isolate and/or anti-O157 IgM antibodies (Table 1). All except one were related to nine index cases, all with documented E. coli O157 infection (by stool culture in eight cases and by anti-O157 LPS IgM antibodies in one case). On the other hand, two household contacts and three HUS patients with non-O157 STEC isolates lacked all anti-O157 LPS antibodies (Table 1). Apparent differences in the frequency of anti-O157 LPS IgM antibodies in various categories of household contacts (parents, siblings, grandparents, other relatives, playmates, and child-minder) are detailed in Table 2. The incidence of anti-O157 LPS IgM was highest in grandparents and siblings, with 31 and 28%, respectively.
(iii) Controls.
Of the 327 controls used in this study, 1.5% (n = 5) of the control persons exceeded the cutoff level for anti-O157 LPS IgM antibodies.
Cumulative evidence of STEC infection in household contacts.
A total of 25 of 95 (26%) household contacts had cumulative evidence of STEC O157 or non-O157 STEC infection by using STEC isolation and stx genotyping, FStx in stool filtrates, and anti-O157 LPS IgM antibodies in serum samples (Table 1). Cumulative evidence of STEC O157 or non-O157 infection was found in one to five household contacts from 42% (11 of 26) of index cases. Altogether, 18 of 25 (72%) infected household contacts were asymptomatic. Based on all diagnostic criteria as listed above, siblings and grandparents had the highest rate of STEC infection, with 40% (10 of 25, including one sister with FStx only) and 37.5% (6 of 16), respectively, followed by mothers, with 24% (6 of 25), and fathers, with 5% (1 of 21) (significant; contingency table analysis of two-by-four table; P < 0.05, chi-square test).
Antibodies to Stx2 and Stx1.
The detection rates of serum antibodies against Stx2 and Stx1 in HUS patients, household contacts, and healthy controls are shown in Table 1. The age distribution of anti-Stx2 and anti-Stx1 IgG (H + L) in controls and household contacts is provided in Fig. 1.
Twenty of 26 (77%) HUS patients and 37 of 95 (39%) household contacts had detectable anti-Stx2 and/or anti-Stx1 IgG (H + L) antibodies, compared with 94 of 327 (29%) controls (P < 10−5, chi-square test, two-by-three contingency table) (Table 1).
HUS patients developed antibodies against Stx2 significantly more often than household contacts and controls {P < 0.01 for HUS patients versus household contacts (18 of 26 [69%] versus 32 of 95 [34%], chi-square test with Bonferroni correction) and P < 0.05 for household contacts versus controls (32 of 95 [34%] versus 69 of 327 [21%]; chi-square test with Bonferroni correction)}. No significant differences were observed when the samples were tested against Stx1, with a frequency of 15% in HUS patients, 8% in household contacts, and 10% in controls (P = 0.58, chi-square test; two-by-three table).
The stx genotype of STEC isolates and the individuals' immune responses revealed that the serum reactivity closely matched the Stx type of the infective strain (Table 1). In HUS patients, 12 of 17 cases (71%) suffering from diarrhea after an infection with Stx2-producing E. coli (Stx2 alone or in combination with Stx1 or Stx2c) showed IgG (H + L) against Stx2 alone (n = 10) or against both toxins (n = 2) (Table 1). In household contacts, in whom diarrhea was not common, four of six cases (67%) suffering from diarrhea following an infection with Stx2-producing E. coli showed an anti-Stx2 response in serum, while only two of seven (29%) household contacts without diarrhea after an infection with Stx2-producing E. coli showed an anti-Stx2 IgG (H + L) response (Table 1). Comparison of the respective two-by-two tables (number with diarrhea versus number with antibody response for household contacts and for patients, respectively) was performed by the LeRoy test for comparison of two tables. Formally, significance was achieved with P = 0.025. This result can, however, only be interpreted as a trend towards a closer association between antibody response and diarrhea in patients because this statistical test must be interpreted as approximate, given the small frequencies of the respective populations. In total, 6 of 13 household contacts with E. coli O157:H7 isolates (harboring the stx2 gene) in stool culture showed IgG (H + L) antibodies against Stx2. Of the two household contacts with E. coli isolates in stool samples carrying the stx1 gene, one showed detectable anti-Stx1 IgG (H + L) in serum (Table 1).
We were interested to know whether differences in the prevalence of anti-Stx antibodies existed between various categories of household contacts, namely, parents, siblings, and more distant relatives that could be used as surrogate markers for the exposure to the source of infection and, possibly, for the relative susceptibility to STEC infections. We estimated the prevalence of antibodies against Stx2 in different categories of family members. The prevalence was greatest in grandparents, with 44% (7 of 16), followed by the parents, including fathers, with 38% (8 of 21), and mothers, with 32% (8 of 25), and then siblings, with 28% (7 of 25). However, the analysis of the contingency table showed no significant result (P = 0.738, chi-square test). One of two uncles and one of three cousins showed anti-Stx2 IgG (H + L), whereas both playmates and the child-minder showed no antibody response against Stx2.
Of special interest are the five serum samples showing anti-Stx1 IgG (H + L) alone, deriving from mothers in four cases and from one brother. However, the combined frequency of anti-Stx2 and anti-Stx1 was higher in mothers, with 48% (12 of 25), and grandparents, with 44% (7 of 16), than in fathers, with 38% (8 of 21), and siblings, with 32% (8 of 25).
Follow-up serum sampling in household contacts showed only one case of seroconversion, in comparison to HUS patients, among whom seroconversion is quite common (35).
