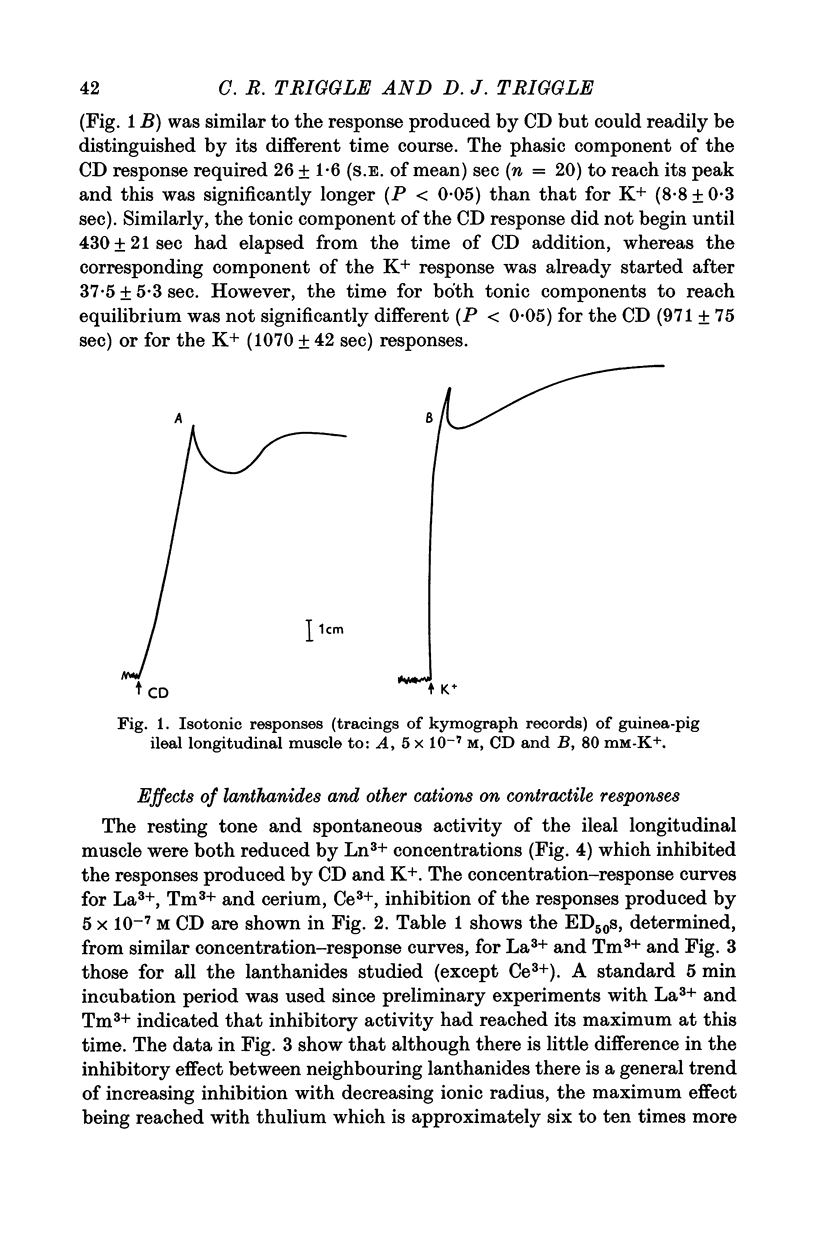
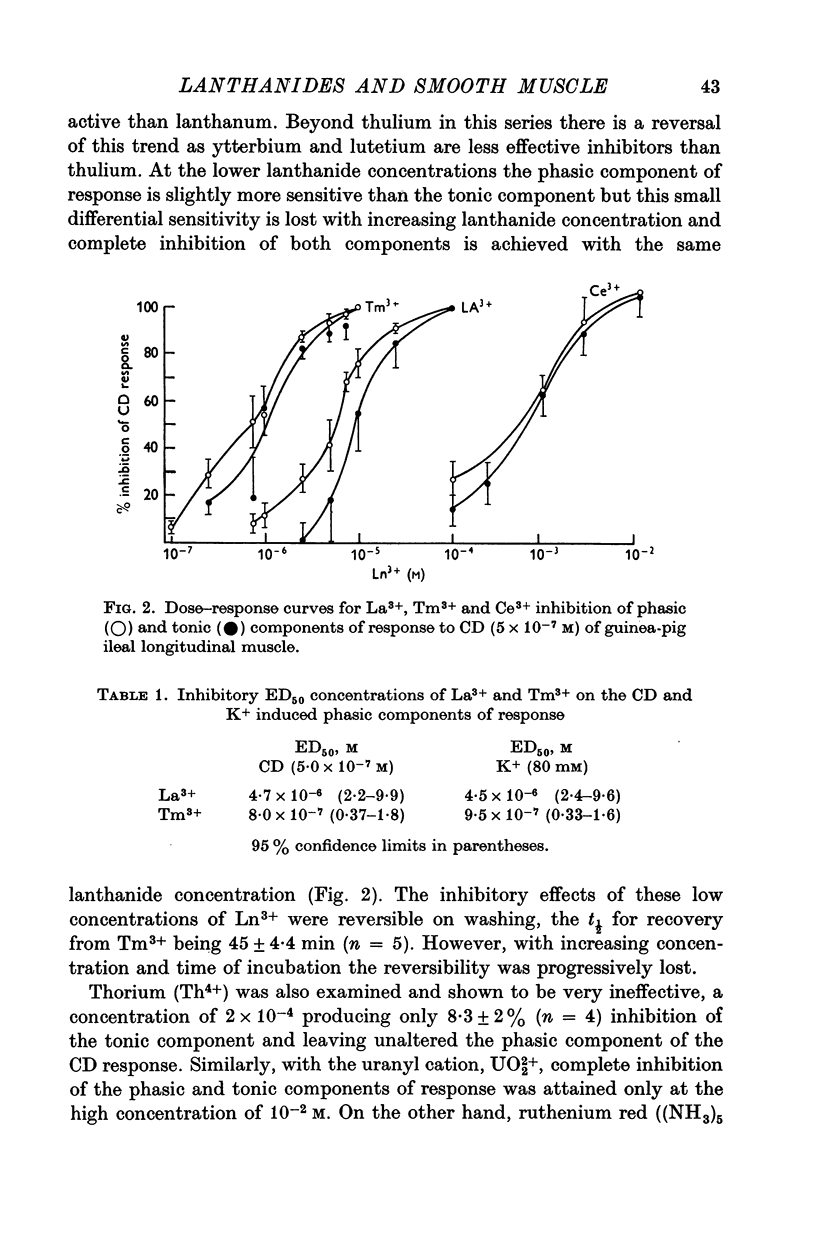
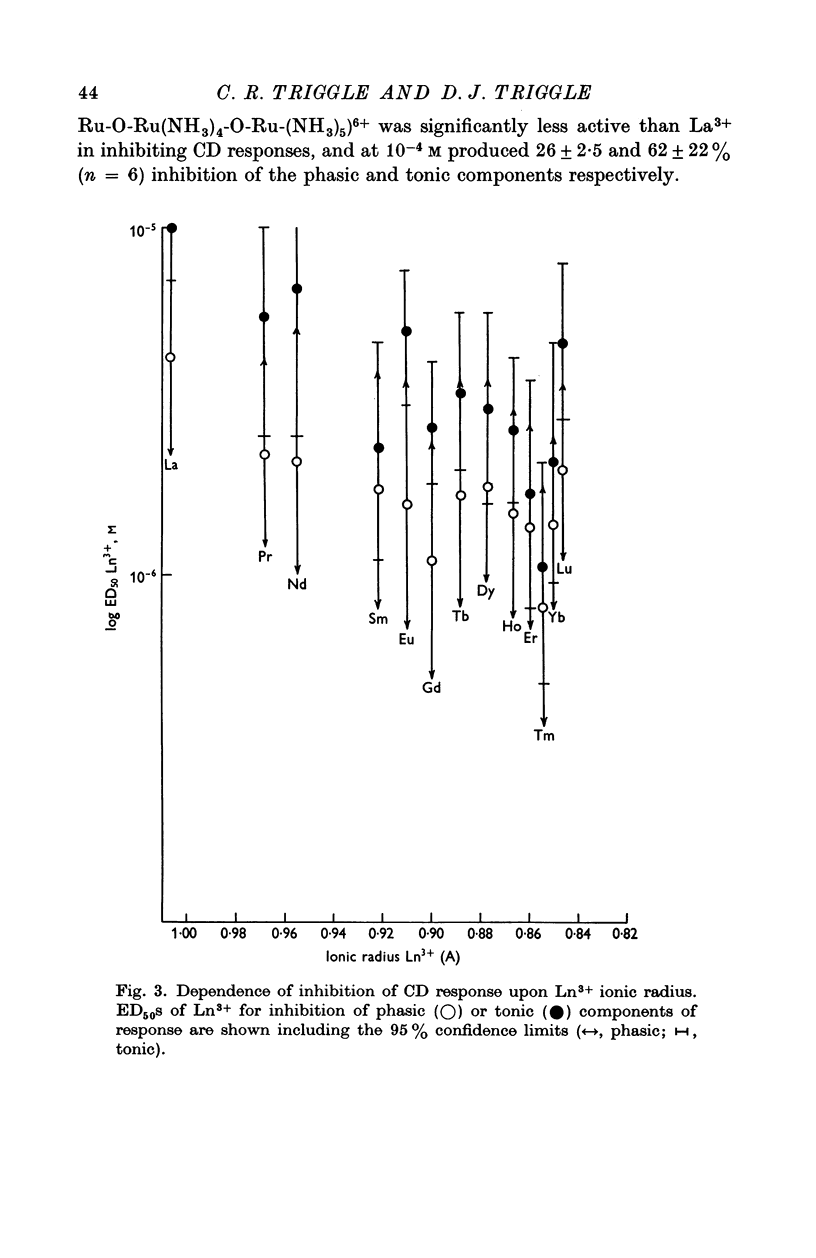
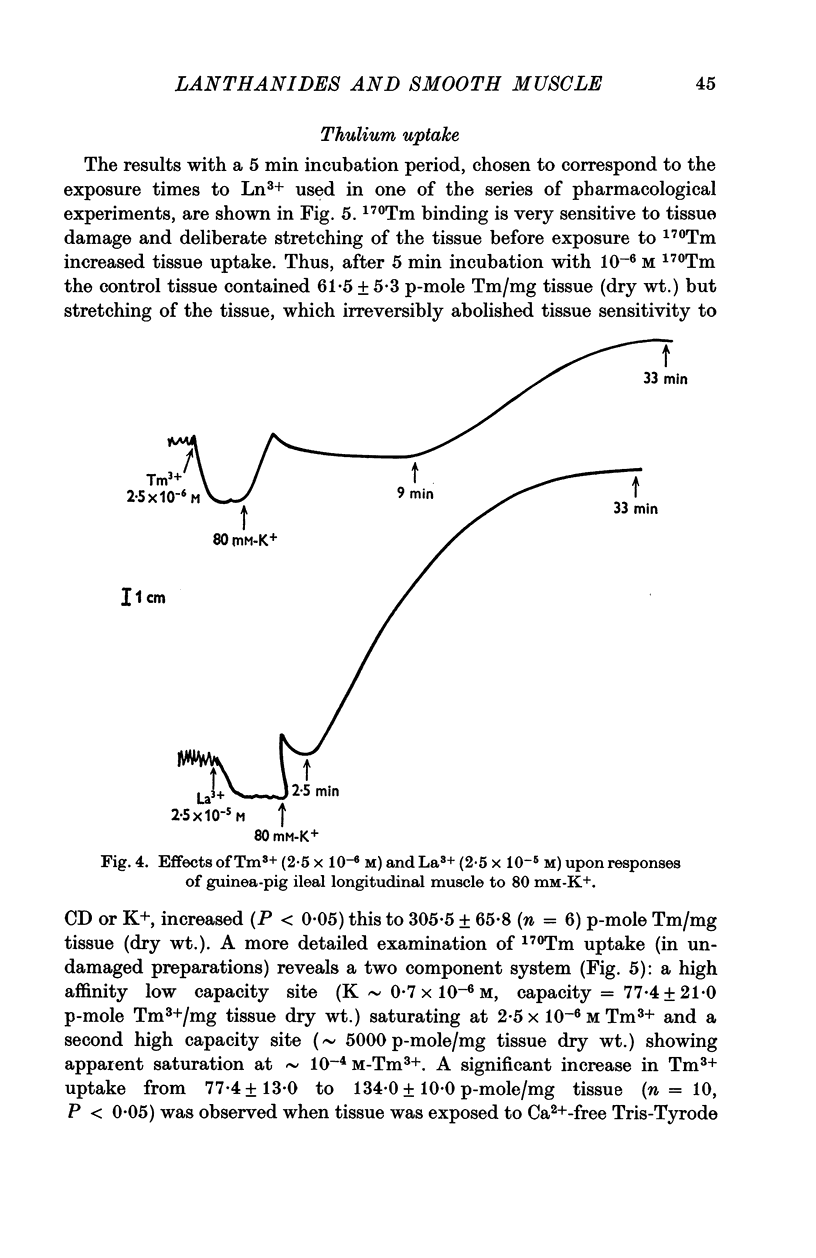
Abstract
1. The inhibitory effects of lanthanide cations (Ln3+) on mechanical responses and 45Ca uptake in guinea-pig ileal longitudinal smooth muscle were studied. 2. Ln3+ strongly inhibited the phasic and tonic component of the response to the muscarinic agonist cis-2-methyl-4-dimethylaminomethyl-1,3-dioxolane methiodide (CD) the two components being affected to the same extent. Inhibition was also obtained for the responses evoked by high K+ but here the effect was mainly on the phasic response, the tonic component was merely delayed. 3. Other members of the Ln3+ series, with the exception of cerium, were found to be more effective than lanthanum in their ability to inhibit the CD response. Thulium, Tm3+, the thirteenth member of the series was the most effective cation. 4. Analysis of 170Tm uptake revealed at least two components. The concentration-dependence of one component, saturating at 2-5 x 10(-6) Tm, corresponded closely to that of the inhibitory effect of Tm3+ on contraction. 5. 170Tm uptake as a function of time showed a secondary rise after 30 min of exposure to the lanthanide. 6. Although 2-5 x 10(-6) M-Tm3+ produced 90% inhibition of the CD and the high K+ induced responses significant reduction of 45Ca uptake by the muscle was only detected when much higher Tm3+ concentrations (greater than or equal 10(-3) M-Tm3+) were used. 7. It is concluded that Ln3+ combine with membrane sites specifically involved in Ca2+ translocation during excitation-contraction coupling.
