Abstract
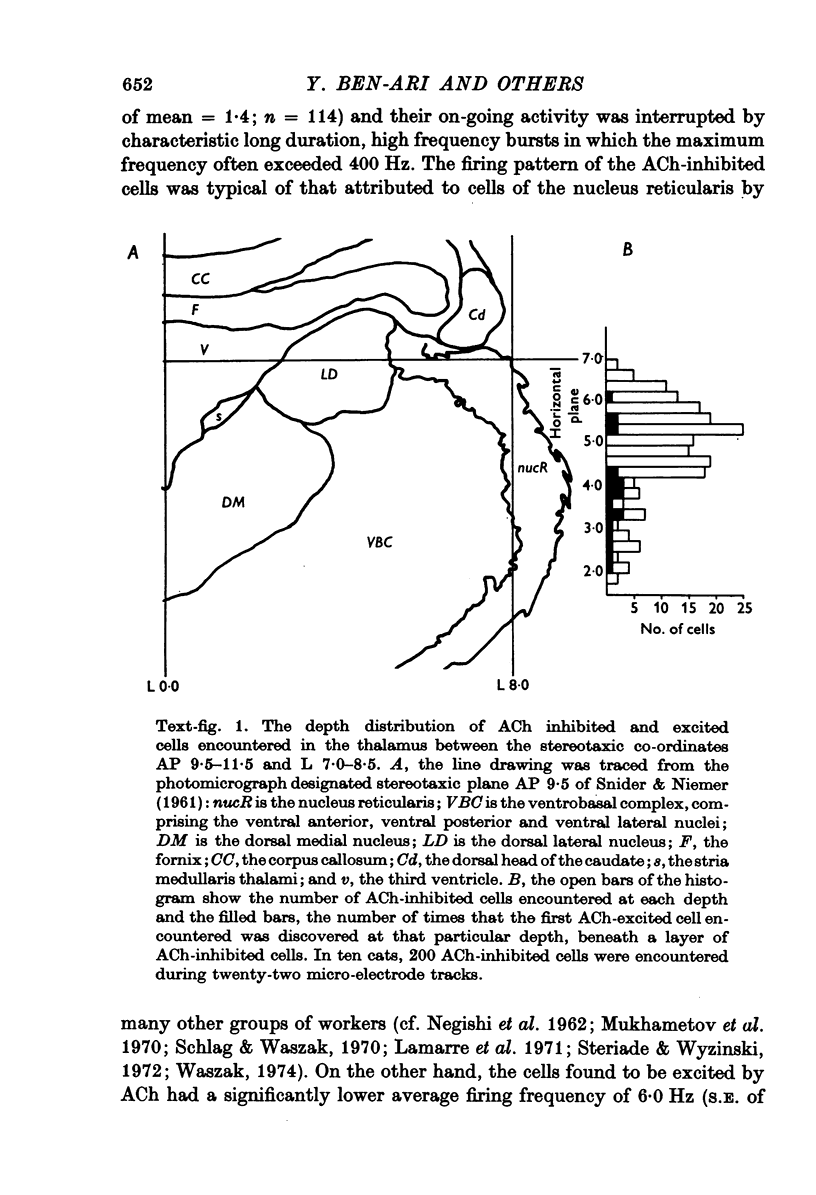
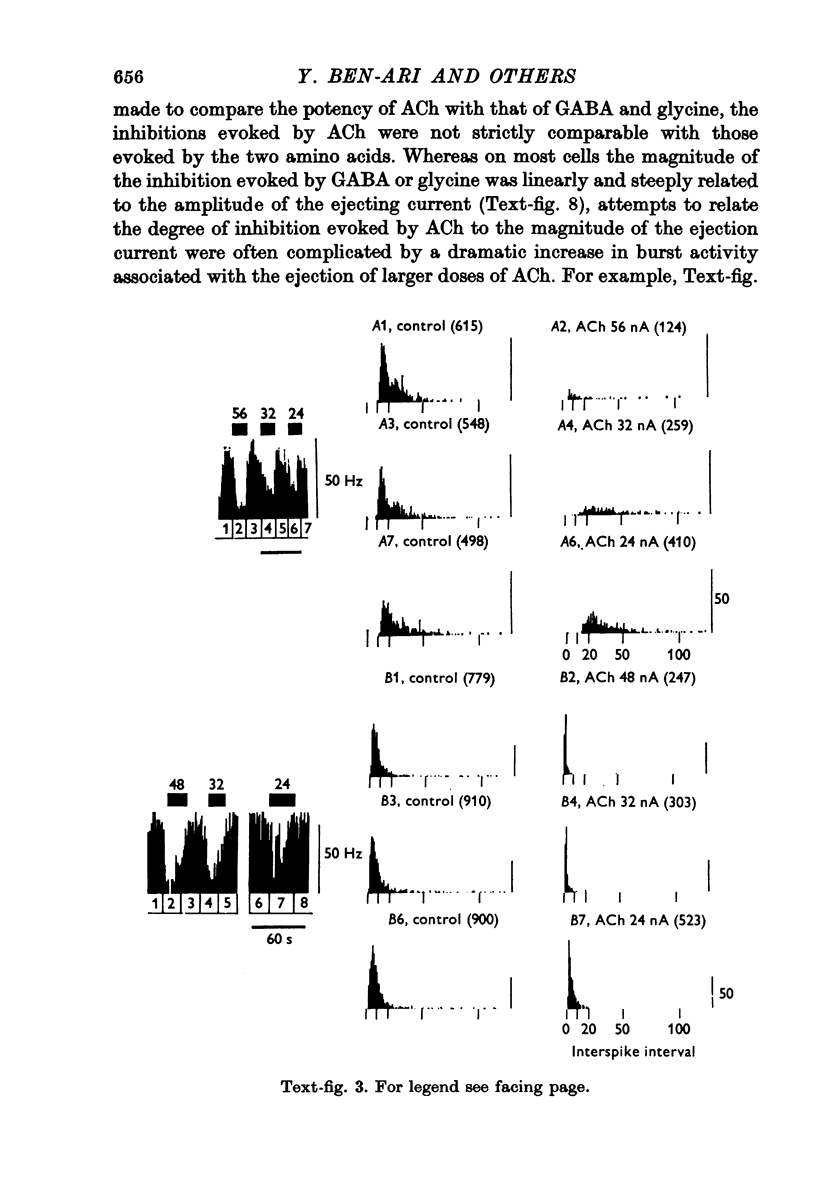
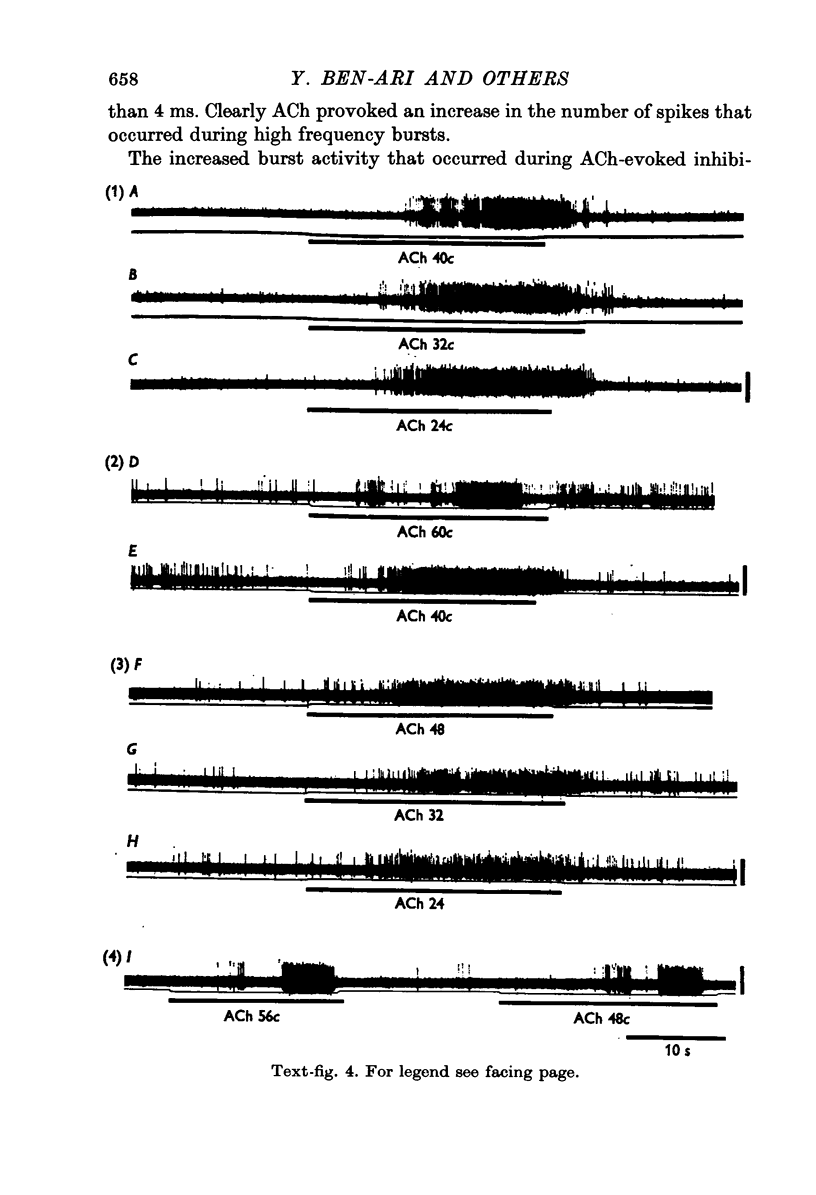
1. Short iontophoretic pulses of acetylcholine (ACh) inhibited almost every spontaneously active cell encountered in the nucleus reticularis thalami of cats anaesthetized with a mixture of halothane, nitrous oxide and oxygen. On 200 cells the mean current needed to eject an effective inhibitory dose of ACh was 67 +/- 2 nA. When the ACh-evoked inhibition was mimicked by gamma-aminobutyric acid (GABA) or glycine on the same cell, the current required to release ACh was found to be approximately twice as great as that required to release an equally effective dose of GABA or glycine. 2. ACh inhibitions developed with a latency which was very much shorter than that for ACh excitation in cells of the ventrobasal complex. The latency of the ACh-evoked inhibition was as rapid as the onset and offset of the excitation of the same cells glutamate and their inhibition by GABA or glycine. 3. The firing pattern of ACh-inhibited neurones in the nucleus reticularis was characterized by periods of prolonged, high frequency bursts, and their mean firing frequency was 22 Hz. Raster dot displays and interspike interval histograms showed that whereas ACh suppressed the spikes that occurred between bursts much more readily than those that occurred during bursts, all spikes were equally sensitive to the depressant action of GABA and glycine. Large doses of ACh provoked or exaggerated burst activity. 