Comparison of household contacts with and without cumulative evidence of STEC infection, according to the criteria defined above, revealed no difference in the presence of anti-Stx2 and/or anti-Stx1 IgG (H + L) per WBA (Table 4).
TABLE 4.
Anti-Stx2 and -Stx1 IgG in household contacts with evidence of STEC infection compared to household contacts without evidence of STEC infection
| Group | Evidence | No. of subjects | No. with IgG against:
|
||
|---|---|---|---|---|---|
| Stx2 | Stx1 | Stx2 and Stx1 | |||
| Evidence of STEC infection | STEC isolate | 15 | 6 | 1 | |
| FStx only | 1 | ||||
| Anti-O157 LPS IgM only | 9 | 2 | 1 | ||
| Total | 25 | 8 | 2 | ||
| No evidence of STEC infection | 70 | 21 | 3 | 3 | |
| Total | 95 | 29 | 5 | 3 | |
Antibodies against the A and/or B subunit of Stx2 and Stx1 in HUS patients, household contacts, and controls.
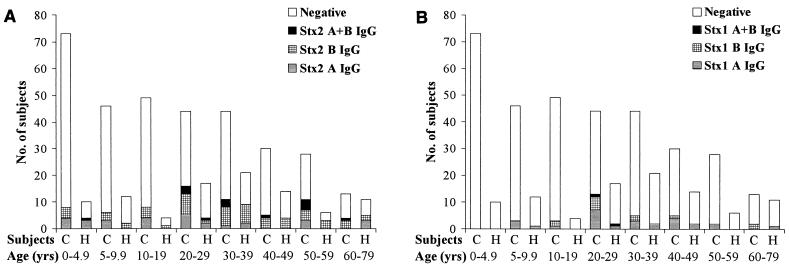
The differences in the immune response pattern to the A and B subunits of Stx2 and Stx1 are summarized in Table 5. The age distribution of an antibody response to the A and/or B subunit of Stx2 and Stx1 in household contacts and controls is presented in Fig. 2A for anti-Stx2 and in Fig. 2B for anti-Stx1 IgG (H + L).
TABLE 5.
Immune response to the A and B subunits of Stx2 and Stx1 in patients with HUS, their household contacts, and controls
| IgG | No. of responders
|
||
|---|---|---|---|
| HUS patients (n = 26) | Household contacts (n = 95) | Controls (n = 327) | |
| Anti-Stx2 | |||
| A subunit | 12 | 7 | 18 |
| B subunit | 2 | 20 | 33 |
| A and B subunits | 2 | 2 | 10 |
| Total | 16 | 29 | 61 |
| Anti-Stx1 | |||
| A subunit | 4 | 13 | |
| B subunit | 11 | ||
| A and B subunits | 2 | 1 | 1 |
| Total | 2 | 5 | 25 |
| Anti-Stx2 and -Stx1 | |||
| Both A subunits | 1 | 3 | 2 |
| Both B subunits | 1 | ||
| Mixeda | 1 | 2 | |
| Mixedb | 3 | ||
| Total | 2 | 3 | 8 |
Subunits A and B of Stx2 and subunit A of Stx1.\
Subunit B of Stx2 and subunit A of Stx1.
FIG. 2.
Age distribution of household contacts (H) and controls (C) showing no antibody response or IgG (H + L) against the A and/or B subunit of Stx2 (A) and Stx1 (B).
A representative Western blot is shown in Fig. 3, demonstrating the anti-Stx antibody response in a HUS patient, family members, and controls. Analysis of the frequency of antibodies against Stx2 revealed that IgG (H + L) against the B subunit of anti-Stx2 was found in household contacts (22 of 32, including two samples reacting with both the A and B subunits) and in controls (49 of 69, including 12 samples reacting with the A and B subunits of Stx2) more frequently than in HUS patients (5 of 18, including three samples with IgG [H + L] against the A and B subunits), not significant for household contacts versus controls, but significant for household contacts versus HUS patients (P < 0.05, Bonferroni-corrected chi-square test) (Table 5).
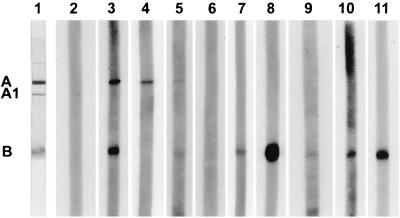
FIG. 3.
Western blot of serum samples from a child with enteropathic HUS and the child's family members and controls using Stx2 as the antigen. Lane 1, blot strip of the PVDF membrane stained with Coomassie blue, showing the A subunit, the A1 fragment, and the B subunit. Lanes 2, 3, 8, and 9 represent human control sera. Lane 2, nonreactive; lane 3, reactive against the A and B subunits; lanes 8 and 9, same control serum reactive against the B subunit of Stx2 at the standard dilution of 1:100 (lane 8) and at a dilution of 1:10,000 (lane 9). Lane 4, Western blot of a 4-year-old patient with enteropathic HUS (reactive against the A subunit of Stx2). Western blots of family members of the patient (lanes 5 to 7, 10, and 11). Lane 5, serum from the 2-year-old brother of the HUS patient (reactive against the A and B subunits of Stx2); lane 6, serum from the 5-year-old brother (nonreactive); and lanes 7, 10, and 11, serum from the 7-year-old brother, 11-year-old sister, and the mother of the patient with HUS (all reactive against the B subunit of Stx2).