Full text
PDF


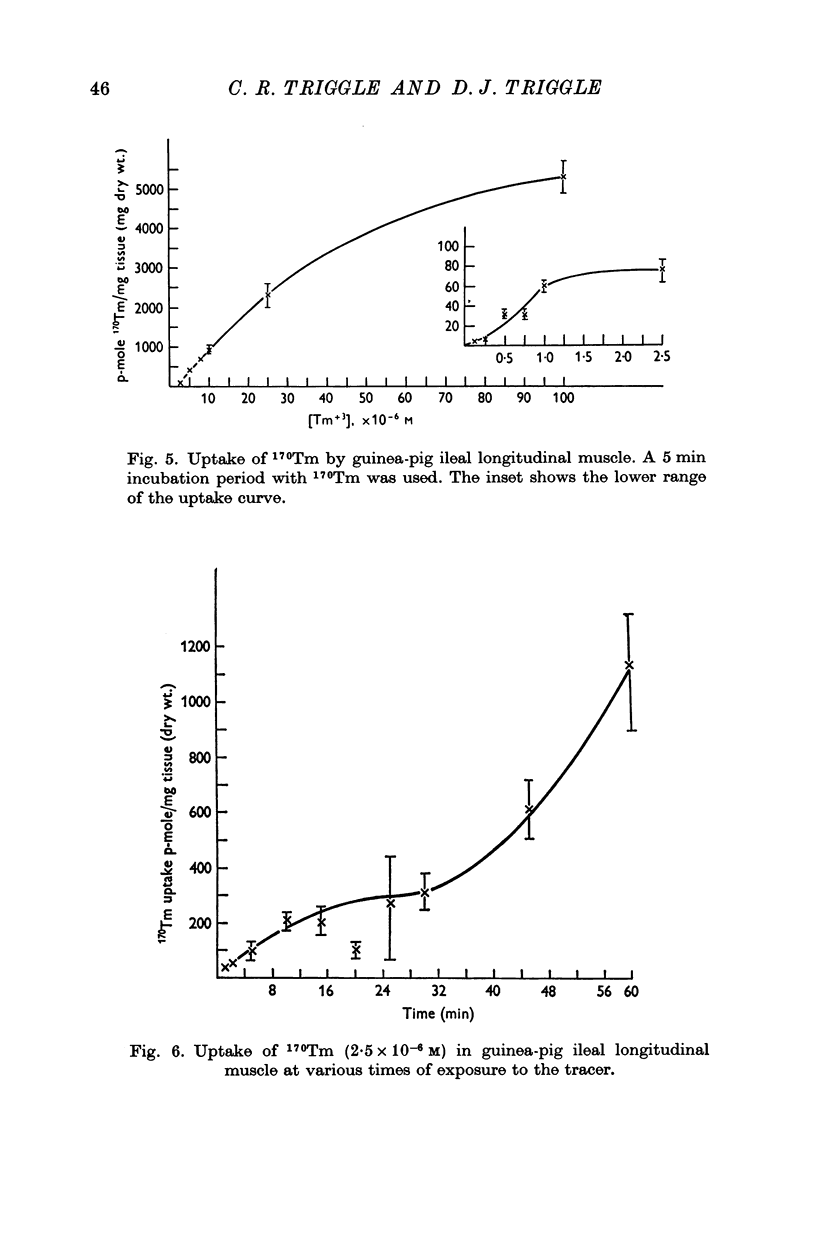

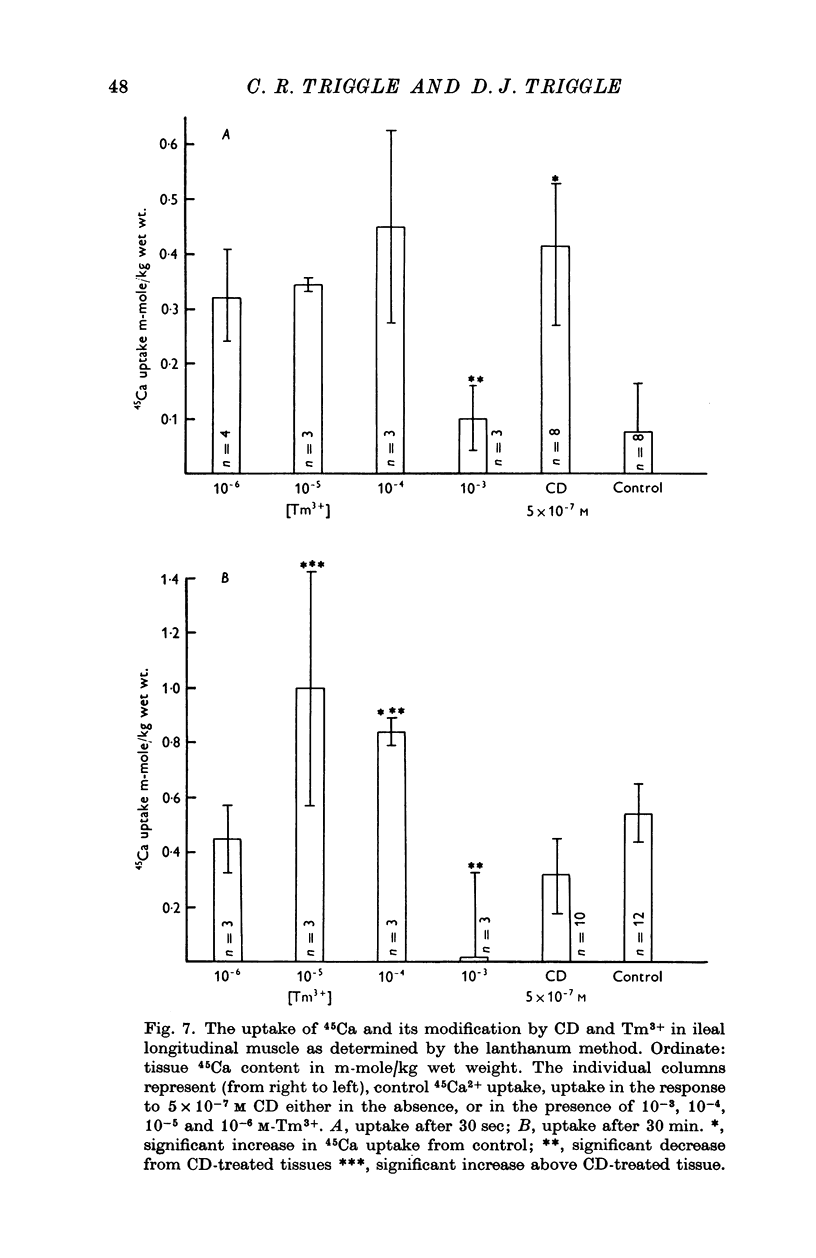
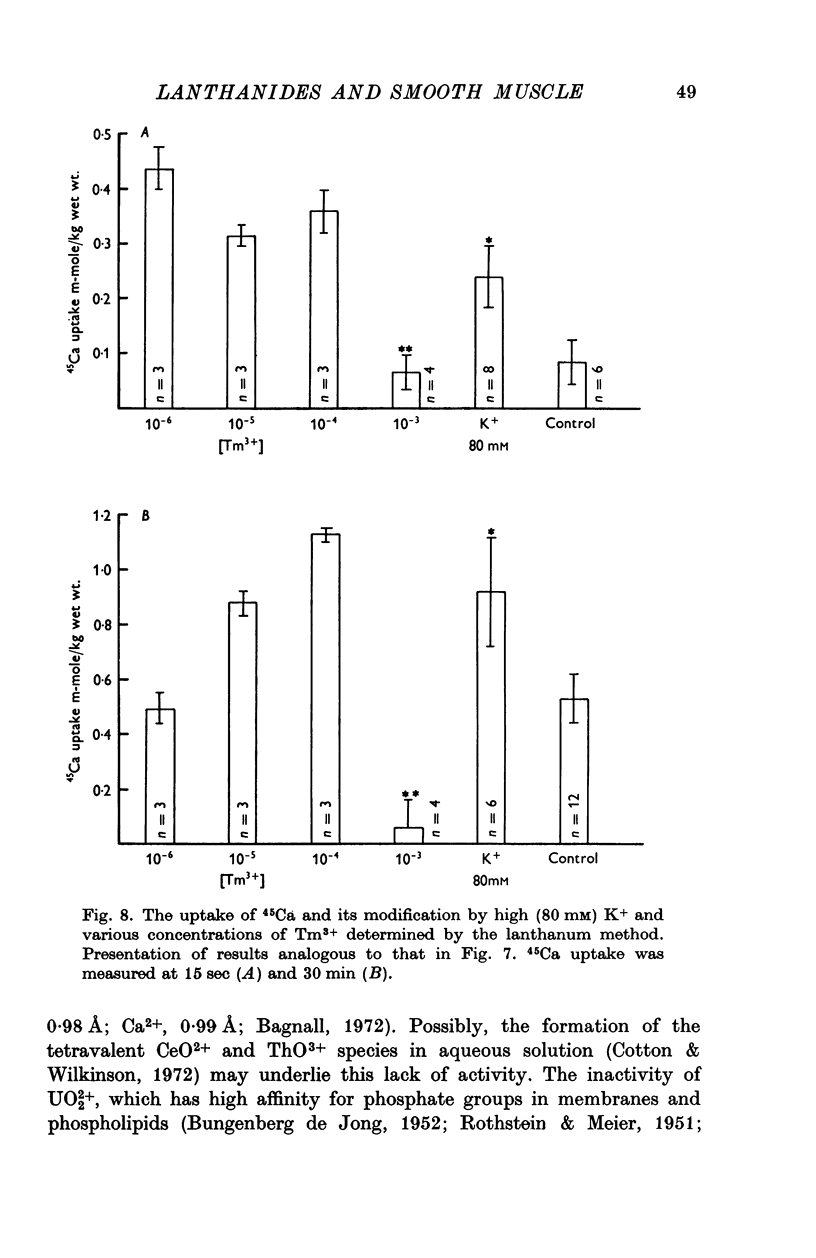
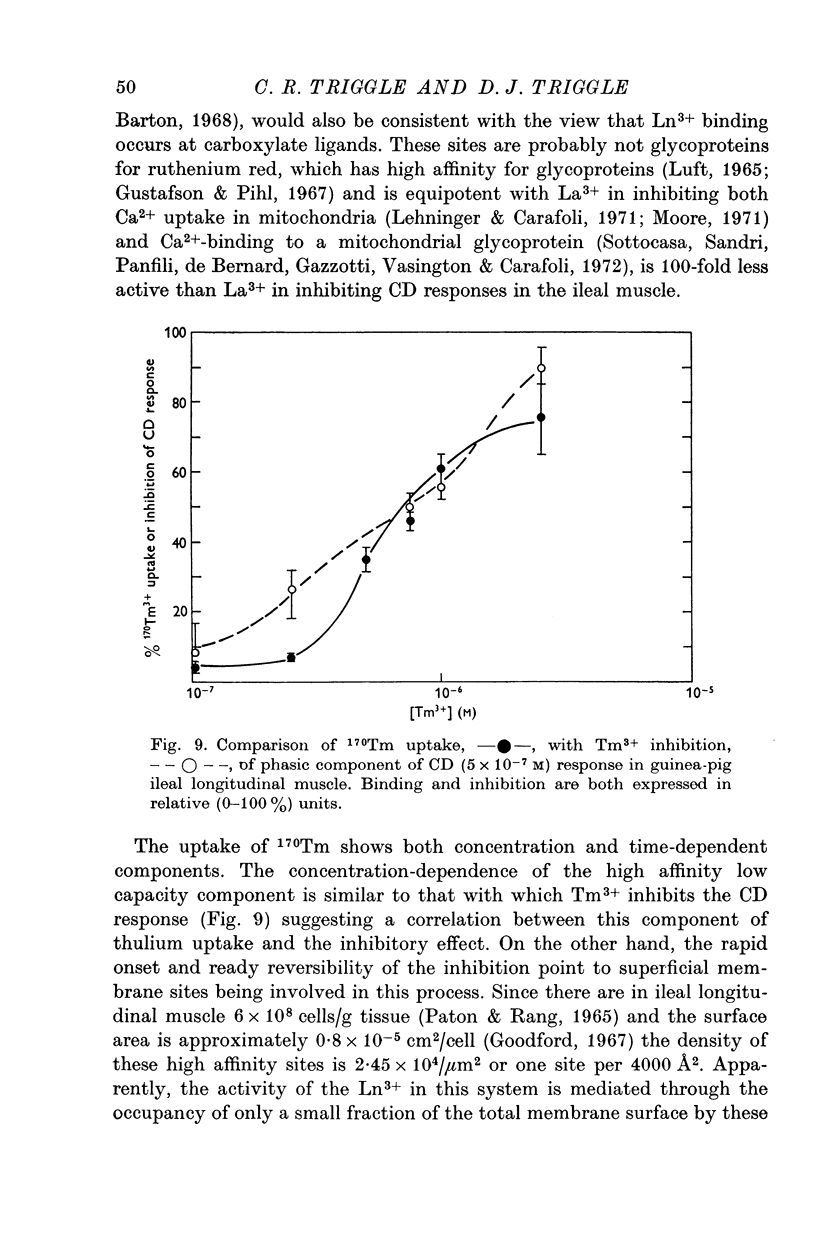




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOHR D. F. ELECTROLYTES AND SMOOTH MUSCLE CONTRACTION. Pharmacol Rev. 1964 Mar;16:85–127. [PubMed] [Google Scholar]
- Bannister L. H. Lanthanum as an intracellular stain. J Microsc. 1972 Jun;95(3):413–419. doi: 10.1111/j.1365-2818.1972.tb01042.x. [DOI] [PubMed] [Google Scholar]
- Barton P. G. The influence of surface charge density of phosphatides on the binding of some cations. J Biol Chem. 1968 Jul 25;243(14):3884–3890. [PubMed] [Google Scholar]
- Bloom S., Brady A. J., Langer G. A. Calcium metabolism and active tension in mechanically disaggregated heart muscle. J Mol Cell Cardiol. 1974 Apr;6(2):137–147. doi: 10.1016/0022-2828(74)90017-0. [DOI] [PubMed] [Google Scholar]
- Chang K. J., Triggle D. J. Quantitative aspects of drug-receptor interactions. I. Ca2+ and cholinergic receptor activation in smooth muscle: a basic model for drug-receptor interactions. J Theor Biol. 1973 Jul;40(1):125–154. doi: 10.1016/0022-5193(73)90168-9. [DOI] [PubMed] [Google Scholar]
- Diamond J. M., Wright E. M. Biological membranes: the physical basis of ion and nonelectrolyte selectivity. Annu Rev Physiol. 1969;31:581–646. doi: 10.1146/annurev.ph.31.030169.003053. [DOI] [PubMed] [Google Scholar]