4. ACh-evoked inhibition was extremely sensitive to blockade by short iontophoretic applications of atropine, which had no effect on the inhibitions evoked on the same cell equipotent doses of GABA or glycine. The ACh-evoked inhibitions were also antagonized by dihydro-beta-erythroidine released with slightly larger currents. When tested on the same cell, small iontophoretic applications of picrotoxin and bicuculline methoiodide blocked the inhibition evoked by GABA but had no effect on that evoked by ACh. Iontophoretic strychnine only rarely affected the inhibition evoked by ACh, while readily blocking the inhibition evoked on the same cell by an equipotent dose of glycine. In two cats, intravenous strychnine (1-2 mg/kg) had no effect on the ACh-evoked inhibition, while greatly reducing the sensitivity of the cell under study to glycine. 5. Only four out of forty-eight ACh-inhibted cells tested were inhibited by iontophoretic applications of either guanosine or adenosine 3':5'-phosphate. 6. Cells of the nucleus reticularis have been shown to have an inhibitory action on the thalamic relay cells, which are excited by ACh. It is suggested that the presence of both ACh excited and inhibited cells in different nuclei of the thalamus could be of considerable functional significance in gating sensory transmission through the thalamus.
Full text
PDF









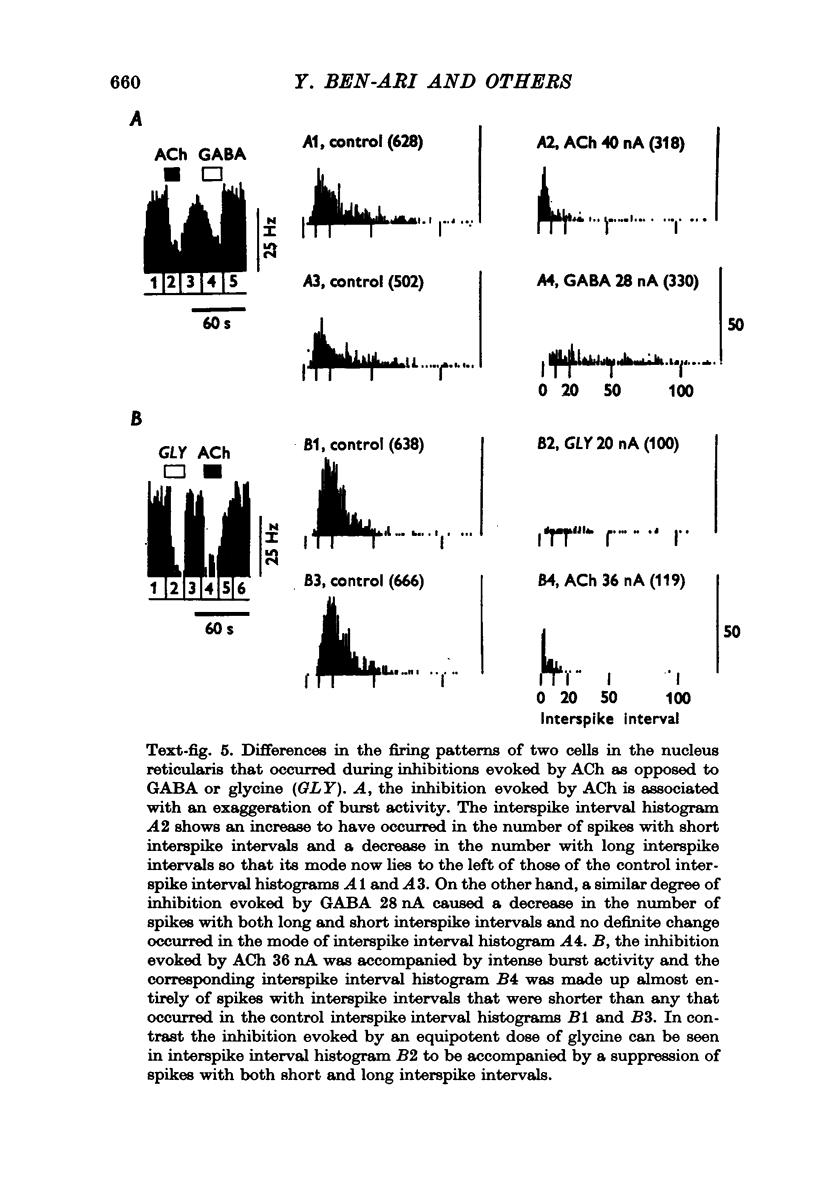
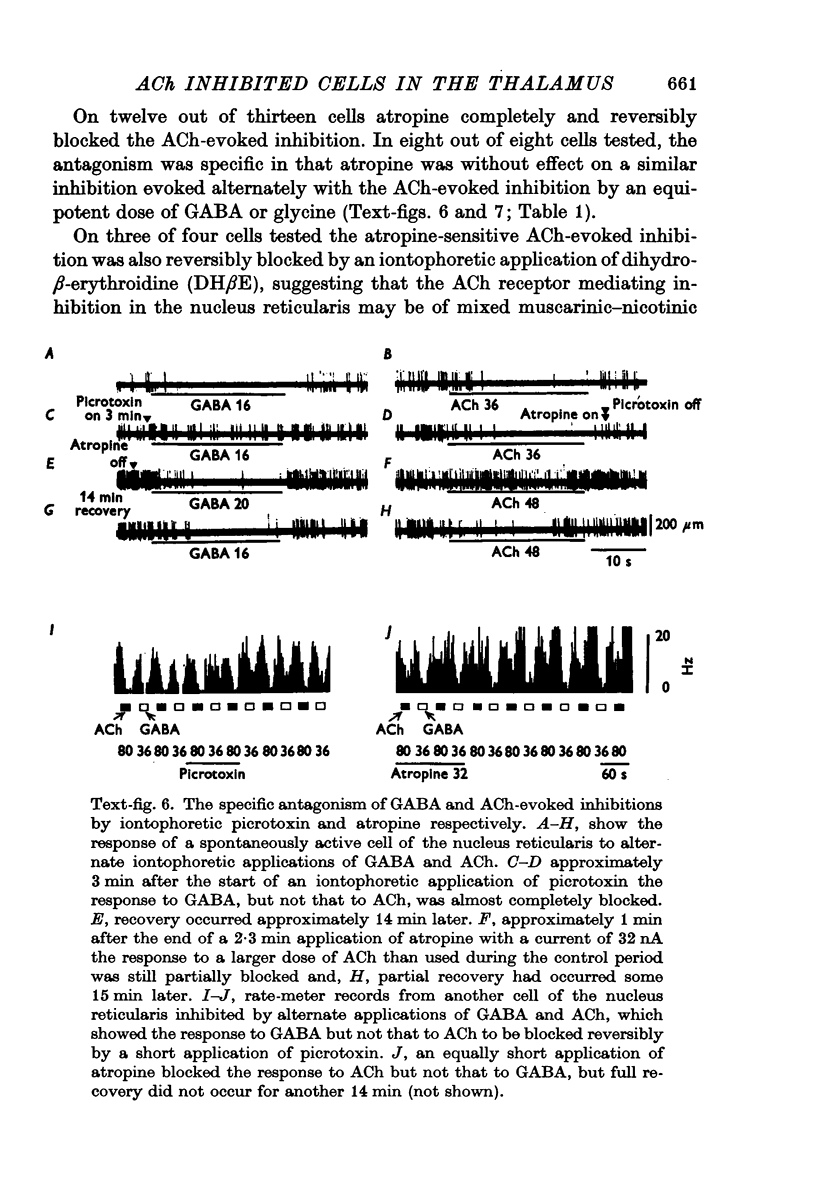

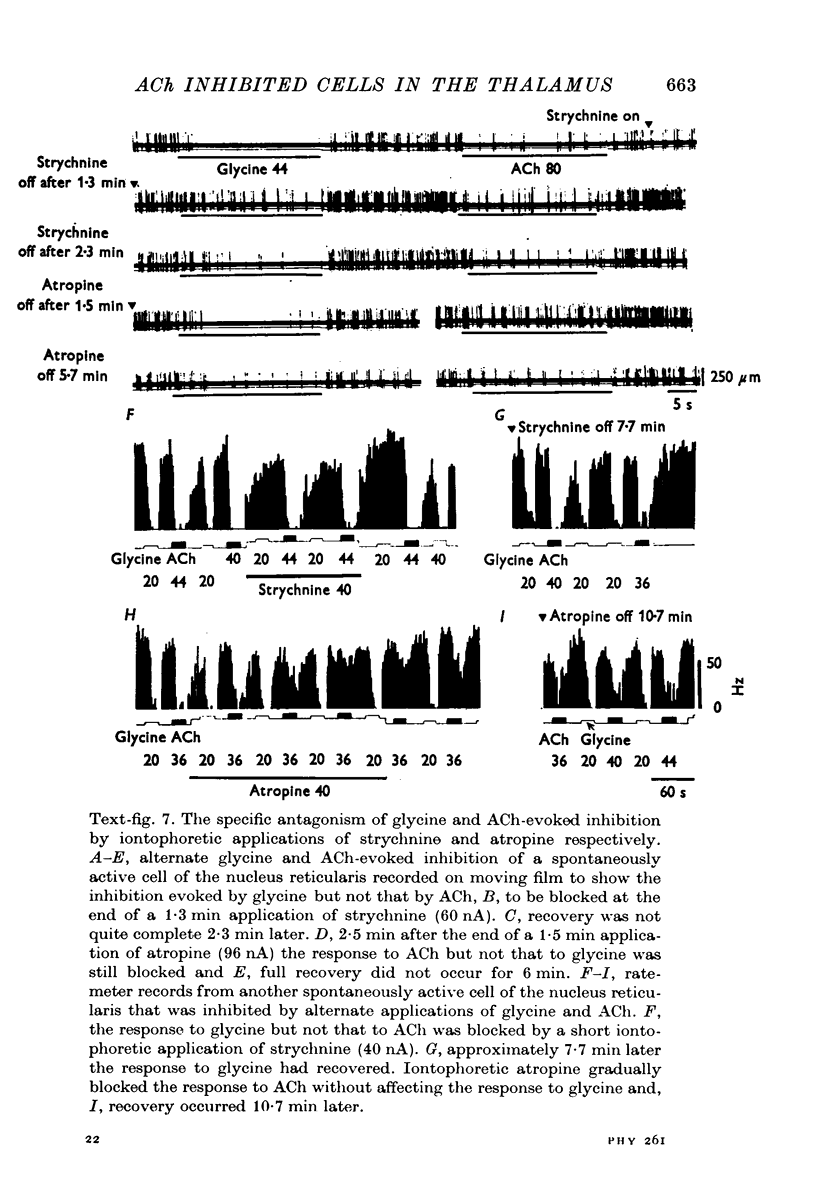
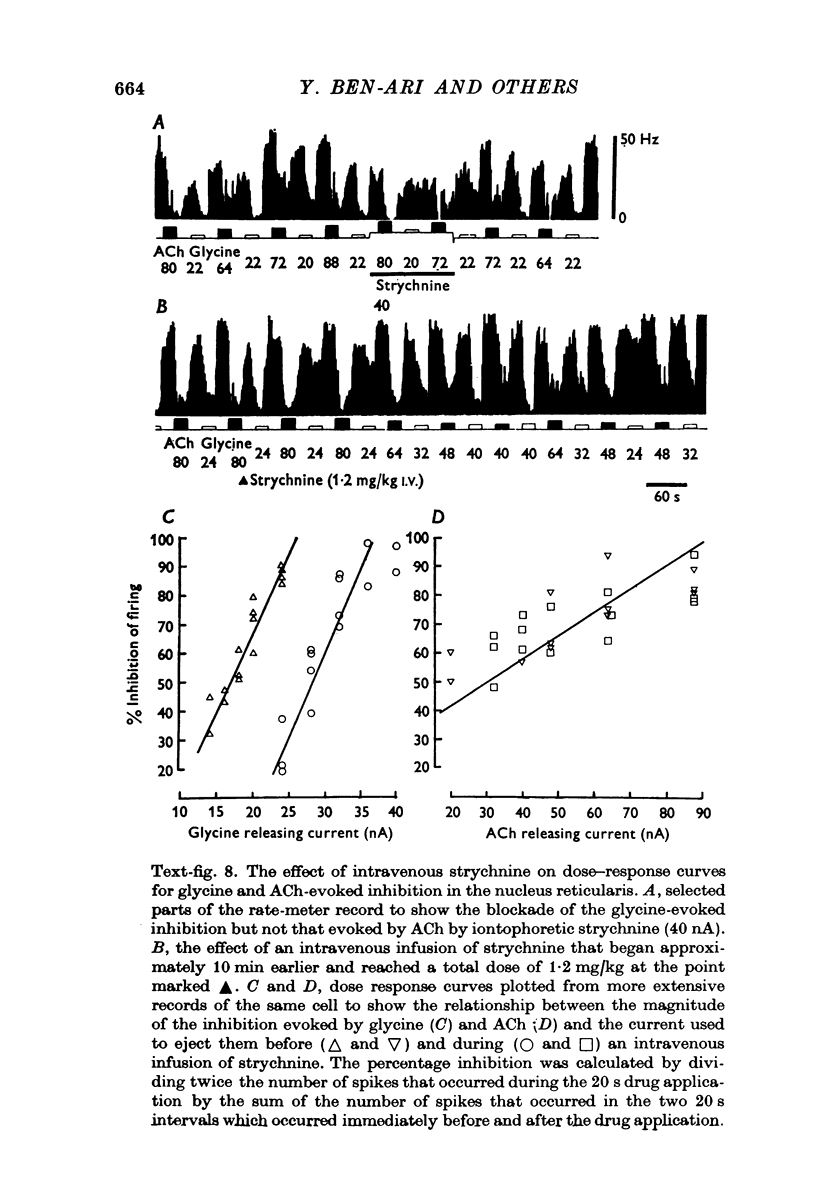







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSEN P., CURTIS D. R. THE EXCITATION OF THALAMIC NEURONES BY ACETYLCHOLINE. Acta Physiol Scand. 1964 May-Jun;61:85–99. doi: 10.1111/j.1748-1716.1964.tb02945.x. [DOI] [PubMed] [Google Scholar]