The age distribution of household contacts and controls showing IgG (H + L) against the A or B subunit of Stx2 and Stx1 or no antibody response is shown in Fig. 1.
It can be stated that there was no significant age difference between the household contacts and controls with an antibody response against the A or B subunit of Stx2 and/or Stx1. The median age of household contacts showing antibodies against the A subunit of Stx2 and/or Stx1 in serum samples (n = 17) was 27 years (median; range, 1.8 to 63 years). The age of household contacts having detectable antibodies against the B subunit of Stx2 and/or Stx1 (n = 23) was 36 years (median; range, 2.4 to 70 years) (no significant difference; Mann-Whitney U test). Controls showing anti-Stx2 and/or anti-Stx1 IgG (H + L) to the A subunit (n = 49) were 27 years (median; range, 3.8 to 60 years) and those showing antibodies to the B subunit of Stx2 and/or Stx1 (n = 61) were 31 years of age (median; range, 0.9 to 77 years) (not significant).
DISCUSSION
This is the first study to integrate microbiological and serological data, using a more sensitive assay than the commonly employed Stx neutralization test or ELISA (30, 42) for investigating household contacts of HUS patients. Importantly, since most HUS-associated, clinically relevant STEC isolates produce Stx2, but—at least in Europe—rarely Stx1, searching for the presence of Stx2-specific antibodies is highly relevant. It has been previously proposed that existing anti-Stx antibodies decrease the risk of HUS. For example, Karmali et al. previously reported that none of four symptomatic individuals (one with HUS, three with diarrhea) after infection with an Stx1-producing E. coli O111 during a farm visit showed neutralizing antibodies against Stx1, while all of seven asymptomatic relatives living on the farm had demonstrable neutralizing antibodies against Stx1 (30). The same investigators also showed that the prevalence of Stx1-neutralizing antibodies was significantly greater in a rural (42%) than among an urban population (7%) in Ontario (42). In the present study, 32% (27 of 85) of asymptomatic household contacts exhibited anti-Stx2 IgG (H + L), suggestive of previous exposure to an Stx2-producing organism.
The true rate of infection of household contacts is unknown and probably underestimated in this and other comparable studies, since stool samples were not repeatedly examined after and—perhaps as important—before a family member developed HUS and came to medical attention (20, 31).
Several studies in recent years reported person-to-person transmission of STEC O157 in institutional settings such as nursing homes, hospitals, and day care centers, and also among family members (3, 28, 34, 41, 48, 53). There are no data available about the frequency of anti-Stx2 and anti-Stx1 antibodies in persons in close household contact with HUS patients. We have recently shown that most patients with HUS mount an IgG (H + L) antibody response to Stx2, detectable by WBA (35). Western blotting affords increased sensitivity and specificity compared with traditional neutralization and toxin-based enzyme immunoassays (16, 30, 35, 42, 43).
In the present study we used the WBA with Stx2 and Stx1 as antigens to establish the prevalence of anti-Stx2 and anti-Stx1 IgG (H + L) antibodies in household contacts of HUS patients and in age-stratified healthy controls. We compared the pattern of anti-Stx antibodies to those of the index cases with HUS and the stx genotypes of the isolated STEC strains.
By using a combination of isolation and molecular and serological techniques, we found that 26% (25 of 95) of household contacts had evidence of STEC infection. Individuals were considered to have STEC infection when they fulfilled one or more of the following diagnostic criteria: (i) isolation of Stx-producing organisms from stool samples (isolation of bacteria and presence of stx genes), (ii) detection of free fecal Stx and/or stx gene sequences in the absence of an isolate, and (iii) elevated serum IgM antibodies against E. coli O157 LPS. In this study, siblings had the highest rate of evidence of STEC infection, with 40% (10 of 25).
Infected household contacts presented with a spectrum of symptoms, including asymptomatic excretion, watery diarrhea (11% of household contacts), hemolytic anemia (2%), and thrombocytopenia with hemolytic anemia (1%). None developed bloody diarrhea or full-blown HUS. We identified symptomatic household contacts for 5 of 26 HUS patients (19%) (one to five household contacts per index case). While the number of HUS patients with infected household contacts varies in previously reported studies (20, 31, 46), depending on study design and technique applied, it becomes apparent that STEC can be carried—and probably transmitted—by asymptomatic persons, albeit only for brief periods of time. This may explain the “sporadic” nature of childhood HUS, where cases of diarrhea—if identified—and HUS merely represent the tip of the iceberg of endemic infection.
The high frequency of FStx in stool samples (39%) observed by Lopez et al. was not confirmed in our investigation (31). In our study we found FStx in stool samples of 17% (16 of 95) of household contacts. However, in 15 of these 16 cases, the detection of FStx was confirmed by STEC isolates (Table 1). STEC was isolated significantly more often in symptomatic (70%) than in asymptomatic household contacts (P < 0.0001; Fisher's exact test). Our results indicate that individuals in closest contact to the HUS patient (siblings and mothers) were more likely to become infected with STEC than fathers, grandparents, and other relatives, suggesting once more the importance of person-to-person transmission in the spread of STEC. For example, 8 of 25 siblings but only 3 of 46 parents showed detectable STEC O157:H7 isolates in stool specimens (P < 0.05, significant; Fisher's exact test).