- Fleckenstein A. Physiologie und Pharmakologie der transmembranären Natrium-, Kalium- und Calcium-Bewegungen. Arzneimittelforschung. 1972 Dec;22(12):2019–2028. [PubMed] [Google Scholar]
- Freeman D. J., Daniel E. E. Calcium movement in vascular smooth muscle and its detection using lanthanum as a tool. Can J Physiol Pharmacol. 1973 Dec;51(12):900–913. doi: 10.1139/y73-139. [DOI] [PubMed] [Google Scholar]
- Goodford P. J. The calcium content of the smooth muscle of the guinea-pig taenia coli. J Physiol. 1967 Sep;192(1):145–157. doi: 10.1113/jphysiol.1967.sp008293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman F. R., Weiss G. B. Dissociation by lanthanum of smooth muscle responses to potassium and acetylcholine. Am J Physiol. 1971 Mar;220(3):759–766. doi: 10.1152/ajplegacy.1971.220.3.759. [DOI] [PubMed] [Google Scholar]
- Hodgson B. J., Kidwai A. M., Daniel E. E. Uptake of lanthanum by smooth muscle. Can J Physiol Pharmacol. 1972 Jul;50(7):730–733. doi: 10.1139/y72-107. [DOI] [PubMed] [Google Scholar]
- Hurwitz L., Suria A. The link between agonist action and response in smooth muscle. Annu Rev Pharmacol. 1971;11:303–326. doi: 10.1146/annurev.pa.11.040171.001511. [DOI] [PubMed] [Google Scholar]