- ANDERSEN P., CURTIS D. R. THE PHARMACOLOGY OF THE SYNAPTIC AND ACETYLCHOLINE-INDUCED EXCITATION OF VENTROBASAL THALAMIC NEURONES. Acta Physiol Scand. 1964 May-Jun;61:100–120. doi: 10.1111/j.1748-1716.1964.tb02946.x. [DOI] [PubMed] [Google Scholar]
- BURGEN A. S., TERROUX K. G. On the negative inotropic effect in the cat's auricle. J Physiol. 1953 Jun 29;120(4):449–464. doi: 10.1113/jphysiol.1953.sp004910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ari Y., Kanazawa I., Kelly J. S. Exclusively inhibitory action of iontophoretic acetylcholine on single neurones of feline thalamus. Nature. 1976 Jan 29;259(5541):327–330. doi: 10.1038/259327a0. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y., Kelly J. S. Dopamine evoked inhibition of single cells of the feline putamen and basolateral amygdala. J Physiol. 1976 Mar;256(1):1–21. doi: 10.1113/jphysiol.1976.sp011308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breuker E., Johnston G. A. Inhibition of acetylcholinesterase by bicuculline and related alkaloids. J Neurochem. 1975 Dec;25(6):903–904. doi: 10.1111/j.1471-4159.1975.tb04427.x. [DOI] [PubMed] [Google Scholar]
- Brownstein M., Kobayashi R., Palkovits M., Saavedra J. M. Choline acetyltransferase levels in diencephalic nuclei of the rat. J Neurochem. 1975 Jan;24(1):35–38. doi: 10.1111/j.1471-4159.1975.tb07624.x. [DOI] [PubMed] [Google Scholar]
- Curtis D. R., Johnston G. A. Amino acid transmitters in the mammalian central nervous system. Ergeb Physiol. 1974;69(0):97–188. doi: 10.1007/3-540-06498-2_3. [DOI] [PubMed] [Google Scholar]
- Davidoff R. A., Aprison M. H. Picrotoxin antagonism of the inhibition of interneurons by glycine. Life Sci. 1969 Jan 1;8(1):107–112. doi: 10.1016/0024-3205(69)90299-9. [DOI] [PubMed] [Google Scholar]