E. coli O157:H7 was isolated in 13 household contacts of six HUS (index) cases (Table 3), which corresponds to the high incidence of E. coli O157-specific IgM antibodies in household contacts, with 17% (16 of 95), whereas only 1.5% (5 of 327) of controls showed detectable anti-O157 IgM. However, whereas all 15 HUS patients with E. coli O157 isolates developed measurable anti-O157 LPS IgM antibodies, only 54% of household contacts with E. coli O157 in stool culture developed anti-O157 LPS antibodies (four of six with nonbloody diarrhea and three of seven asymptomatic household contacts). This did not correspond to the study of Heuvelink et al., who found a significant correlation for the presence of serum IgM class antibodies against O157 LPS to (bloody) diarrhea in household contacts (20).
Interestingly, in our study 9 of 80 (11%) household contacts without an isolate had detectable anti-O157 LPS antibodies, although all nine were asymptomatic, but they derived, all except one, from E. coli O157-associated HUS (index) cases. This reflects the recent exposure of these individuals to E. coli O157, and suggests that these household contacts may have been the source of the organism for the HUS patients, or become colonized from them (34). However, the anti-O157 LPS antibody response represents a sum of responses to the O-specific polysaccharide and the R3 core, and it seems likely that the increase in median IgM titers to the O157 LPS among controls above 5 years of age reflects, in part, recent exposure to organisms with R3 cores (12). This suggests that anti-O157 LPS antibodies might have a protective role in age groups who are commonly not affected by extraintestinal STEC complications like HUS (12). As part of a future study, it would be interesting to separately measure the O antigen and R3 core responses in these individuals.
Other investigators postulated that a primary exposure to STEC is insufficient to stimulate a detectable antibody response to Stx1 and/or Stx2 in many HUS patients (43; T. Takeda, H. Nakao, T. Yamanaka, T. Igarashi, Y. Takeda, and N. Kobayashi, letter, J. Infect. 27:211-213, 1993). Reymond et al. suggested that the failure to develop anti-Stx antibodies renders an individual susceptible to reinfection. This may explain why the same persons may have a second STEC infection, as reported in a few instances (47, 52; T. D. Piscione, M. A. Karmali, D. Stephens, R. Donckerwolcke, P. I. Tarr, E. Harvey, and G. S. Arbus. Abstr. 3rd Int. Symp. Workshop Shiga Toxin [Verocytotoxin]-Producing E. coli Infect., abstr. 213/I, 1997).
Recently we reported that 71% of children with HUS due to Stx2-producing E. coli strains develop an anti-Stx2-directed immune response (35). In this investigation the frequency of an anti-Stx2 response following infection by Stx2-producing E. coli O157 or non-O157 strains was 12 of 17 (71%) in HUS patients and 6 of 13 (46%) in household contacts (Table 1). However, four of six with diarrhea and two of seven asymptomatic household contacts with Stx2-producing E. coli infection developed anti-Stx2 IgG (H + L). The results of the present study clearly show that the overall frequency of anti-Stx2 response is highest in HUS patients (69%) and higher in household contacts (34%) than in controls (21%).
The peak age incidence of STEC infection, with its complications such as HUS, is in early childhood and in the elderly (10, 17, 29, 38, 48), suggesting that there might develop an age-related increase in immunity. This is supported by the fact of anti-Stx2 and -Stx1 IgG (H + L) with the lowest frequency in controls in early childhood (0.1 to 4.9 years) with 11%, an increase in subsequent age groups, and a peak of 59% (26 of 44) in the third decade of life (Fig. 1). However, in the control group aged from 60 to 79 years, 38% had detectable anti-Stx2 IgG (H + L), although HUS also affects the elderly population (10, 29). In previous studies it was shown that immunization of laboratory animals with Stx1 B subunit protects them against systemic challenge with Stx1 or Shiga toxin (4). Furthermore we could show that immunization of rabbits with Stx2 toxoid provides protection against challenge not only by Stx2 but also by Stx1, although neutralizing antibody titers were elevated for Stx2 and not for Stx1 (K. Ludwig, M. A. Karmali, M. Winkler, and M. Petric. Abstr. 3rd Int. Symp. Workshop Shiga Toxin [Verocytotoxin]-Producing E. coli Infect., abstr. 163/IV, 1997). However, even nine household contacts aged from 2 months to 7.6 years without detectable anti-Stx2 or anti-Stx1 IgG (H + L) antibodies were asymptomatic, including three siblings with either Stx2-producing E. coli O157:H7 in stool culture (two cases) or FStx only (one case).
The observed frequency of antibodies to Stx2 and Stx1 reflects the prevalence of stx2 and stx1 genes in STEC strains endemic in our population and was found among HUS patients and household contacts as well as in STEC-induced diarrhea (25). Consistent with Stx2 being the most frequently expressed toxin by serogroup O157, a higher frequency of anti-Stx2 than of anti-Stx1 was seen in HUS patients (69% versus 15%), in household contacts (34% versus 8%), and in controls (21% versus 10%). Comparing the household contacts without any symptoms (n = 85) and the household contacts with diarrhea (n = 10), it was shown that 32% and 50%, respectively, had detectable anti-Stx2 (Table 1).
The IgG (H + L) antibody used in this study detected not only IgG but also, potentially, IgM and IgA. It is likely, however, that among the symptomatic household contacts and the patients with HUS, we are dealing with IgM and IgG; in the control group, all of whom were asymptomatic, we are dealing with IgG. Nevertheless, focusing on the high frequency of anti-Stx2 and -Stx1 antibodies in selected age groups of household contacts and controls, future studies are necessary to differentiate between recent and previous infection.