- Kohlhardt M., Bauer B., Krause H., Fleckenstein A. Differentiation of the transmembrane Na and Ca channels in mammalian cardiac fibres by the use of specific inhibitors. Pflugers Arch. 1972;335(4):309–322. doi: 10.1007/BF00586221. [DOI] [PubMed] [Google Scholar]
- Langer G. A., Frank J. S. Lanthanum in heart cell culture. Effect on calcium exchange correlated with its localization. J Cell Biol. 1972 Sep;54(3):441–455. doi: 10.1083/jcb.54.3.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer G. A., Serena S. D., Nudd L. M. Cation exchange in heart cell culture: correlation with effects on contractile force. J Mol Cell Cardiol. 1974 Apr;6(2):149–161. doi: 10.1016/0022-2828(74)90018-2. [DOI] [PubMed] [Google Scholar]
- Lehninger A. L., Carafoli E. The interaction of La 3+ with mitochondria in relation to respiration-coupled Ca 2+ transport. Arch Biochem Biophys. 1971 Apr;143(2):506–515. doi: 10.1016/0003-9861(71)90235-9. [DOI] [PubMed] [Google Scholar]
- Mayer C. J., van Breemen C., Casteels T. The action of lanthanum and D600 on the calcium exchange in the smooth muscle cells of the guinea-pig Taenia coli. Pflugers Arch. 1972;337(4):333–350. doi: 10.1007/BF00586650. [DOI] [PubMed] [Google Scholar]
- Moore C. L. Specific inhibition of mitochondrial Ca++ transport by ruthenium red. Biochem Biophys Res Commun. 1971 Jan 22;42(2):298–305. doi: 10.1016/0006-291x(71)90102-1. [DOI] [PubMed] [Google Scholar]