- Dreifuss J. J., Kelly J. S., Krnjević K. Cortical inhibition and gamma-aminobutyric acid. Exp Brain Res. 1969;9(2):137–154. doi: 10.1007/BF00238327. [DOI] [PubMed] [Google Scholar]
- Duggan A. W., Hall J. G. Inhibition of thalamic neurones by acetylcholine. Brain Res. 1975 Dec 19;100(2):445–449. doi: 10.1016/0006-8993(75)90499-0. [DOI] [PubMed] [Google Scholar]
- EDWARDS C., KUFFLER S. W., TRAUTWEIN W. Changes in membrane characteristics of heart muscle during inhibition. J Gen Physiol. 1956 Sep 20;40(1):135–145. doi: 10.1085/jgp.40.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfraind J. M. Micro-electrophoretic studies in the cat pulvinar region: effect of acetylcholine. Exp Brain Res. 1975 Mar 27;22(3):243–254. doi: 10.1007/BF00234767. [DOI] [PubMed] [Google Scholar]
- Hill R. G., Simmonds M. A., Straughan D. W. Convulsive properties of d-tubocurarine and cortical inhibition. Nature. 1972 Nov 3;240(5375):51–52. doi: 10.1038/240051a0. [DOI] [PubMed] [Google Scholar]
- Jones E. G. Some aspects of the organization of the thalamic reticular complex. J Comp Neurol. 1975 Aug 1;162(3):285–308. doi: 10.1002/cne.901620302. [DOI] [PubMed] [Google Scholar]
- Jordan L. M., Phillis J. W. Acetylcholine inhibition in the intact and chronically isolated cerebral cortex. Br J Pharmacol. 1972 Aug;45(4):584–595. doi: 10.1111/j.1476-5381.1972.tb08116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krnjević K., Pumain R., Renaud L. The mechanism of excitation by acetylcholine in the cerebral cortex. J Physiol. 