As previously reported and confirmed in this study, STEC infections can be entirely asymptomatic. Moreover, asymptomatic infection can induce a detectable immune response to E. coli LPS as well as to Stx, albeit less frequently than in symptomatic infections, especially with HUS. Asymptomatic STEC infection in household contacts represents a potential source of infection via person-to-person transmission. This supports the need to implement strict hygienic measures among family members and other household contacts to prevent spreading the responsible STEC strain and the occurrence of secondary cases of STEC disease.
Of all subjects with anti-Stx2 IgG (H + L), 69% of the household contacts and 71% of the controls showed antibodies against the B subunit of IgG (H + L), in contrast to 28% of HUS patients (Table 5).
The lack of disease in household contacts with B subunit-specific antibodies, as well as the significantly higher median of anti-O157 LPS IgM antibodies in controls (n = 438) beyond 4.9 years of age, suggests a protective role for anti-Stx B subunit and anti-O157 LPS antibodies. If confirmed, active immunization against Stx2 and E. coli O157 LPS may be feasible and effective.
Acknowledgments
K.L. was supported by a grant from Stifterverband für die Deutsche Wissenschaft (Sanitätsrat-Dr.-Emil-Alexander-Huebner-und-Gemahlin-Stiftung), Essen, Germany.
We thank Maike Westphal for technical assistance. Some parts of the anti-Stx IgG (H + L) antibody results appear in the doctoral thesis of Volkan Sarkim. The help of colleagues (M. J. Kemper, H. Altrogge, K. Timmermann, and M. Dietz) and the staff nurses of the pediatric nephrology unit and the outpatient department of the University Children's Hospitals in Hamburg and Erlangen in patient care and sample collection is gratefully acknowledged.
REFERENCES
- 1.Aleksic, S., H. Karch, and J. Bockemühl. 1992. A biotyping scheme for Shiga-like (Vero) toxin-producing Escherichia coli O157 and a list of serological cross-reactions between O157 and other gram-negative bacteria. Int. J. Med. Microbiol. Virol. Parasitol. Infect. Dis. 276:221-230. [DOI] [PubMed] [Google Scholar]
- 2.Barrett, T. J., J. H. Green, P. M. Griffin, A. T. Pavia, S. M. Ostroff, and I. K. Wachsmuth. 1991. Enzyme-linked immunosorbent assays for detecting antibodies to Shiga-like toxin I, Shiga-like toxin II, and Escherichia coli O157:H7 lipopolysaccharide in human serum. Curr. Microbiol. 23:189-195. [Google Scholar]
- 3.Belongia, E. A., M. T. Osterholm, J. T. Soler, D. A. Ammend, J. E. Braun, and K. L. MacDonald. 1993. Transmission of Escherichia coli 0157:H7 infection in Minnesota child day-care facilities. JAMA 269:883-888. [PubMed] [Google Scholar]
- 4.Bielaszewska, M., I. Clarke, M. A. Karmali, and M. Petric. 1997. Localization of intravenously administered verocytotoxins (Shiga-like toxins) 1 and 2 in rabbits immunized with homologous and heterologous toxoids and toxin subunits. Infect. Immun. 65:2509-2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bitzan, M., E. Moebius, K. Ludwig, D. E. Müller-Wiefel, J. Heesemann, and H. Karch. 1991. High incidence of serum antibodies to Escherichia coli O157 lipopolysaccharide in children with hemolytic uremic syndrome. J. Pediatr. 119:380-385. [DOI] [PubMed] [Google Scholar]
- 6.Bitzan, M., K. Ludwig, M. Klemt, H. König, J. Büren, and D. E. Müller-Wiefel. 1993. The role of Escherichia coli O157 infections in the classical (enteropathic) haemolytic uraemic syndrome: results of a Central European, multicentre study. Epidemiol. Infect. 110:183-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Böhm, H., and H. Karch. 1992. DNA fingerprinting of Escherichia coli O157:H7 strains by pulsed-field gel electrophoresis. J. Clin. Microbiol. 30:2169-2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caprioli, A., I. Luzzi, L. Seganti, M. Marchetti, M. A. Karmali, I. Clarke, and B. Boyd. 1994. Frequency and nature of verocytotoxin 2 (VT2) neutralizing activity (NA) in human and animal sera, p. 353-356. In M. A. Karmali and A. G. Goglio (ed.), Recent advances in verocytotoxin-producing Escherichia coli infections. Elsevier Sciences, Amsterdam, The Netherlands.