- PATON W. D., RANG H. P. THE UPTAKE OF ATROPINE AND RELATED DRUGS BY INTESTINAL SMOOTH MUSCLE OF THE GUINEA-PIG IN RELATION TO ACETYLCHOLINE RECEPTORS. Proc R Soc Lond B Biol Sci. 1965 Aug 24;163:1–44. doi: 10.1098/rspb.1965.0058. [DOI] [PubMed] [Google Scholar]
- RANG H. P. STIMULANT ACTIONS OF VOLATILE ANAESTHETICS ON SMOOTH MUSCLE. Br J Pharmacol Chemother. 1964 Apr;22:356–365. doi: 10.1111/j.1476-5381.1964.tb02040.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROTHSTEIN A., MEIER R. The relationship of the cell surface to metabolism. VI. The chemical nature of uranium-complexing groups of the cell surface. J Cell Physiol. 1951 Oct;38(2):245–270. doi: 10.1002/jcp.1030380209. [DOI] [PubMed] [Google Scholar]
- Reed K. C., Bygrave F. L. Accumulation of lanthanum by rat liver mitochondria. Biochem J. 1974 Feb;138(2):239–252. doi: 10.1042/bj1380239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sottocasa G., Sandri G., Panfili E., De Bernard B., Gazzotti P., Vasington F. D., Carafoli E. Isolation of a soluble Ca 2+ binding glycoprotein from ox liver mitochondria. Biochem Biophys Res Commun. 1972 May 26;47(4):808–813. doi: 10.1016/0006-291x(72)90564-5. [DOI] [PubMed] [Google Scholar]
- Triggle D. J. Adrenergic receptors. Annu Rev Pharmacol. 1972;12:185–196. doi: 10.1146/annurev.pa.12.040172.001153. [DOI] [PubMed] [Google Scholar]