1971 May;215(1):247–268. doi: 10.1113/jphysiol.1971.sp009467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo J. F., Lee T. P., Reyes P. L., Walton K. G., Donnelly T. E., Jr, Greengard P. Cyclic nucleotide-dependent protein kinases. X. An assay method for the measurement of quanosine 3',5'-monophosphate in various biological materials and a study of agents regulating its levels in heart and brain. J Biol Chem. 1972 Jan 10;247(1):16–22. [PubMed] [Google Scholar]
- Lamarre Y., Filion M., Cordeau J. P. Neuronal discharges of the ventrolateral nucleus of the thalamus during sleep and wakefulness in the cat. I. Spontaneous activity. Exp Brain Res. 1971 Jun 29;12(5):480–498. doi: 10.1007/BF00234244. [DOI] [PubMed] [Google Scholar]
- McCance I., Phillis J. W., Tebécis A. K., Westerman R. A. The pharmacology of acetylcholine-excitation of thalamic neurones. Br J Pharmacol Chemother. 1968 Mar;32(3):652–662. doi: 10.1111/j.1476-5381.1968.tb00464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCance I., Phillis J. W., Westerman R. A. Acetylcholine-sensitivity of thalamic neurones: its relationship to synaptic transmission. Br J Pharmacol Chemother. 1968 Mar;32(3):635–651. doi: 10.1111/j.1476-5381.1968.tb00463.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLennan H., Huffman R. D., Marshall K. C. Patterns of excitation of thalamic neurones by amino-acids and by acetylcholine. Nature. 1968 Jul 27;219(5152):387–388. doi: 10.1038/219387a0. [DOI] [PubMed] [Google Scholar]
- Minderhoud J. M. An anatomical study of the efferent connections of the thalamic reticular nucleus. Exp Brain Res. 1971 May 26;112(4):435–446. doi: 10.1007/BF00234497. [DOI] [PubMed] [Google Scholar]