- 9.Caprioli, A., I. Luzzi, F. Rosmini, C. Resti, A. Edefonti, F. Perfumo, C. Farina, A. Goglio, A. Gianviti, and G. Rizzoni. 1994. Community-wide outbreak of hemolytic-uremic syndrome associated with non-O157 verocytotoxin-producing Escherichia coli. J. Infect. Dis. 169:208-211. [DOI] [PubMed] [Google Scholar]
- 10.Carter, A. O., A. A. Borczyk, J. A. Carlson, B. Harvey, J. C. Hockin, M. A. Karmali, C. Krishnan, D. A. Korn, and H. Lior. 1987. A severe outbreak of Escherichia coli O157:H7-associated hemorrhagic colitis in a nursing home. N. Engl. J. Med. 317:1496-1500. [DOI] [PubMed] [Google Scholar]
- 11.Chart, H., H. R. Smith, S. M. Scotland, B. Rowe, D. V. Milford, and C. M. Taylor. 1991. Serological identification of Escherichia coli O157:H7 infection in haemolytic uraemic syndrome. Lancet 337:138-140. [DOI] [PubMed] [Google Scholar]
- 12.Currie, C. G., K. McCallum, and I. R. Poxton. 2001. Mucosal and systemic antibody responses to the lipopolysaccharide of Escherichia coli O157 in health and disease. J. Med. Microbiol. 50:345-354. [DOI] [PubMed] [Google Scholar]
- 13.Fong, J. S., J. P. de Chadarevian, and B. S. Kaplan. 1982. Hemolytic uremic syndrome: current concepts and management. Pediatr. Clin. North Am. 29:835-856. [DOI] [PubMed] [Google Scholar]
- 14.Fukushima, H., T. Hashizume, Y. Morita, J. Tanaka, K. Azuma, Y. Mizumoto, M. Kaneno, M. Matsuura, K. Konma, and T. Kitani. 1999. Clinical experiences in Sakai City Hospital during the massive outbreak of enterohemorrhagic Escherichia coli O157 infections in Sakai City, 1996. Pediatr. Int. 41:213-217. [DOI] [PubMed] [Google Scholar]
- 15.Garred, O., B. van Deurs, and K. Sandvig. 1995. Furin-induced cleavage and activation of Shiga toxin. J. Biol. Chem. 270:10817-10821. [DOI] [PubMed] [Google Scholar]
- 16.Greatorex, J. S., and G. M. Thorne. 1994. Humoral immune responses to Shiga-like toxins and Escherichia coli O157 lipopolysaccharide in hemolytic-uremic syndrome patients and healthy subjects. J. Clin. Microbiol. 32:1172-1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Griffin, P. M., and R. V. Tauxe. 1991. The epidemiology of infections caused by Escherichia coli O157:H7, other enterohemorrhagic E. coli, and the associated hemolytic uremic syndrome. Epidemiol. Rev. 13:60-98. [DOI] [PubMed] [Google Scholar]
- 18.Gunzer, F., H. Böhm, H. Rüssmann, M. Bitzan, S. Aleksic, and H. Karch. 1992. Molecular detection of sorbitol-fermenting Escherichia coli O157 in patients with hemolytic-uremic syndrome. J. Clin. Microbiol. 30:1807-1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Head, S. C., M. Petric, S. E. Richardson, M. E. Roscoe, and M. A. Karmali. 1988. Purification and characterization of verocytotoxin 2. FEMS Microbiol. Lett. 51:211-216. [Google Scholar]
- 20.Heuvelink, A. E., N. C. van-de-Kar, T. J. van-der-Velden, H. Chart, and L. A. Monnens. 1999. Verocytotoxin-producing Escherichia coli infection in household members of children with hemolytic-uremic syndrome in the Netherlands. Pediatr. Infect. Dis. J. 18:709-714. [DOI] [PubMed] [Google Scholar]
- 21.Huang, A., S. de Grandis, J. Friesen, M. A. Karmali, M. Petric, R. Congi, and J. L. Brunton. 1986. Cloning and expression of the genes specifying Shiga-like toxin production in Escherichia coli H19. J. Bacteriol. 166:375-379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karch, H., H. Rüssmann, H. Schmidt, A. Schwarzkopf, and J. Heesemann. 1995. Long-term shedding and clonal turnover of enterohemorrhagic Escherichia coli O157 in diarrheal diseases. J. Clin. Microbiol. 33:1602-1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karch, H., C. Janetzki-Mittmann, S. Aleksic, and M. Datz. 1996. Isolation of enterohemorrhagic Escherichia coli O157 strains from patients with hemolytic-uremic syndrome by using immunomagnetic separation, DNA-based methods, and direct culture. J. Clin. Microbiol. 34:516-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karch, H., H. I. Huppertz, J. Bockemühl, H. Schmidt, A. Schwarzkopf, and R. Lissner. 1997. Shiga toxin-producing Escherichia coli infections in Germany. J. Food. Prot. 60:1454-1457. [DOI] [PubMed] [Google Scholar]
- 25.Karch, H., M. Bielaszewska, M. Bitzan, and H. Schmidt. 1999. Epidemiology and diagnosis of Shiga toxin-producing Escherichia coli infections. Diagn. Microbiol. Infect. Dis. 34:229-243. [DOI] [PubMed] [Google Scholar]
- 26.Karmali, M. A., B. T. Steele, M. Petric, and C. Lim. 1983. Sporadic cases of hemolytic uremic syndrome associated with fecal cytotoxin and cytotoxin-producing Escherichia coli. Lancet i:619-620. [DOI] [PubMed]