- Van Breemen C. Blockade of membrane calcium fluxes by lanthanum in relation to vascular smooth muscle contractility. Arch Int Physiol Biochim. 1969 Oct;77(4):710–716. doi: 10.3109/13813456909059783. [DOI] [PubMed] [Google Scholar]
- Van Breemen C., Farinas B. R., Gerba P., McNaughton E. D. Excitation-contraction coupling in rabbit aorta studied by the lanthanum method for measuring cellular calcium influx. Circ Res. 1972 Jan;30(1):44–54. doi: 10.1161/01.res.30.1.44. [DOI] [PubMed] [Google Scholar]
- Van Breemen C. Permselectivity of a porous phospholipid-cholesterol artificial membrane. Calcium and lanthanum effects. Biochem Biophys Res Commun. 1968 Sep 30;32(6):977–983. doi: 10.1016/0006-291x(68)90124-1. [DOI] [PubMed] [Google Scholar]
- Weiss G. B., Goodman F. R. Effects of lanthanum on contraction, calcium distribution and Ca45 movements in intestinal smooth muscle. J Pharmacol Exp Ther. 1969 Sep;169(1):46–55. [PubMed] [Google Scholar]
- van Breemen C., De Weer P. Lanthanum inhibition of 45Ca efflux from the squid giant axon. Nature. 1970 May 23;226(5247):760–761. doi: 10.1038/226760a0. [DOI] [PubMed] [Google Scholar]
- van Breemen C., Farinas B. R., Casteels R., Gerba P., Wuytack F., Deth R. Factors controlling cytoplasmic Ca 2+ concentration. Philos Trans R Soc Lond B Biol Sci. 1973 Mar 15;265(867):57–71. doi: 10.1098/rstb.1973.0009. [DOI] [PubMed] [Google Scholar]