- Mukhametov L. M., Rizzolatti G., Tradardi V. Spontaneous activity of neurones of nucleus reticularis thalami in freely moving cats. J Physiol. 1970 Oct;210(3):651–667. doi: 10.1113/jphysiol.1970.sp009233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson P. G., Peacock J. H. Acetylcholine responses in L cells. Science. 1972 Sep 15;177(4053):1005–1007. doi: 10.1126/science.177.4053.1005. [DOI] [PubMed] [Google Scholar]
- Nelson P. G., Peacock J. H., Amano T. Responses of neuroblastoma cells to iontophoretically applied acetylcholine. J Cell Physiol. 1971 Jun;77(3):353–362. doi: 10.1002/jcp.1040770309. [DOI] [PubMed] [Google Scholar]
- Olivier A., Parent A., Poirier L. J. Identification of the thalamic nuclei on the basis of their cholinesterase content in the monkey. J Anat. 1970 Jan;106(Pt 1):37–50. [PMC free article] [PubMed] [Google Scholar]
- Phillis J. W. The pharmacology of thalamic and geniculate neurons. Int Rev Neurobiol. 1971;14:1–48. doi: 10.1016/s0074-7742(08)60182-8. [DOI] [PubMed] [Google Scholar]
- Phillis J. W., York D. H. Cholinergic inhibition in the cerebral cortex. Brain Res. 1967 Aug;5(4):517–520. doi: 10.1016/0006-8993(67)90025-x. [DOI] [PubMed] [Google Scholar]
- Phillis J. W., York D. H. Pharmacological studies on a cholinergic inhibition in the cerebral cortex. Brain Res. 1968 Sep;10(3):297–306. doi: 10.1016/0006-8993(68)90201-1. [DOI] [PubMed] [Google Scholar]
- Phillis J. W., York D. H. Strychnine block of neural and drug-induced inhibition in the cerebral cortex. Nature. 1967 Dec 2;216(5118):922–923. doi: 10.1038/216922a0. [DOI] [PubMed] [Google Scholar]