- 27.Karmali, M. A., M. Petric, C. Lim, P. C. Fleming, G. S. Arbus, and H. Lior. 1985. The association between hemolytic uremic syndrome and infection by verotoxin-producing Escherichia coli. J. Infect. Dis. 151:775-782. [DOI] [PubMed] [Google Scholar]
- 28.Karmali, M. A., G. S. Arbus, N. Ish-Shalom, P. C. Fleming, D. Malkin, M. Petric, R. Cheung, S. Louie, G. R. Humphreys, and M. Strachan. 1988. A family outbreak of hemolytic-uremic syndrome associated with verotoxin-producing Escherichia coli serotype O157:H7. Pediatr. Nephrol. 2:409-414. [DOI] [PubMed] [Google Scholar]
- 29.Karmali, M. A. 1989. Infection by verocytotoxin-producing Escherichia coli. Clin. Microbiol. Rev. 2:15-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karmali, M. A., M. Petric, M. Winkler, M. Bielaszewska, J. Brunton, N. van-de-Kar, T. Morooka, G. B. Nair, S. E. Richardson, and G. S. Arbus. 1994. Enzyme-linked immunosorbent assay for detection of immunoglobulin G antibodies to Escherichia coli Vero cytotoxin 1. J. Clin. Microbiol. 32:1457-1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lopez, E. L., M. Diaz, S. Devoto, S. Grinstein, M. Woloj, B. E. Murray, E. Rubeglio, F. Mendilaharzu, M. Turco, M. Vasquez, L. K. Pickering, and T. Cleary. 1991. Evidence of infection with organisms producing Shiga-like toxins in household contacts of children with the hemolytic uremic syndrome. Pediatr. Infect. Dis. J. 10:20-24. [DOI] [PubMed] [Google Scholar]
- 32.Lopez, E. L., V. Prado-Jimenez, M. O'Ryan-Gallardo, and M. M. Contrini. 2000. Shigella and Shiga toxin-producing Escherichia coli causing bloody diarrhea in Latin America. Infect. Dis. Clin. North Am. 14:41-65. [DOI] [PubMed] [Google Scholar]
- 33.Ludwig, K., M. Bitzan, S. Zimmermann, M. Kloth, H. Ruder, and D. E. Müller-Wiefel. 1996. Immune response to non-O157 Vero toxin-producing Escherichia coli in patients with hemolytic uremic syndrome. J. Infect. Dis. 174:1028-1039. [DOI] [PubMed] [Google Scholar]
- 34.Ludwig, K., H. Ruder, M. Bitzan, S. Zimmermann, and H. Karch. 1997. Outbreak of Escherichia coli O157:H7 infection in a large family. Eur. J. Clin. Microbiol. Infect. Dis. 16:238-241. [DOI] [PubMed] [Google Scholar]
- 35.Ludwig, K., M. A. Karmali, V. Sarkim, C. Bobrowski, M. Petric, H. Karch, D. E. Müller-Wiefel, and Arbeitsgemeinschaft für Pädiatrische Nephrologie. 2001. Antibody response to Shiga toxins Stx2 and Stx1 in children with enteropathic hemolytic-uremic syndrome. J. Clin. Microbiol. 39:2272-2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Merril, C. R., D. Goldman, S. A. Sedman, and M. H. Ebert. 1981. Ultrasensitive stain for proteins in polyacrylamide gels shows regional variation in cerebrospinal fluid proteins. Science 211:1437-1438. [DOI] [PubMed] [Google Scholar]
- 37.O'Brien, A. D., V. L. Tesh, A. Donohue-Rolfe, M. P. Jackson, S. Olsnes, K. Sandvig, A. A. Lindberg, and G. T. Keusch. 1992. Shiga toxin: biochemistry, genetics, mode of action, and role in pathogenesis. Curr. Top. Microbiol. Immunol. 180:65-94. [DOI] [PubMed] [Google Scholar]
- 38.Pai, C. H., N. Ahmed, H. Lior, W. M. Johnson, H. V. Sims, and D. E. Woods. 1988. Epidemiology of sporadic diarrhea due to verocytotoxin-producing Escherichia coli: a two-year prospective study. J. Infect. Dis. 157:1054-1057. [DOI] [PubMed] [Google Scholar]
- 39.Pavia, A. T., C. R. Nichols, D. P. Green, R. V. Tauxe, S. Mottice, K. D. Greene, J. G. Wells, R. L. Siegler, E. D. Brewer, D. Hannon, and P. A. Blake. 1990. Hemolytic-uremic syndrome during an outbreak of Escherichia coli O157:H7 infections in institutions for mentally retarded persons: clinical and epidemiologic observations. J. Pediatr. 116:544-551. [DOI] [PubMed] [Google Scholar]
- 40.Petric, M., M. A. Karmali, S. Richardson, and R. Cheung. 1987. Purification and biological properties of Escherichia coli verocytotoxin. FEMS Microbiol. Lett. 41:63-68. [Google Scholar]
- 41.Reida, P., M. Wolff, H. W. Pohls, W. Kuhlmann, A. Lehmacher, S. Aleksic, H. Karch, and J. Bockemühl. 1994. An outbreak due to enterohaemorrhagic Escherichia coli O157:H7 in a children day care centre characterized by person-to-person transmission and environmental contamination. Int. J. Med. Microbiol. Virol. Parasitol. Infect. Dis. 281:534-543. [DOI] [PubMed] [Google Scholar]
- 42.Reymond, D., R. P. Johnson, M. A. Karmali, M. Petric, M. Winkler, S. Johnson, K. Rahn, S. Renwick, J. Wilson, R. C. Clarke, and J. Spika. 1996. Neutralizing antibodies to Escherichia coli Vero cytotoxin 1 and antibodies to O157 lipopolysaccharide in healthy farm family members and urban residents. J. Clin. Microbiol. 34:2053-2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reymond, D., M. A. Karmali, I. Clarke, M. Winkler, and M. Petric. 1997. Comparison of the western blot assay with the neutralizing-antibody and enzyme-linked immunosorbent assays for measuring antibody to verocytotoxin 1. J. Clin. Microbiol. 35:609-613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Richardson, S. E., T. A. Rotman, V. Jay, C. R. Smith, L. E. Becker, M. Petric, N. F. Olivieri, and M. A. Karmali. 1992. Experimental verocytotoxemia in rabbits. Infect. Immun. 60:4154-4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Riley, L. W., R. S. Remis, S. D. Helgerson, H. B. McGee, J. G. Wells, B. R. Davis, R. J. Hebert, E. S. Olcott, L. M. Johnson, N. T. Hargrett, P. A. Blake, and M. L. Cohen. 1983. Hemorrhagic colitis associated with a rare Escherichia coli serotype. N. Engl. J. Med. 308:681-685. [DOI] [PubMed] [Google Scholar]
- 46.Rivas, M., L. E. Voyer, M. Tous, M. F. De-Mena, N. Leardini, R. Wainsztein, R. Callejo, B. Quadri, S. Corti, and V. Prado. 1996. Verocytotoxin-producing Escherichia coli infection in family members of children with hemolytic uremic syndrome. Medicina (Buenos Aires) 56:119-125. [PubMed] [Google Scholar]
- 47.Robson, W. L., A. K. Leung, and D. J. Miller-Hughes. 1993. Recurrent hemorrhagic colitis caused by Escherichia coli O157:H7. Pediatr. Infect. Dis. J. 12:699-701. [DOI] [PubMed] [Google Scholar]
- 48.Rowe, P. C., E. Orrbine, G. A. Wells, and P. N. McLaine. 1991. Epidemiology of hemolytic uremic syndrome in Canadian children from 1986 to 1988. J. Pediatr. 119:218-224. [DOI] [PubMed] [Google Scholar]
- 49.Rowe, P. C., E. Orrbine, M. Ogborn, G. A. Wells, W. Winther, H. Lior, D. Manuel, and P. N. McLaine. 1994. Epidemic Escherichia coli O157:H7 gastroenteritis and hemolytic-uremic syndrome in a Canadian Inuit community: intestinal illness in family members as a risk factor. J. Pediatr. 124:21-26. [DOI] [PubMed] [Google Scholar]
- 50.Rüssmann, H., H. Schmidt, J. Heesemann, A. Caprioli, and H. Karch. 1994. Variants of Shiga-like toxin II constitute a major toxin component in Escherichia coli O157 strains from patients with haemolytic uraemic syndrome. J. Med. Microbiol. 40:338-343. [DOI] [PubMed] [Google Scholar]
- 51.Schmidt, H., H. Rüssmann, A. Schwarzkopf, S. Aleksic, J. Heesemann, and H. Karch. 1994. Prevalence of attaching and effacing Escherichia coli in stool samples from patients and controls. Int. J. Med. Microbiol. Virol. Parasitol. Infect. Dis. 281:201-213. [DOI] [PubMed] [Google Scholar]
- 52.Siegler, R. L., P. M. Griffin, T. J. Barrett, and N. A. Strockbine. 1993. Recurrent hemolytic uremic syndrome secondary to Escherichia coli O157:H7 infection. Pediatrics 91:666-668. [PubMed] [Google Scholar]
- 53.Spika, J. S., J. E. Parsons, D. Nordenberg, J. G. Wells, R. A. Gunn, and P. A. Blake. 1986. Hemolytic uremic syndrome and diarrhea associated with Escherichia coli O157:H7 in a day care center. J. Pediatr. 109:287-291. [DOI] [PubMed] [Google Scholar]
- 54.Starr, M., V. Bennett-Wood, A. K. Bigham, T. F. de-Koning-Ward, A. M. Bordun, D. Lightfoot, K. A. Bettelheim, C. L. Jones, and R. M. Robins-Browne. 1998. Hemolytic-uremic syndrome following urinary tract infection with enterohemorrhagic Escherichia coli: case report and review. Clin. Infect. Dis. 27:310-315. [DOI] [PubMed] [Google Scholar]
- 55.Strockbine, N. A., L. R. M. Marques, J. W. Newland, H. W. Smith, R. K. Holmesand, and A. D. O'Brien. 1986. Two toxin-converting phages from Escherichia coli O157:H7 strain 933 encode antigenically distinct toxins with similar biological activities. Infect. Immun. 53:135-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tarr, P. I., M. A. Neill, C. R. Clausen, S. L. Watkins, D. L. Christie, and R. O. Hickman. 1990. Escherichia coli O157:H7 and the hemolytic uremic syndrome: importance of early cultures in establishing the etiology. J. Infect. Dis. 162:553-556. [DOI] [PubMed] [Google Scholar]
- 57.Towbin, H., T. Staehlin, and J. Gordon. 1979. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. USA 76:4350-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.van de Kar, N. C. A. J., H. G. R. Roelofs, H. L. Muytjens, J. J. M. Tolboom, H. Chart, and A. H. Monnens. 1994. Verocytotoxin-producing Escherichia coli infection in patients with hemolytic uremic syndrome and their family-members in the Netherlands, p. 45-48. In M. A. Karmali and A. G. Goglio (ed.), Recent advances in verocytotoxin-producing Escherichia coli infections. Elsevier Science B. V., Amsterdam, The Netherlands.
- 59.Zhang, W. L., M. Bielaszewska, A. Liesegang, H. Tschäpe, H. Schmidt, M. Bitzan, and H. Karch. 2000. Molecular characteristics and epidemiological significance of Shiga toxin-producing Escherichia coli O26 strains. J. Clin. Microbiol. 38:2134-2140. [DOI] [PMC free article] [PubMed] [Google Scholar]