- Pong S. F., Graham L. T. N-methyl bicuculline, a convulsant more potent than bicuculline. Brain Res. 1972 Jul 20;42(2):486–490. doi: 10.1016/0006-8993(72)90547-1. [DOI] [PubMed] [Google Scholar]
- Purpura D. P., McMurtry J. G., Maekawa K. Synaptic events in ventrolateral thalamic neurons during suppression of recruiting responses by brain stem reticular stimulation. Brain Res. 1966 Jan;1(1):63–76. doi: 10.1016/0006-8993(66)90105-3. [DOI] [PubMed] [Google Scholar]
- RANDIC M., SIMINOFF R., STRAUGHAN D. W. ACETYLCHOLINE DEPRESSION OF CORTICAL NEURONS. Exp Neurol. 1964 Mar;9:236–242. doi: 10.1016/0014-4886(64)90020-2. [DOI] [PubMed] [Google Scholar]
- SUZUKI H., TAIRA N. Effect of reticular stimulation upon synaptic transmission in cat's lateral geniculate body. Jpn J Physiol. 1961 Dec 15;11:641–655. doi: 10.2170/jjphysiol.11.641. [DOI] [PubMed] [Google Scholar]
- Scheibel M. E., Scheibel A. B. The organization of the nucleus reticularis thalami: a Golgi study. Brain Res. 1966 Jan;1(1):43–62. doi: 10.1016/0006-8993(66)90104-1. [DOI] [PubMed] [Google Scholar]
- Schlag J., Waszak M. Characteristics of unit responses in nucleus reticularis thalami. Brain Res. 1970 Jul 14;21(2):286–288. doi: 10.1016/0006-8993(70)90371-9. [DOI] [PubMed] [Google Scholar]
- Schlag J., Waszak M. Electrophysiological properties of units of the thalamic reticular complex. Exp Neurol. 1971 Jul;32(1):79–97. doi: 10.1016/0014-4886(71)90167-1. [DOI] [PubMed] [Google Scholar]
- Schneyer L. H., Young J. A., Schneyer C. A. Salivary secretion of electrolytes. Physiol Rev. 1972 Jul;52(3):720–777. doi: 10.1152/physrev.1972.52.3.720. [DOI] [PubMed] [Google Scholar]
- Shute C. C., Lewis P. R. The ascending cholinergic reticular system: neocortical, olfactory and subcortical projections. Brain. 1967 Sep;90(3):497–520. doi: 10.1093/brain/90.3.497. [DOI] [PubMed] [Google Scholar]
- Steriade M., Wyzinski P. Cortically elicited activities in thalamic reticularis neurons. Brain Res. 1972 Jul 20;42(2):514–520. doi: 10.1016/0006-8993(72)90552-5. [DOI] [PubMed] [Google Scholar]
- Stone T. W. Cholinergic mechanisms in the rat somatosensory cerebral cortex. J Physiol. 1972 Sep;225(2):485–499. doi: 10.1113/jphysiol.1972.sp009951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone T. W., Taylor D. A., Bloom F. E. Cyclic AMP and cyclic GMP may mediate opposite neuronal responses in the rat cerebral cortex. Science. 1975 Mar 7;187(4179):845–847. doi: 10.1126/science.163488. [DOI] [PubMed] [Google Scholar]
- TAUC L., GERSCHENFELD H. M. A cholinergic mechanism of inhibitory synaptic transmission in a molluscan nervous system. J Neurophysiol. 1962 Mar;25:236–262. doi: 10.1152/jn.1962.25.2.236. [DOI] [PubMed] [Google Scholar]
- Waszak M. Firing pattern of neurons in the rostral and ventral part of nucleus reticularis thalami during EEG spindles. Exp Neurol. 1974 Apr;43(1):38–58. doi: 10.1016/0014-4886(74)90132-0. [DOI] [PubMed] [Google Scholar]
- Weight F. F., Padjen A. Acetylcholine and slow synaptic inhibition in frog sympathetic ganglion cells. Brain Res. 1973 May 30;55(1):225–228. doi: 10.1016/0006-8993(73)90506-4. [DOI] [PubMed] [Google Scholar]
- ten Bruggencate G., Engberg I. Iontophoretic studies in Deiters' nucleus of the inhibitory actions of GABA and related amino acids and the interactions of strychnine and picrotoxin. Brain Res. 1971 Feb 5;25(3):431–448. doi: 10.1016/0006-8993(71)90453-7. [DOI] [PubMed] [Google Scholar]